Fine-Tuning Intrinsic and Doped Hydrogenated Amorphous Silicon Thin-Film Anodes Deposited by PECVD to Enhance Capacity and Stability in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparation and Characterization of Hydrogenated Amorphous Silicon
2.2. Preparation of the aSi:H Electrodes, Assembly in Lithium-Ion Batteries and Electrochemical Characterization
3. Results and Discussion
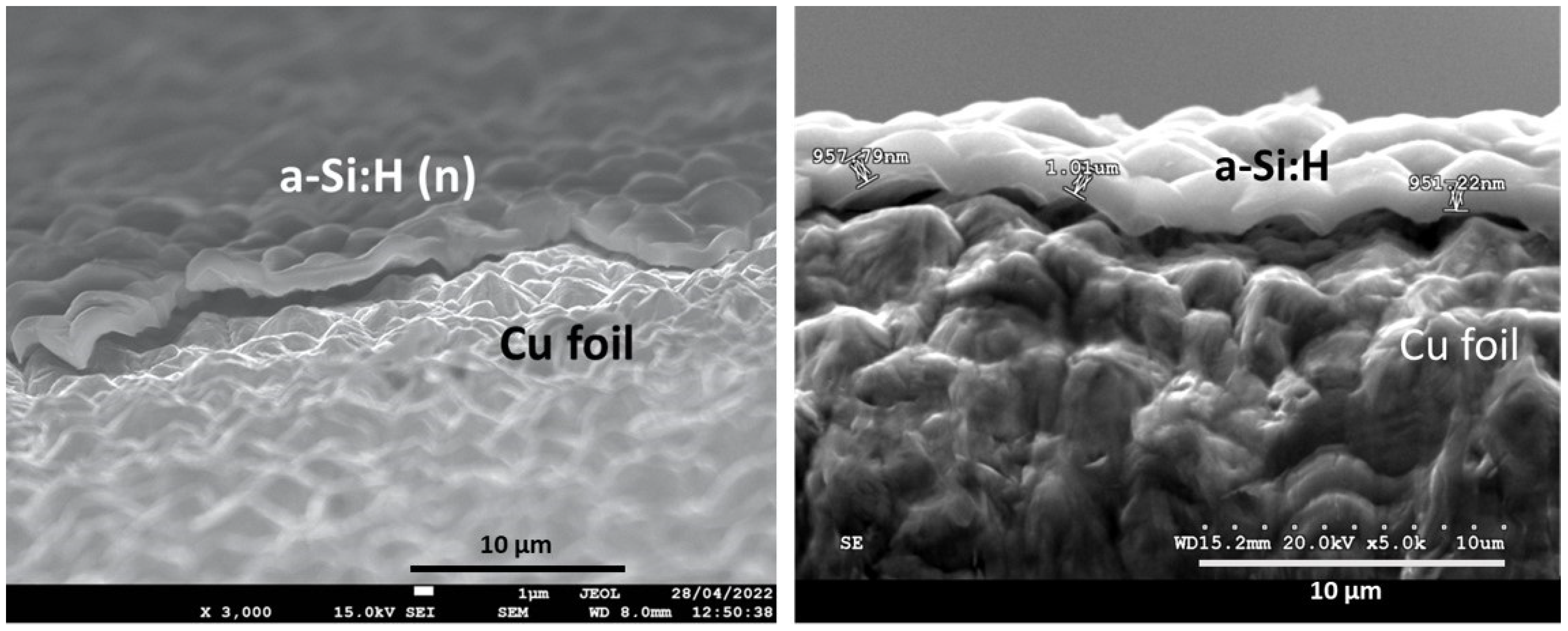
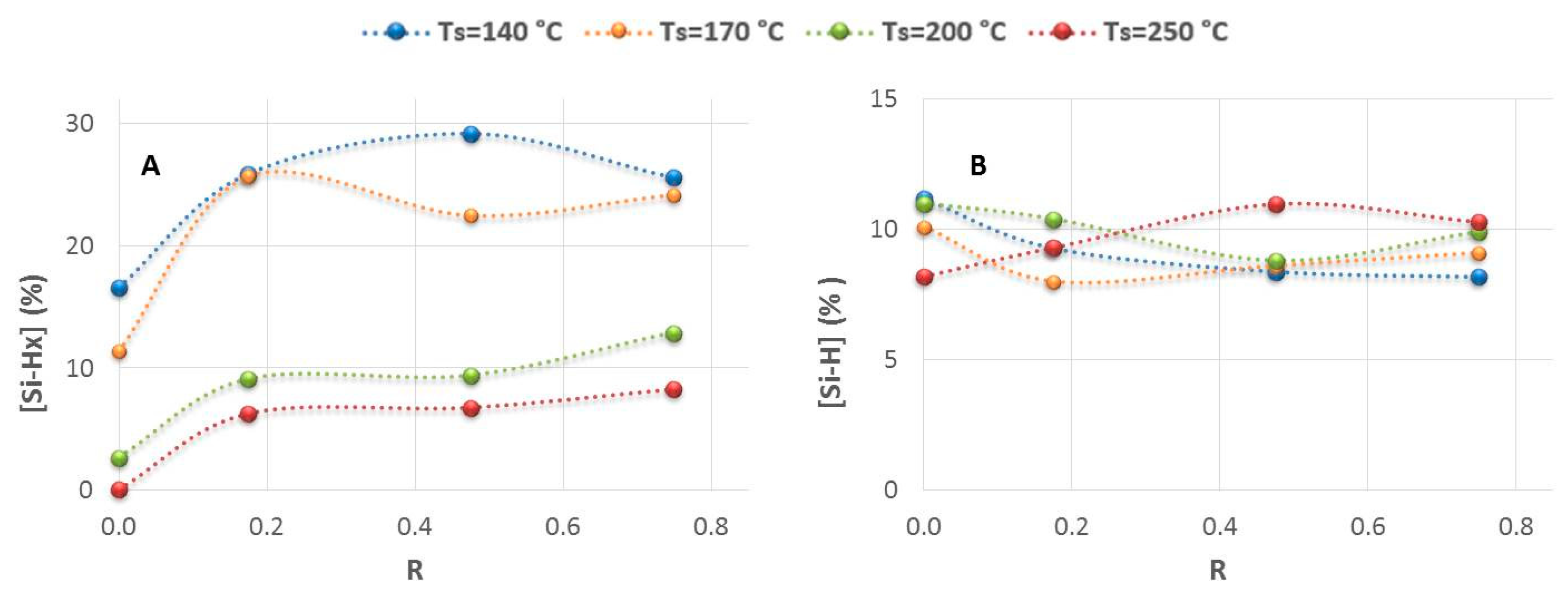
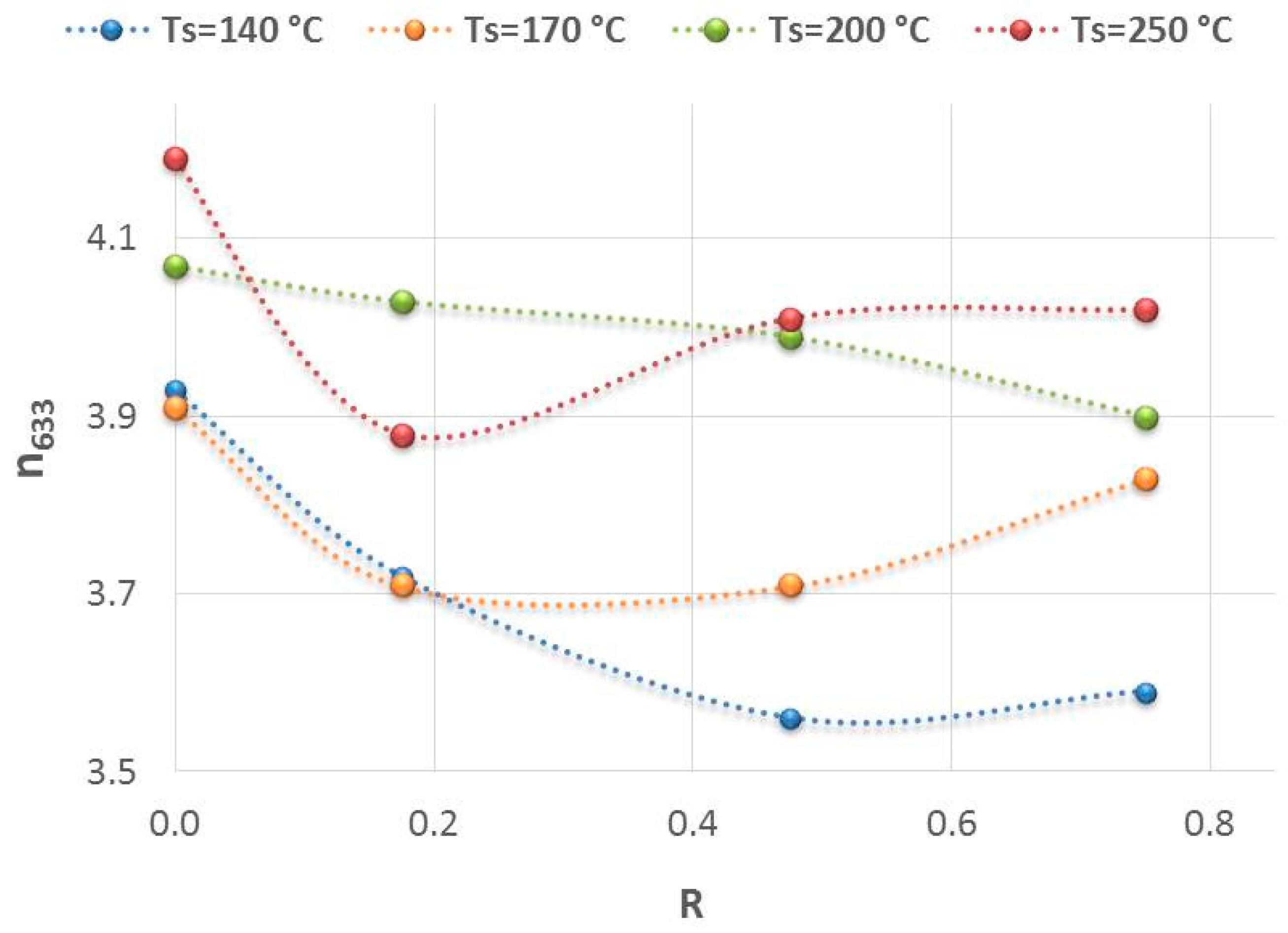
3.1. The Hydrogenated Amorphous Silicon Thin-Films’ Properties
3.2. Assembly of aSi:H Films in Lithium-Ion Batteries
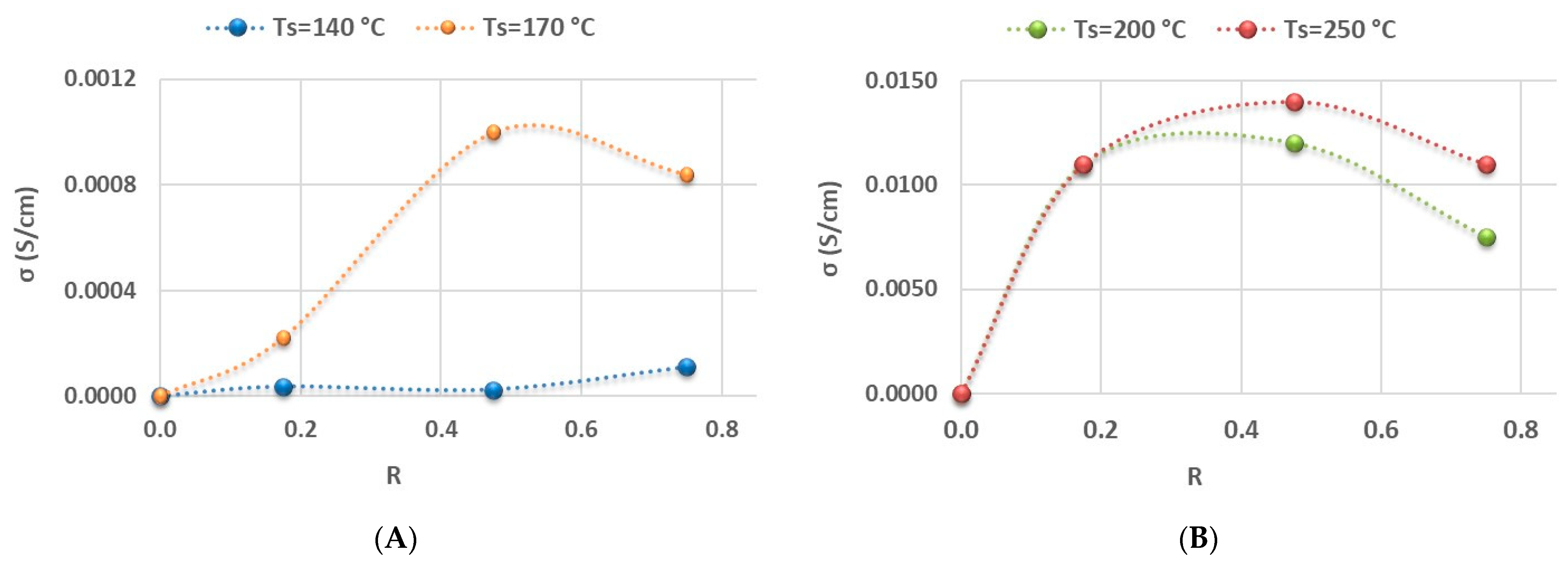
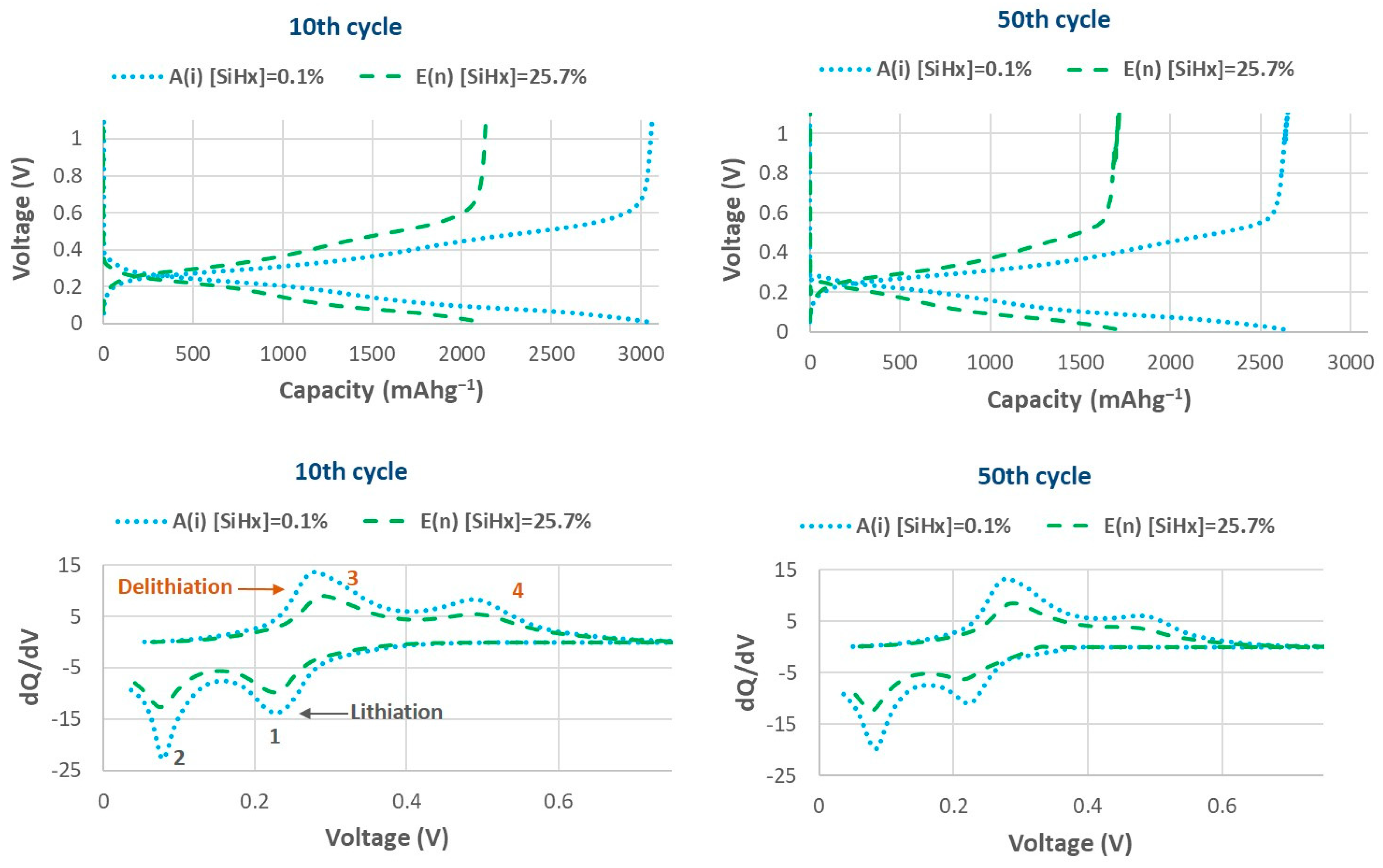
3.2.1. Effect of Electrical Properties on the Specific Capacity and Capacity Retention of LIBs
3.2.2. Effect of the Structural Properties (Si-Hx Concentration and Refractive Index) on Specific Capacity and Capacity Retention
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, Y.; Wan, L.; Guo, Y. Silicon-based nanomaterials for lithium-ion batteries. Chin. Sci. Bull. 2012, 57, 4104–4110. [Google Scholar] [CrossRef]
