Hydroxyapatite Nanorods Based Drug Delivery Systems for Bumetanide and Meloxicam, Poorly Water Soluble Active Principles
Abstract
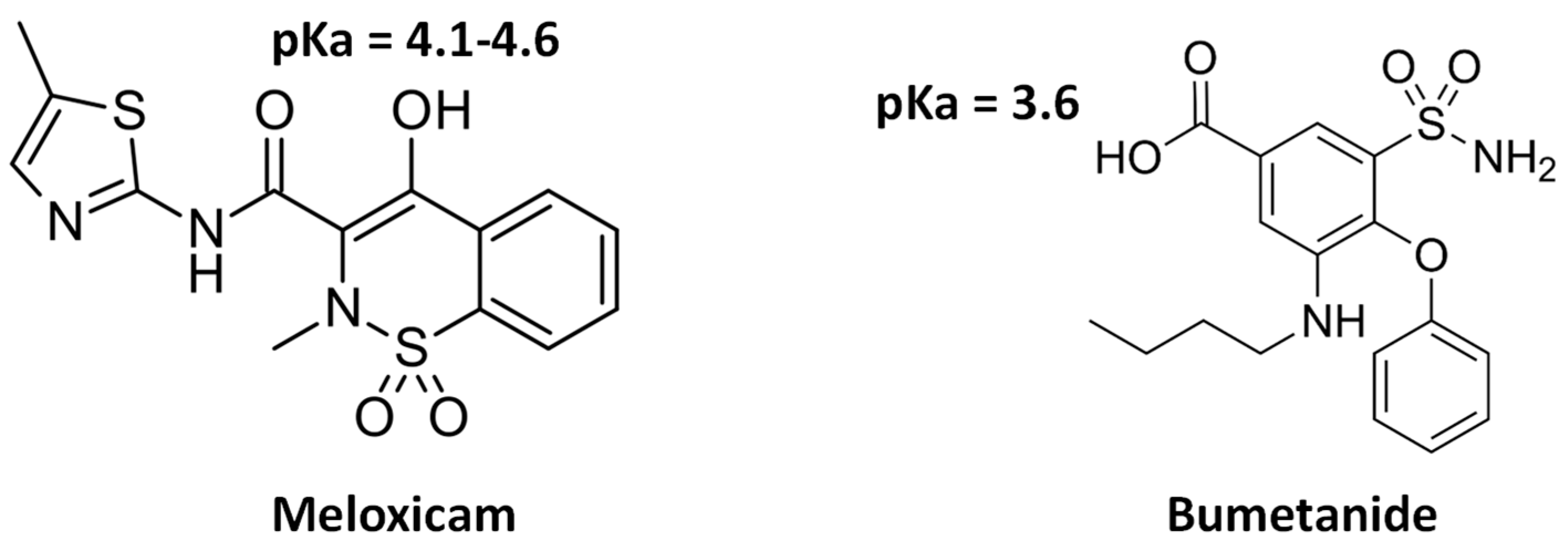
