Increasing the Particle Size and Magnetic Property of Iron Oxide Nanoparticles through a Segregated Nucleation and Growth Process
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Reagents
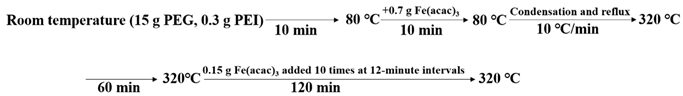
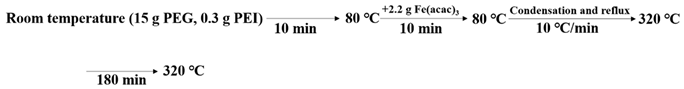
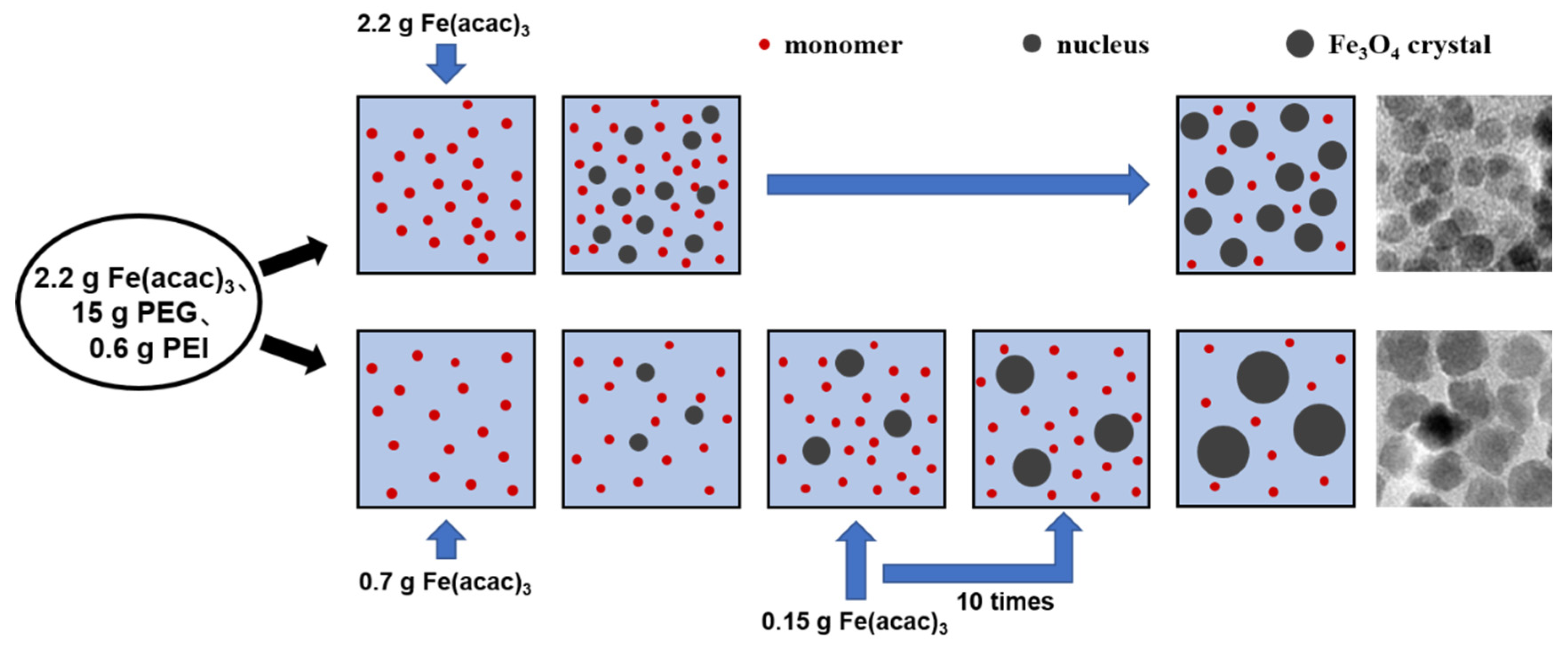
2.2. Preparation of IONs
2.3. Characterization
3. Results and Discussion
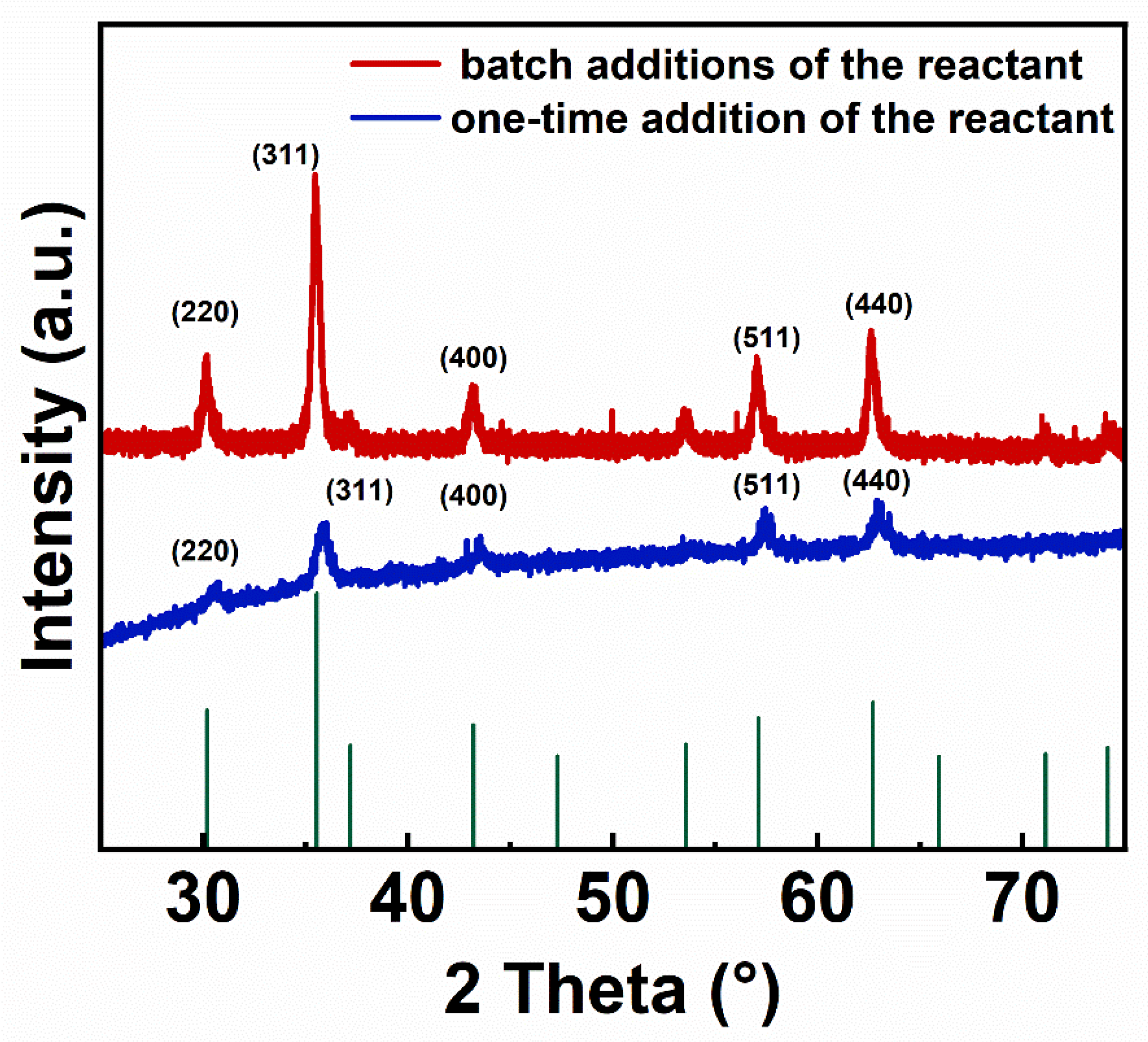
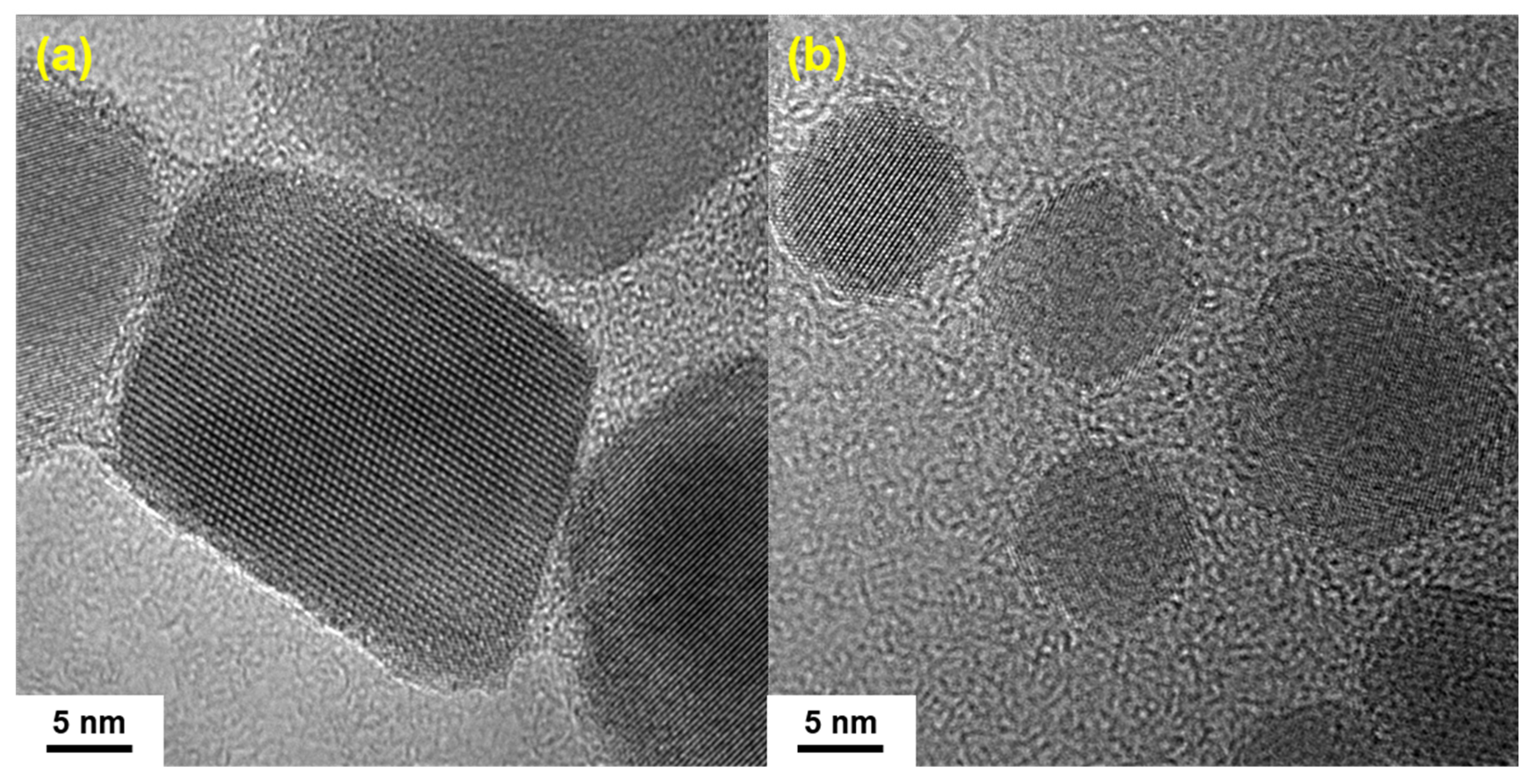
3.1. X-ray Diffraction and Fourier Transform Infrared Spectroscopy of IONs
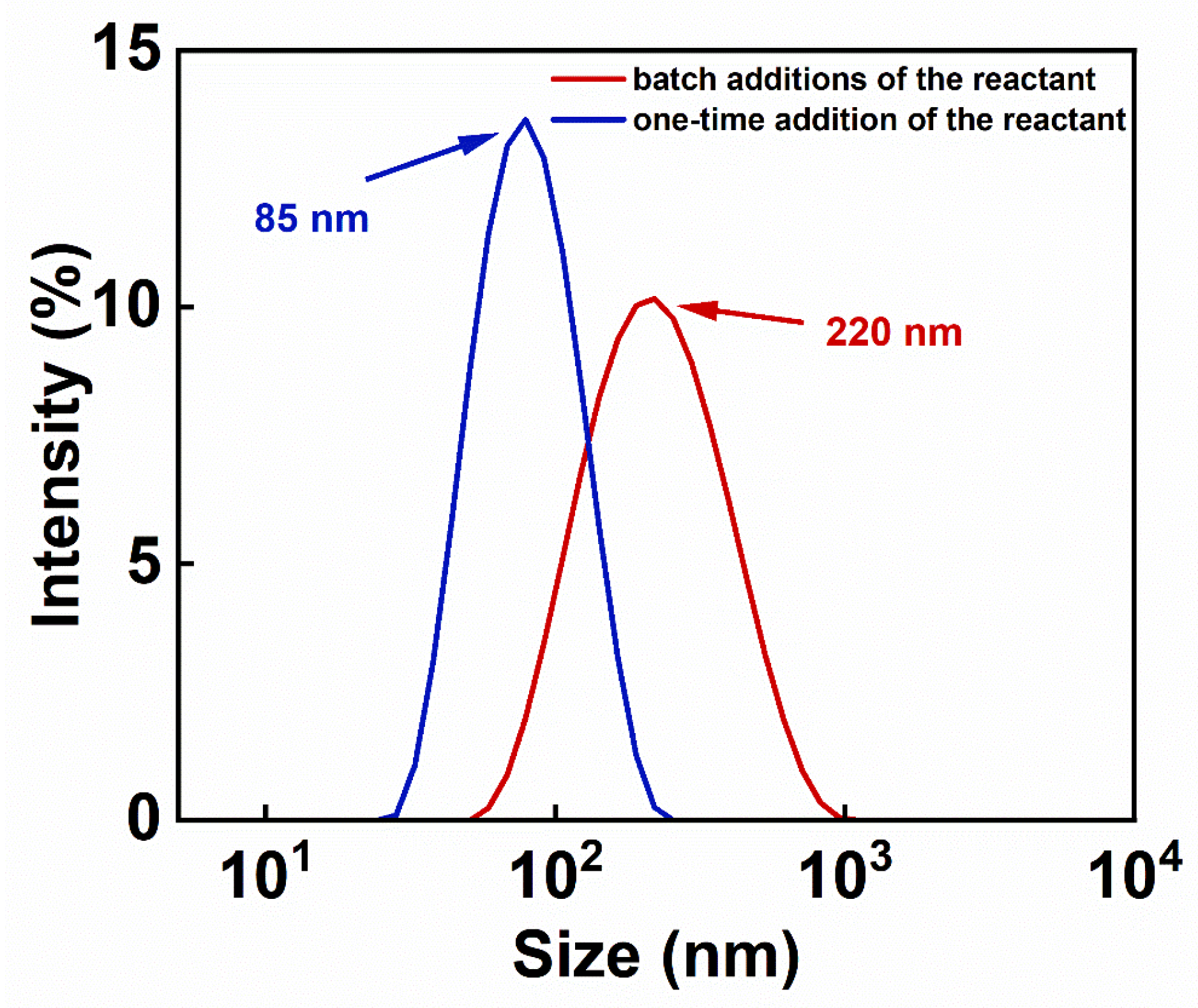
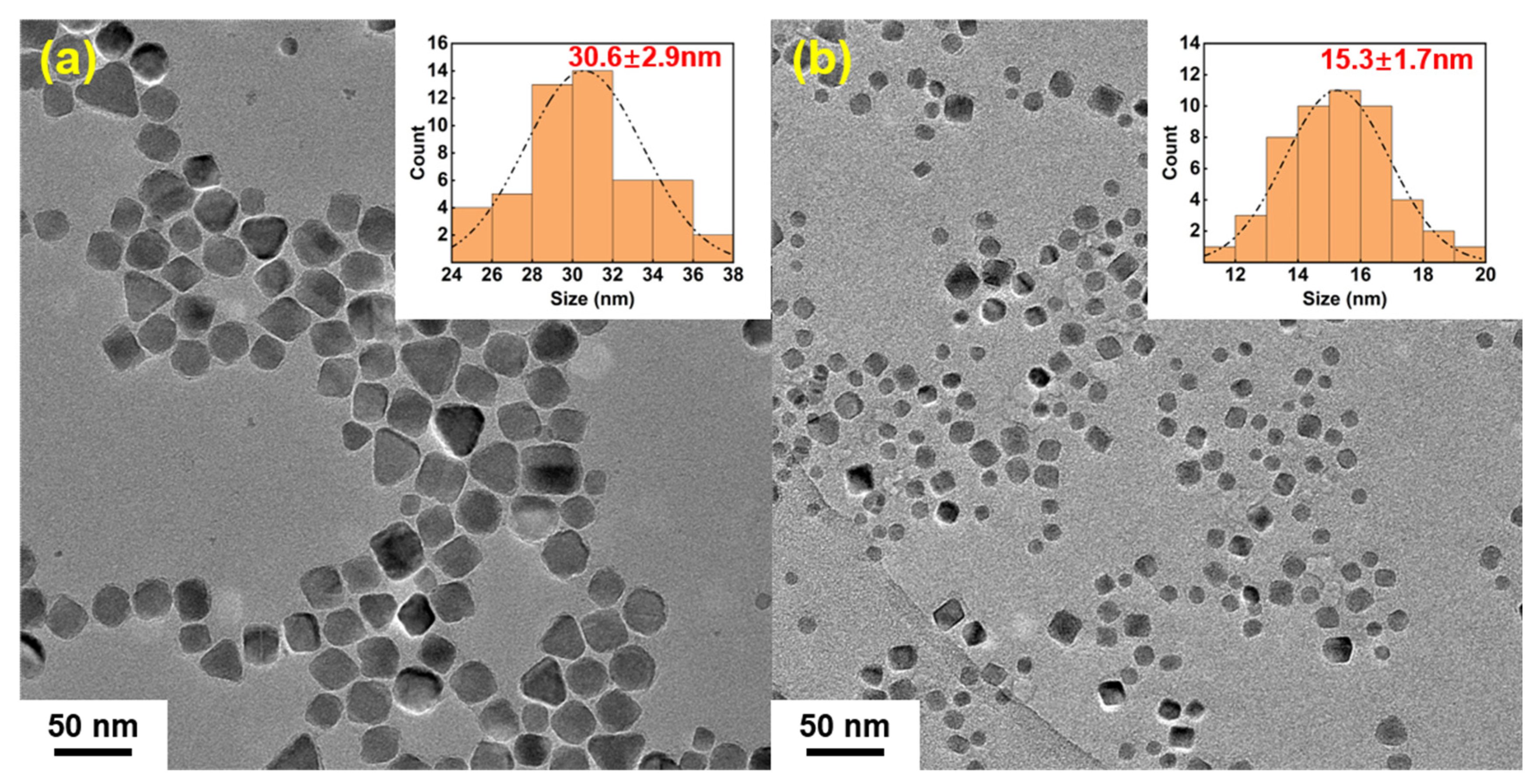
3.2. Analysis of Colloidal Stability and Particle Size Distribution of IONs
3.3. Hysteresis Loop Analysis of IONs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Awogbemi, O.; Von Kallon, D.V. Recent advances in the application of nanomaterials for improved biodiesel, biogas, biohydrogen, and bioethanol production. Fuel 2024, 358, 130261. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ul Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Zhao, X.; Xuan, J.; Li, Q.; Gao, F.; Xun, X.; Liao, Q.; Zhang, Y. Roles of Low-Dimensional Nanomaterials in Pursuing Human-Machine-Thing Natural Interaction. Adv. Mater. 2023, 35, e2207437. [Google Scholar] [CrossRef]