- Salah, M.; Hall, C.; Murphy, P.; Francis, C.; Kerr, R.; Stoehr, B.; Rudd, S.; Fabretto, M. Doped and reactive silicon thin film anodes for lithium ion batteries: A review. J. Power Sources 2021, 506, 230194. [Google Scholar] [CrossRef]
- Liang, B.; Liu, Y.; Xu, Y. Silicon-based materials as high capacity anodes for next generation lithium ion batteries. J. Power Sources 2014, 267, 469–490. [Google Scholar] [CrossRef]
- Szczech, J.R.; Jin, S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 2011, 4, 56–72. [Google Scholar] [CrossRef]
- Barrio, R.; González, N.; Portugal, Á.; Morant, C.; Gandía, J.J. Hydrogenated Amorphous Silicon-Based Nanomaterials as Alternative Electrodes to Graphite for Lithium-Ion Batteries. Nanomaterials 2022, 12, 4400. [Google Scholar] [CrossRef]
- Pinilla, S.; Barrio, R.; González, N.; Casero, R.P.; Márquez, F.; Sanz, J.M.; Morant, C. Role of Hydrogen in the Preparation of Amorphous Silicon Nanowires by Metal-Assisted Chemical Etching. J. Phys. Chem. C 2018, 122, 22667–22674. [Google Scholar] [CrossRef]