:1. Introduction
2. Materials and Methods
2.1. Hydroxyapatite Synthesis
2.2. Hybrid Preparation
2.3. Physical–Chemical Characterizations
2.4. Pharmaceutical Measurements
2.4.1. Drug Loading
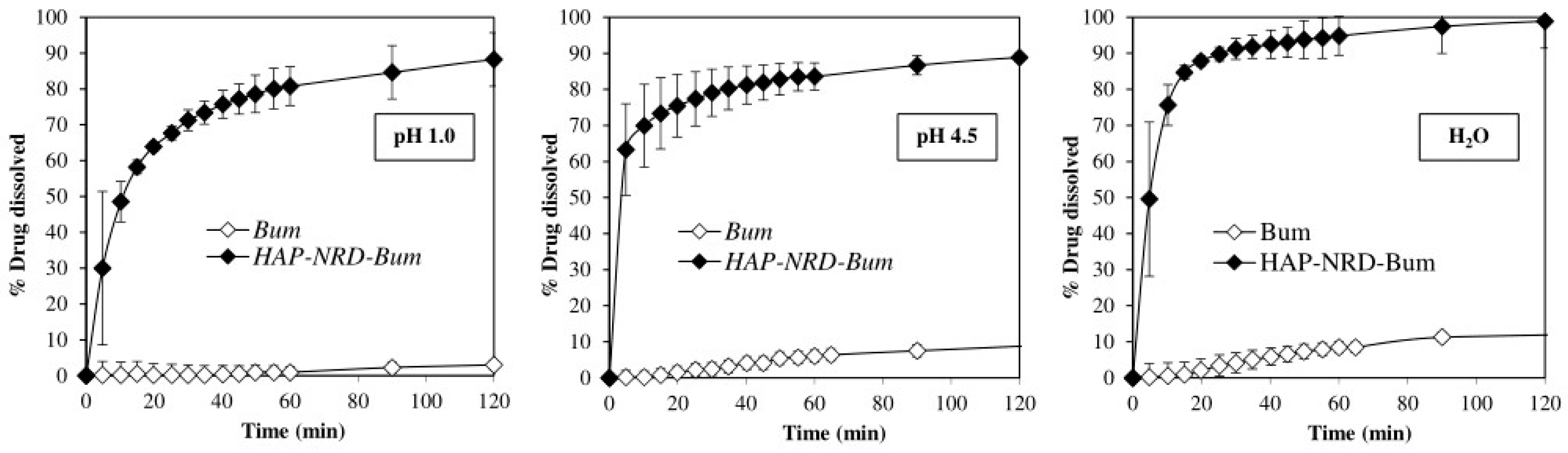
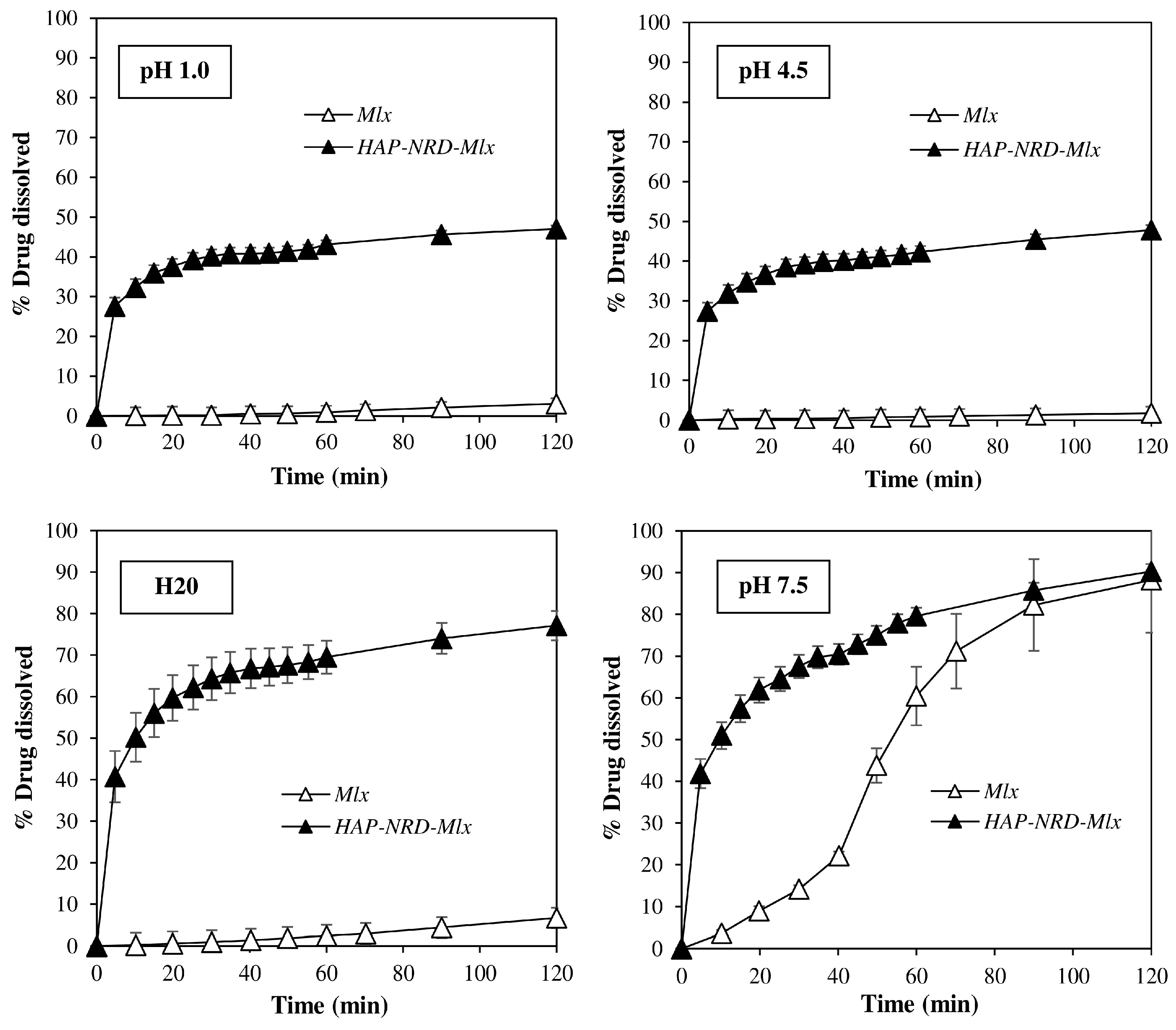
2.4.2. Dissolution Test
2.4.3. Solubility