- Joseph, T.M.; Mahapatra, D.K.; Esmaeili, A.; Piszczyk, L.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Otto, M. Multifunctional nanocomposites modulating the tumor microenvironment for enhanced cancer immunotherapy. Bioact. Mater. 2024, 31, 440–462. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gao, F.; Zhang, Q.; Yang, J. Covalent Organic Frameworks: Recent Progress in Biomedical Applications. ACS Nano 2023, 17, 1879–1905. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, F.; Cao, J.; Dou, Q.; Wang, J.; Wang, J.; Yang, L.; Chen, W. Research advances of nanomaterials for the acceleration of fracture healing. Bioact. Mater. 2024, 31, 368–394. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Zhang, H.; Huang, H.; Sun, L.; Ma, L.; Du, Y.; Pei, C.; Zhang, Q.; Li, H.; et al. Activating Layered Metal Oxide Nanomaterials via Structural Engineering as Biodegradable Nanoagents for Photothermal Cancer Therapy. Small 2021, 17, 2007486. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Xu, P.A.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Abuawad, A.; Ashhab, Y.; Offenhaeusser, A.; Krause, H.-J. DNA Sensor for the Detection of Brucella spp. Based on Magnetic Nanoparticle Markers. Int. J. Mol. Sci. 2023, 24, 17272. [Google Scholar] [CrossRef] [PubMed]