- Nunomura, S.; Uchida, G. Nanostructure and doping effects of a-Si:H and μc-Si:H anode in lithium-ion battery performance. Mater. Lett. 2023, 349, 134777. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, F.; Li, C.; Li, T.; Zhang, S.; Wang, C.; Zhang, Z.; Wang, R. Progress and perspectives on two-dimensional silicon anodes for lithium-ion batteries. ChemPhysMater 2022, 2, 1–19. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kawasaki, H.; Yoshida, W.; Irie, T.; Konishi, K.; Nakano, K.; Uto, T.; Adachi, D.; Kanematsu, M.; Uzu, H.; et al. Silicon heterojunction solar cell with interdigitated back contacts for a photoconversion efficiency over 26%. Nat. Energy 2017, 2, 17032. [Google Scholar] [CrossRef]
- De Wolf, S.; Descoeudres, A.; Holman, Z.C.; Ballif, C.J.G. High-efficiency silicon heterojunction solar cells: A review. Green 2012, 2, 7–24. [Google Scholar] [CrossRef]
- Hosseini, M.J.M.; Nawrocki, R.A. A review of the progress of thin-film transistors and their technologies for flexible electronics. Micromachines 2021, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Lee, C.-H.; Cui, B.; Sazonov, A. Flexible semi-transparent a-Si:H pin solar cells for functional energy-harvesting applications. Mater. Sci. Eng. B 2018, 229, 1–5. [Google Scholar] [CrossRef]