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.Z.; Lee, Y.W.; Nagaraj, H.; Rotello, V.M. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020, 156, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Alawak, M.; Brüssler, J.; Bakowsky, U.; El Sayed, M.M.H. Nanoenabled Bioseparations: Current Developments and Future Prospects. Biomed. Res. Int. 2019, 2019, 4983291. [Google Scholar] [CrossRef] [PubMed]
- Krasitskaya, V.V.; Kudryavtsev, A.N.; Yaroslavtsev, R.N.; Velikanov, D.A.; Bayukov, O.A.; Gerasimova, Y.V.; Stolyar, S.V.; Frank, L.A. Starch-Coated Magnetic Iron Oxide Nanoparticles for Affinity Purification of Recombinant Proteins. Int. J. Mol. Sci. 2022, 23, 5410. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Fehn, S.; Steegmüller, T.; Rauwolf, S.; Löwe, H.; Pflüger-Grau, K.; Berensmeier, S. Immobilization of PETase enzymes on magnetic iron oxide nanoparticles for the decomposition of microplastic PET. Nanoscale Adv. 2021, 3, 4395–4399. [Google Scholar] [CrossRef] [PubMed]
- Altalbawy, F.M.A.; Ali, E.; Fenjan, M.N.; Mustafa, Y.F.; Mansouri, S.; Bokov, D.O.; Idiyevna, S.G.; Misra, N.; Alawadi, A.H.; Alsalamy, A. Aptamer-Magnetic Nanoparticle Complexes for Powerful Biosensing: A Comprehensive Review. Crit. Rev. Anal. Chem. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H. Synthesis of iron oxide nanorods and nanocubes in an imidazolium ionic liquid. Chem. Eng. J. 2009, 147, 71–78. [Google Scholar] [CrossRef]
- Zhang, R.R.; Lu, K.; Xiao, L.; Hu, X.L.; Cai, W.; Liu, L.J.; Liu, Y.; Li, W.H.; Zhou, H.; Qian, Z.Y.; et al. Exploring atherosclerosis imaging with contrast-enhanced MRI using PEGylated ultrasmall iron oxide nanoparticles. Front. Bioeng. Biotech. 2023, 11, 1279446. [Google Scholar] [CrossRef]
- Sun, S.H.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G.X. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Han, G.H.; Wang, S.; Chong, C.G.; Han, D.; Tan, J.; Zhang, B.L. The distribution of the iron oxide nanoparticles modified with polyethylene glycol in rat brains. Mater. Chem. Phys. 2021, 260, 124108. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.H.; Yu, T.; Jiang, C.Z.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mat. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Hufschmid, R.; Arami, H.; Ferguson, R.M.; Gonzales, M.; Teeman, E.; Brush, L.N.; Browning, N.D.; Krishnan, K.M. Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition. Nanoscale 2015, 7, 11142–11154. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.W.; Zhuang, Z.B.; Peng, Q.; Li, Y.D. Room-temperature soft magnetic iron oxide nanocrystals: Synthesis, characterization, and size-dependent magnetic properties. Chem. Mater. 2008, 20, 5029–5034. [Google Scholar] [CrossRef]
- Park, J.; An, K.J.; Hwang, Y.S.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Han, D.; Zhang, B.L.; Su, L.C.; Han, G.H.; Wang, S. Synthesis of superparamagnetic iron oxide nanoparticles with different particle sizes and its magneto-calorific effects under alternating current magnetic field. J. Mater. Eng. 2019, 47, 84–90. [Google Scholar] [CrossRef]
- Erdemir, D.; Lee, A.Y.; Myerson, A.S. Nucleation of Crystals from Solution: Classical and Two-Step Models. Acc. Chem. Res. 2009, 42, 621–629. [Google Scholar] [CrossRef]
- LaMer, V.K.; Dinegar, R.H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Lee, J.; Yang, J.; Kwon, S.G.; Hyeon, T. Nonclassical nucleation and growth of inorganic nanoparticles. Nat. Rev. Mater. 2016, 1, 16034. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Sun, J. Heterogeneous Nucleation in Protein Crystallization. Biomimetics 2023, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Huang, N.; Wang, J.; Wang, T. A general and facile strategy for precisely controlling the crystal size of monodispersed metal-organic frameworks via separating the nucleation and growth. Chem. Commun. 2018, 54, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, B.L.; Su, L.C.; Nie, W.; Han, D.; Han, G.H.; Zhang, H.; Chong, C.G.; Tan, J. Subcellular distributions of iron oxide nanoparticles in rat brains affected by different surface modifications. J. Biomed. Mater. Res. A 2019, 107, 1988–1998. [Google Scholar] [CrossRef] [PubMed]
- Sosso, G.C.; Chen, J.; Cox, S.J.; Fitzner, M.; Pedevilla, P.; Zen, A.; Michaelides, A. Crystal Nucleation in Liquids: Open Questions and Future Challenges in Molecular Dynamics Simulations. Chem. Rev. 2016, 116, 7078–7116. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Hyeon, T. Formation Mechanisms of Uniform Nanocrystals via Hot-Injection and Heat-Up Methods. Small 2011, 7, 2685–2702. [Google Scholar] [CrossRef] [PubMed]
- Leon Felix, L.; Rodriguez Martinez, M.A.; Pacheco Salazar, D.G.; Huamani Coaquira, J.A. One-step synthesis of polyethyleneimine-coated magnetite nanoparticles and their structural, magnetic and power absorption study. RSC Adv. 2020, 10, 41807–41815. [Google Scholar] [CrossRef]