- Shah, A.V.; Schade, H.; Vanecek, M.; Meier, J.; Vallat-Sauvain, E.; Wyrsch, N.; Kroll, U.; Droz, C.; Bailat, J. Thin-film silicon solar cell technology. Prog. Photovolt. Res. Appl. 2004, 12, 113–142. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Wu, Z.; Luo, D.; Yu, H.-Y.; Lu, Z.-H. Review and perspective of materials for flexible solar cells. Mater. Rep. Energy 2021, 1, 100001. [Google Scholar] [CrossRef]
- Ramanujam, J.; Bishop, D.M.; Todorov, T.K.; Gunawan, O.; Rath, J.; Nekovei, R.; Artegiani, E.; Romeo, A. Flexible CIGS, CdTe and a-Si:H based thin film solar cells: A review. Prog. Mater. Sci. 2019, 110, 100619. [Google Scholar] [CrossRef]
- Barrio, R.; Gonzalez, N.; Gandía, J.J. SiNx:H Films for Efficient Bulk Passivation of Nonconventional Wafers for Silicon Heterojunction Solar Cells. JOM 2021, 73, 2781–2789. [Google Scholar] [CrossRef]
- Manfredotti, C.; Fizzotti, F.; Boero, M.; Pastorino, P.; Polesello, P.; Vittone, E. Influence of hydrogen-bonding configurations on the physical properties of hydrogenated amorphous silicon. Phys. Rev. B 1994, 50, 18046–18053. [Google Scholar] [CrossRef]
- Smets, A.H.M.; Kessels, W.M.M.; van de Sanden, M.C.M. Vacancies and voids in hydrogenated amorphous silicon. Appl. Phys. Lett. 2003, 82, 1547–1549. [Google Scholar] [CrossRef]
- Zhao, Y.; Procel, P.; Smets, A.; Mazzarella, L.; Han, C.; Yang, G.; Cao, L.; Yao, Z.; Weeber, A.; Zeman, M.; et al. Effects of (i)a-Si:H deposition temperature on high-efficiency silicon heterojunction solar cells. Prog. Photovolt. Res. Appl. 2022, 31, 1170–1180. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Tang, F.; Xiang, Y.; Wu, C.; Lin, B. Roles of hydrogen in structural stability and electronic property of bulk hydrogenated amorphous silicon. Comput. Mater. Sci. 2023, 216, 111846. [Google Scholar] [CrossRef]
- Langford, A.; Fleet, M.; Mahan, A. Correction for multiple reflections in infrared spectra of amorphous silicon. Sol. Cells 1989, 27, 373–383. [Google Scholar] [CrossRef]
- Lucovsky, G.; Nemanich, R.J.; Knights, J.C. Structural interpretation of the vibrational spectra ofa-Si: H alloys. Phys. Rev. B 1979, 19, 2064–2073. [Google Scholar] [CrossRef]
- Duan, W.; Lambertz, A.; Bittkau, K.; Qiu, D.; Qiu, K.; Rau, U.; Ding, K. A route towards high-efficiency silicon heterojunction solar cells. Prog. Photovolt. Res. Appl. 2022, 30, 384–392. [Google Scholar] [CrossRef]