- Gerulova, K.; Kucmanova, A.; Sanny, Z.; Garaiova, Z.; Seiler, E.; Caplovicova, M.; Caplovic, Ľ.; Palcut, M. Fe3O4-PEI Nanocomposites for Magnetic Harvesting of Chlorella vulgaris, Chlorella ellipsoidea, Microcystis aeruginosa, and Auxenochlorella protothecoides. Nanomaterials 2022, 12, 1786. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, S.; Wang, Q.; Wang, L.; Dong, J.; Zhang, B. Increasing the Particle Size and Magnetic Property of Iron Oxide Nanoparticles through a Segregated Nucleation and Growth Process. Nanomaterials 2024, 14, 827. https://doi.org/10.3390/nano14100827
Liu Y, Wang S, Wang Q, Wang L, Dong J, Zhang B. Increasing the Particle Size and Magnetic Property of Iron Oxide Nanoparticles through a Segregated Nucleation and Growth Process. Nanomaterials. 2024; 14(10):827. https://doi.org/10.3390/nano14100827
Chicago/Turabian StyleLiu, Yiyang, Sheng Wang, Qin Wang, Liping Wang, Jianghui Dong, and Baolin Zhang. 2024. "Increasing the Particle Size and Magnetic Property of Iron Oxide Nanoparticles through a Segregated Nucleation and Growth Process" Nanomaterials 14, no. 10: 827. https://doi.org/10.3390/nano14100827