- Brodsky, M.H.; Cardona, M.; Cuomo, J.J. I nfrared and Raman spectra of the silicon-hydrogen bonds in amorphous silicon prepared by glow discharge and sputtering. Phys. Rev. B 1977, 16, 3556. [Google Scholar] [CrossRef]
- Jeon, M.; Yoshiba, S.; Kamisako, K. Hydrogenated amorphous silicon film as intrinsic passivation layer deposited at various temperatures using RF remote-PECVD technique. Curr. Appl. Phys. 2010, 10, S237–S240. [Google Scholar] [CrossRef]
- Müllerová, J.; Fischer, M.; Netrvalová, M.; Zeman, M.; Šutta, P. Influence of deposition temperature on amorphous structure of PECVD deposited a-Si:H thin films. Open Phys. 2011, 9, 1301–1308. [Google Scholar] [CrossRef]
- Ganji, J.; Kosarian, A.; Kaabi, H. Mathematical Evaluation of a-Si:H Film Formation in rf-PECVD Systems. Silicon 2020, 12, 723–734. [Google Scholar] [CrossRef]
- Remeš, Z.; Vaněček, M.; Torres, P.; Kroll, U.; Mahan, A.; Crandall, R. Optical determination of the mass density of amorphous and microcrystalline silicon layers with different hydrogen contents. J. Non-Cryst. Solids 1998, 227–230, 876–879. [Google Scholar] [CrossRef]
- Linke, J.; Rinder, J.; Hahn, G.; Terheiden, B. Correlation between the optical bandgap and the monohydride bond density of hydrogenated amorphous silicon. J. Non-Cryst. Solids X 2020, 5, 100044. [Google Scholar] [CrossRef]
- Wang, M.; Ye, H.; Zhai, C. Amorphization-induced energy loss of amorphous Si anodes for Li-ion batteries. Scr. Mater. 2022, 221, 114958. [Google Scholar] [CrossRef]
- Heubner, C.; Schneider, M.; Michaelis, A. Investigation of charge transfer kinetics of Li-Intercalation in LiFePO 4. J. Power Sources 2015, 288, 115–120. [Google Scholar] [CrossRef]
- Hummelshøj, J.S.; Luntz, A.C.; Nørskov, J.K. Theoretical evidence for low kinetic overpotentials in Li-O2 electrochemistry. J. Chem. Phys. 2013, 138, 034703. [Google Scholar] [CrossRef] [PubMed]

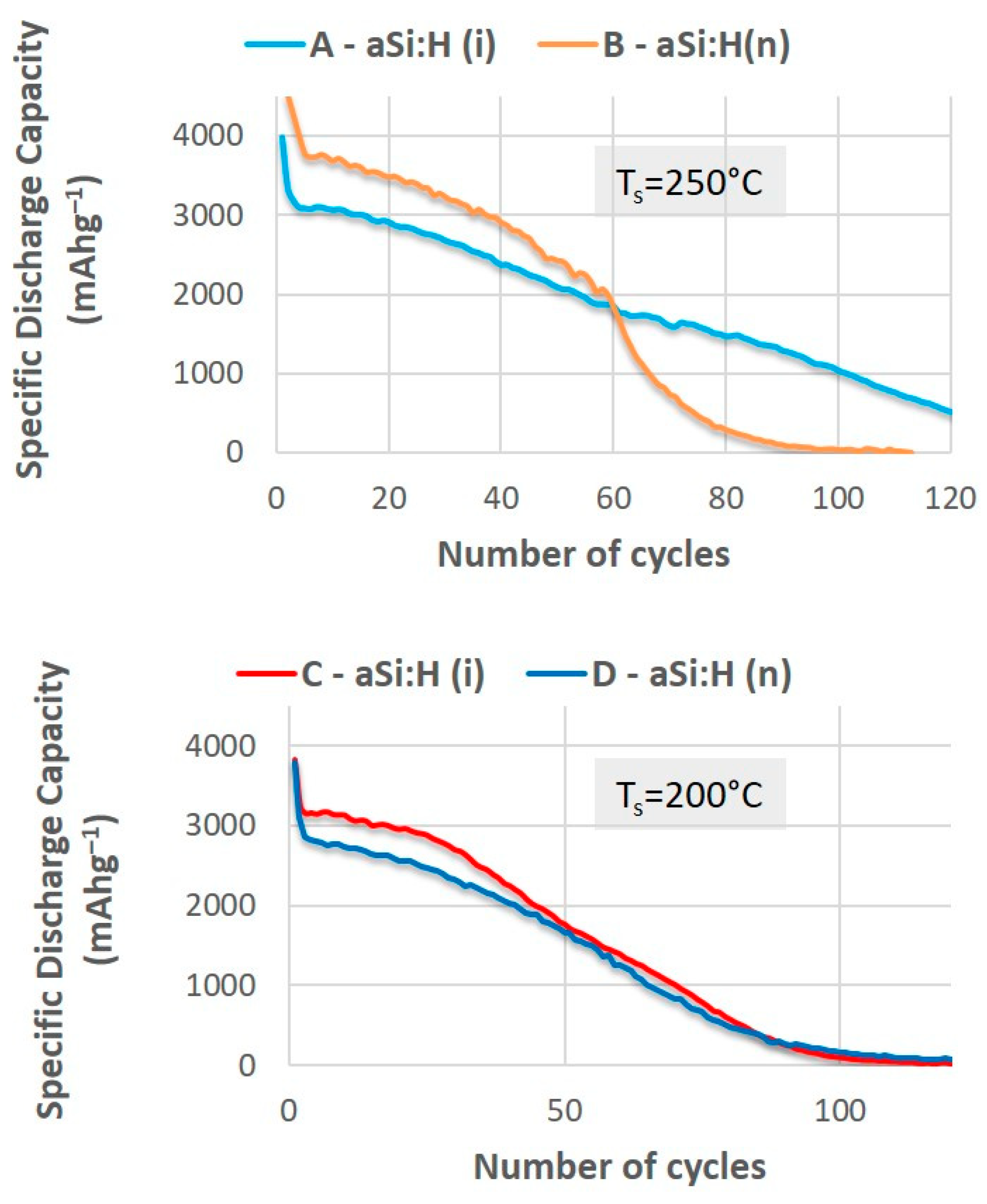
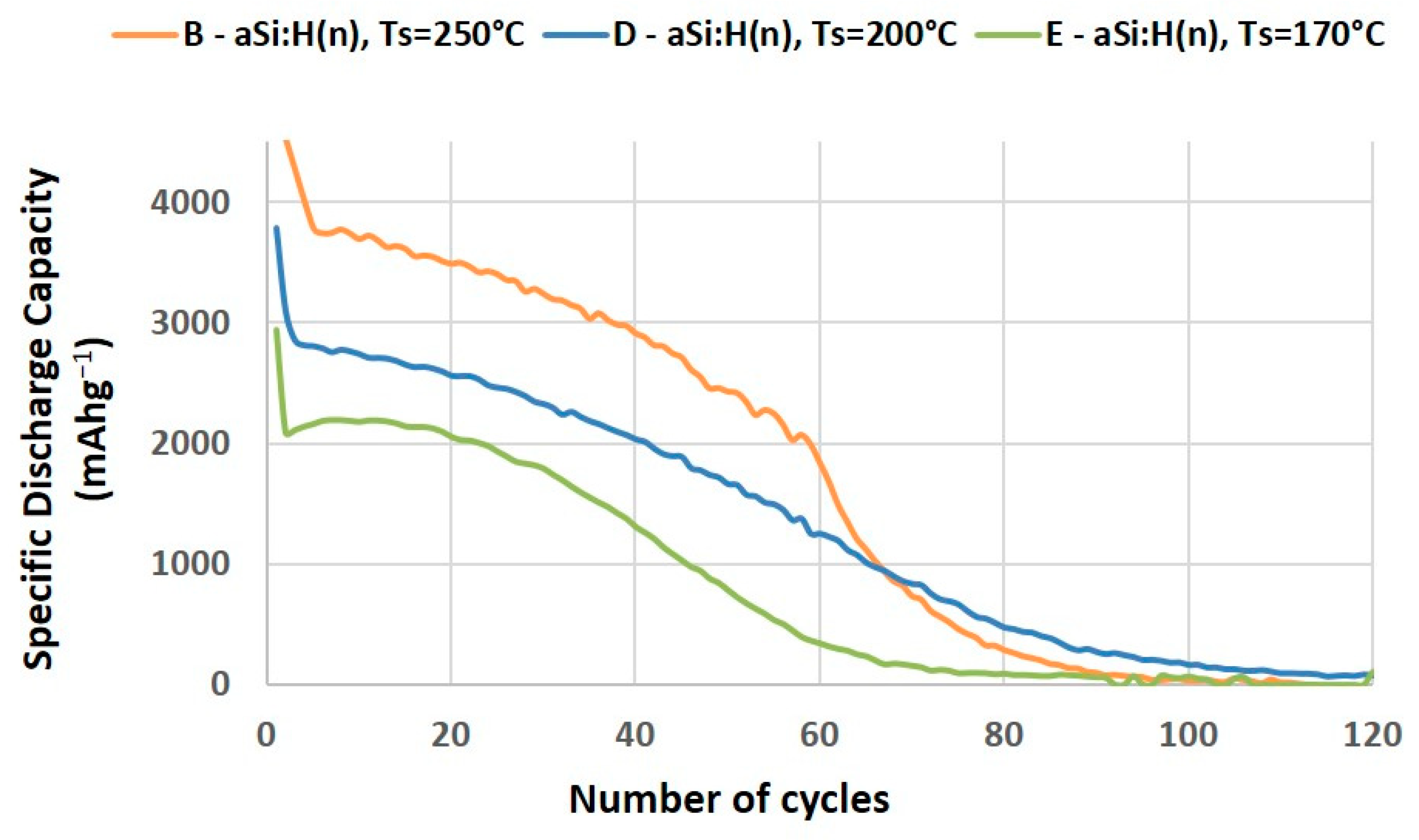
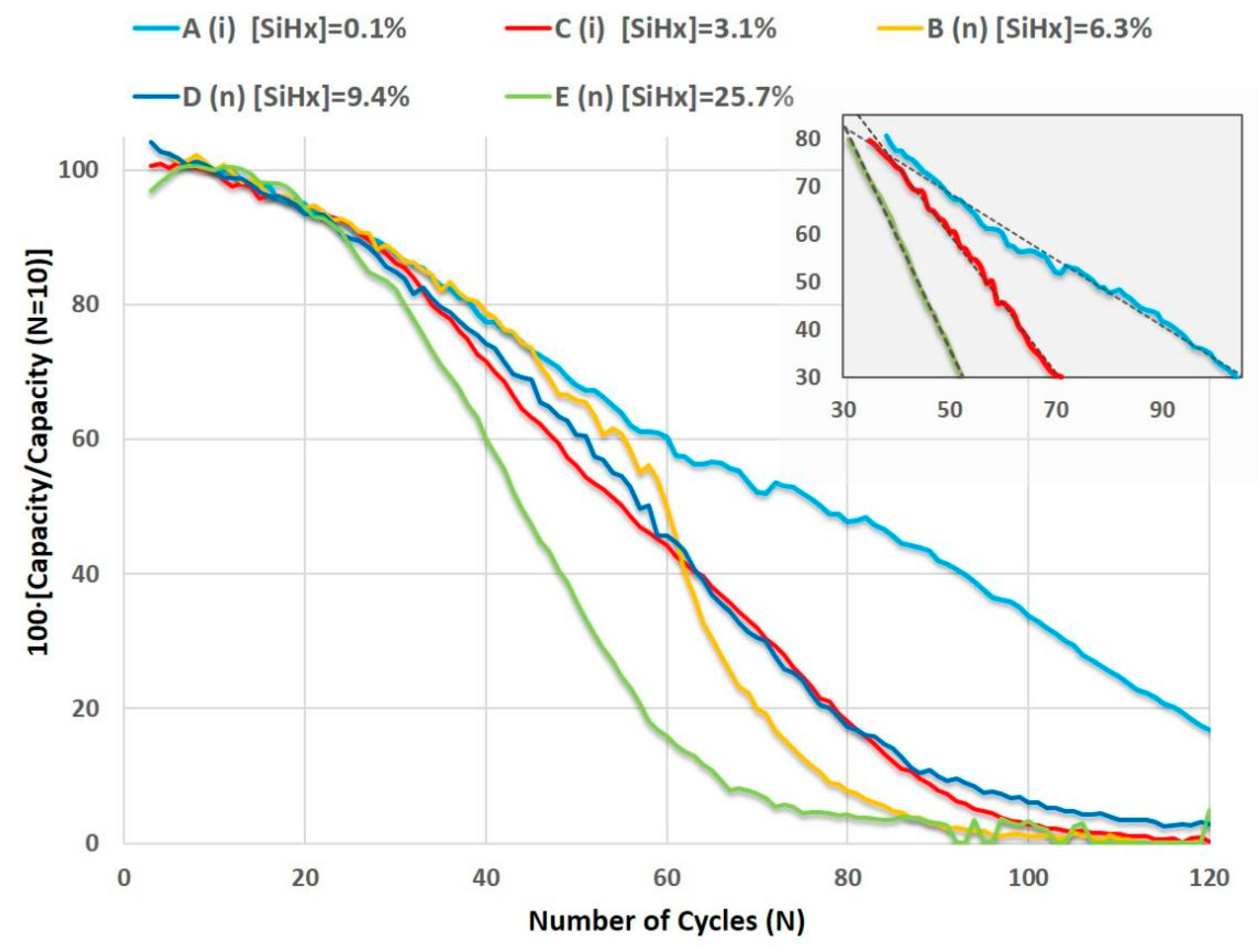
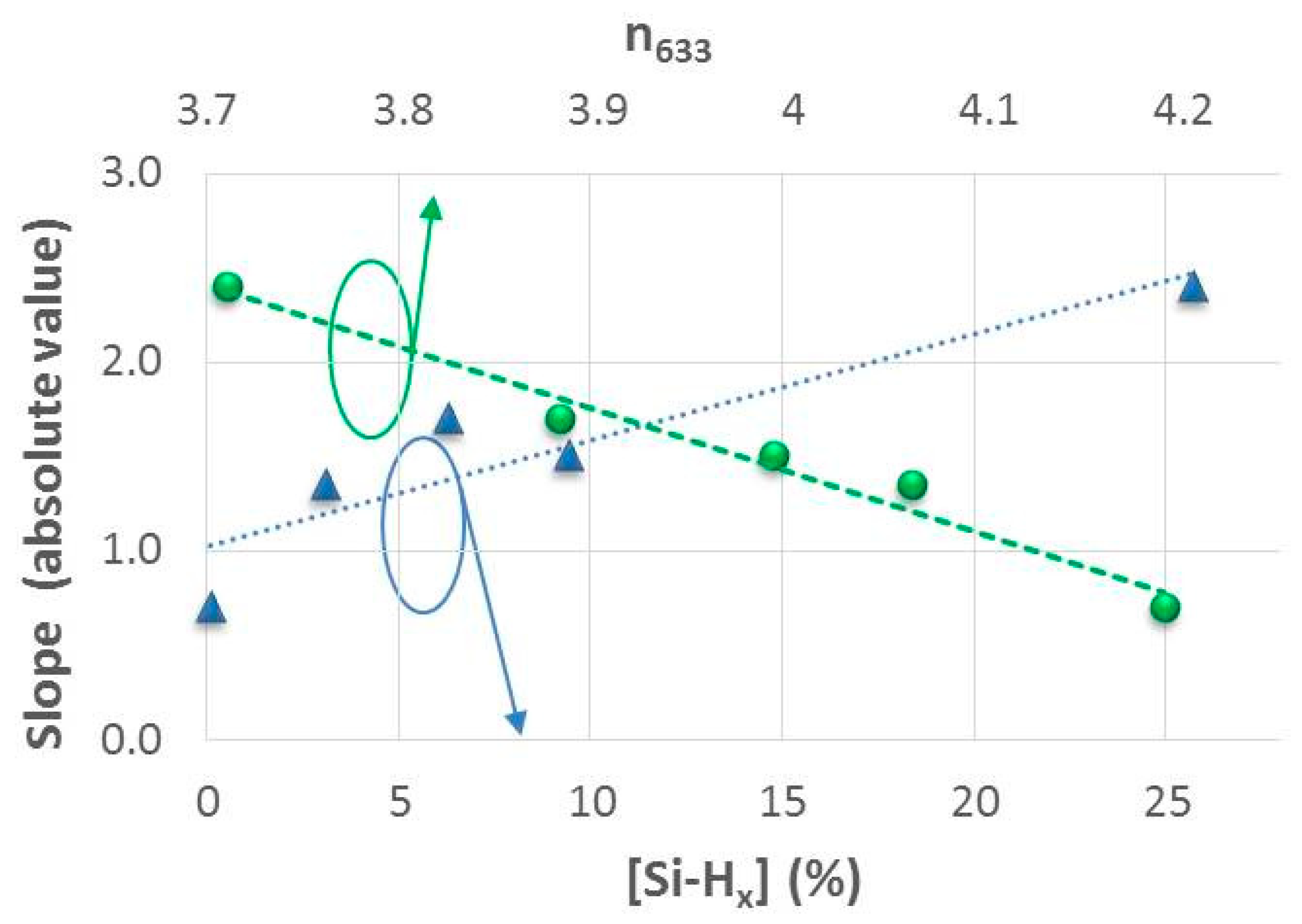
| Sample | TS (°C) | Φ(PH3/H2) (sccm) | R | mact. ± 0.05 (mg) | n633 | [Si-H] (%) | [Si-Hx] (%) | RSM | σ (S/cm) | Ea (eV) |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 250 | 0 | 0 | 0.37 | 4.19 | 7.4 | 0.1 | 0.01 | 4.0 × 10−10 | -- |
| B | 250 | 3.5 | 0.18 | 0.40 | 3.88 | 9.3 | 6.3 | 0.21 | 1.1 × 10−2 | 0.20 |
| C | 200 | 0 | 0 | 0.34 | 4.06 | 8.8 | 3.1 | 0.12 | 4.0 × 10−10 | -- |
| D | 200 | 9.5 | 0.48 | 0.43 | 3.99 | 8.8 | 9.4 | 0.31 | 1.2 × 10−2 | 0.20 |
| E | 170 | 3.5 | 0.18 | 0.37 | 3.71 | 8.0 | 25.7 | 0.62 | 2.2 × 10−4 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, N.; García, T.; Morant, C.; Barrio, R. Fine-Tuning Intrinsic and Doped Hydrogenated Amorphous Silicon Thin-Film Anodes Deposited by PECVD to Enhance Capacity and Stability in Lithium-Ion Batteries. Nanomaterials 2024, 14, 204. https://doi.org/10.3390/nano14020204
González N, García T, Morant C, Barrio R. Fine-Tuning Intrinsic and Doped Hydrogenated Amorphous Silicon Thin-Film Anodes Deposited by PECVD to Enhance Capacity and Stability in Lithium-Ion Batteries. Nanomaterials. 2024; 14(2):204. https://doi.org/10.3390/nano14020204
Chicago/Turabian StyleGonzález, Nieves, Tomás García, Carmen Morant, and Rocío Barrio. 2024. "Fine-Tuning Intrinsic and Doped Hydrogenated Amorphous Silicon Thin-Film Anodes Deposited by PECVD to Enhance Capacity and Stability in Lithium-Ion Batteries" Nanomaterials 14, no. 2: 204. https://doi.org/10.3390/nano14020204