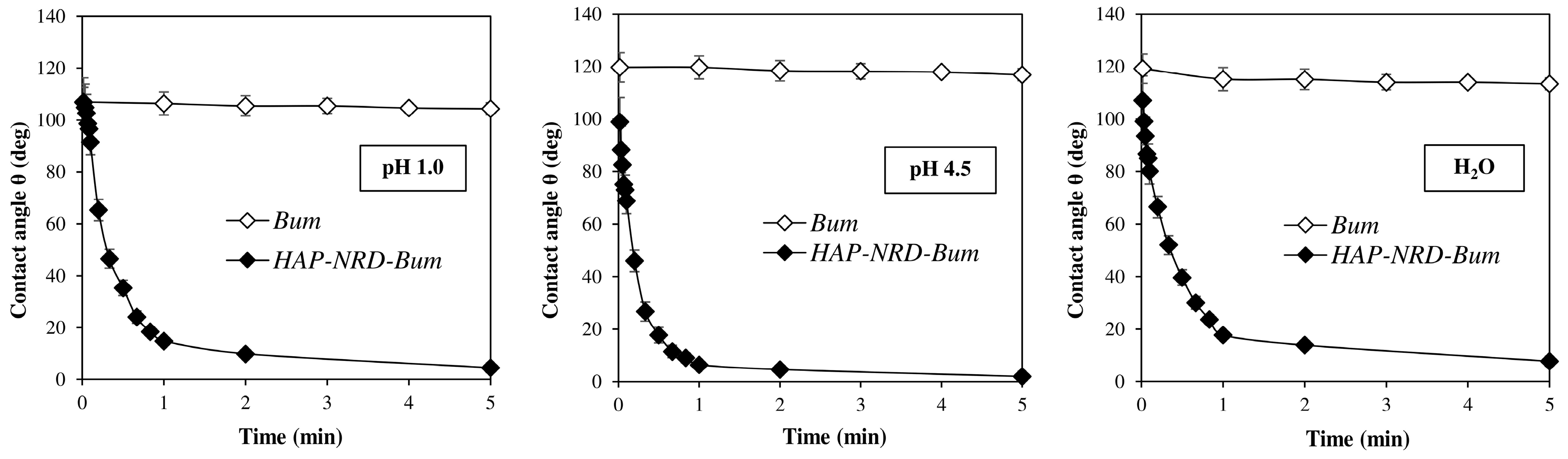
2.4.4. Contact Angle
3. Results and Discussion
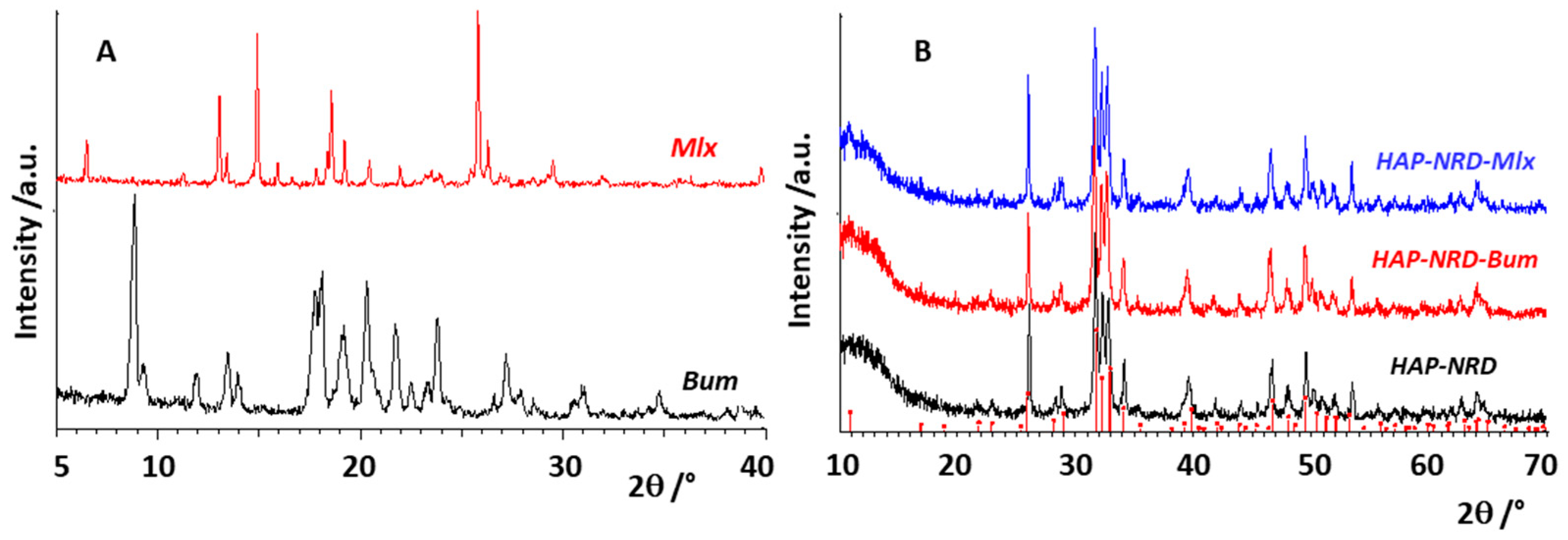
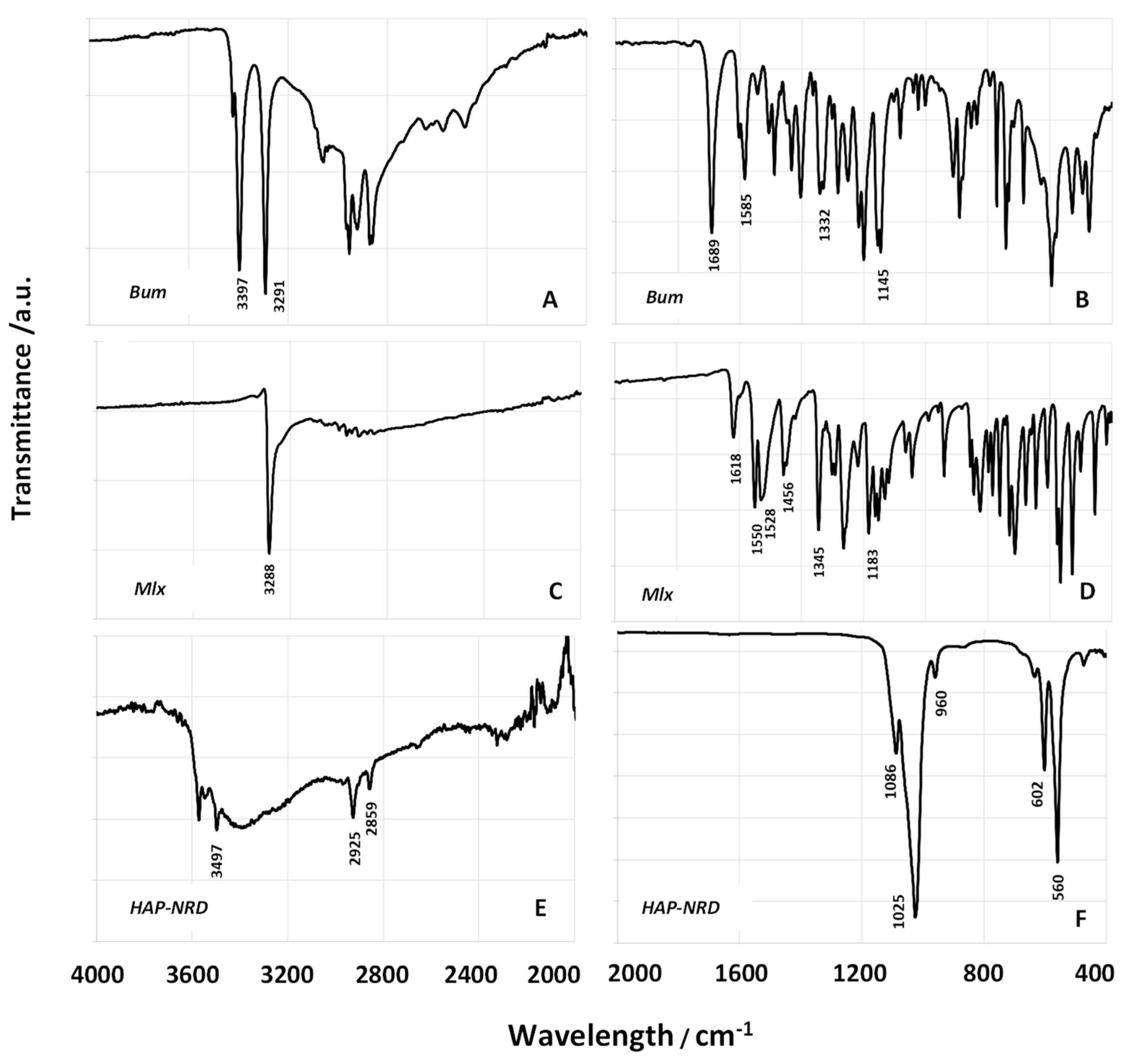
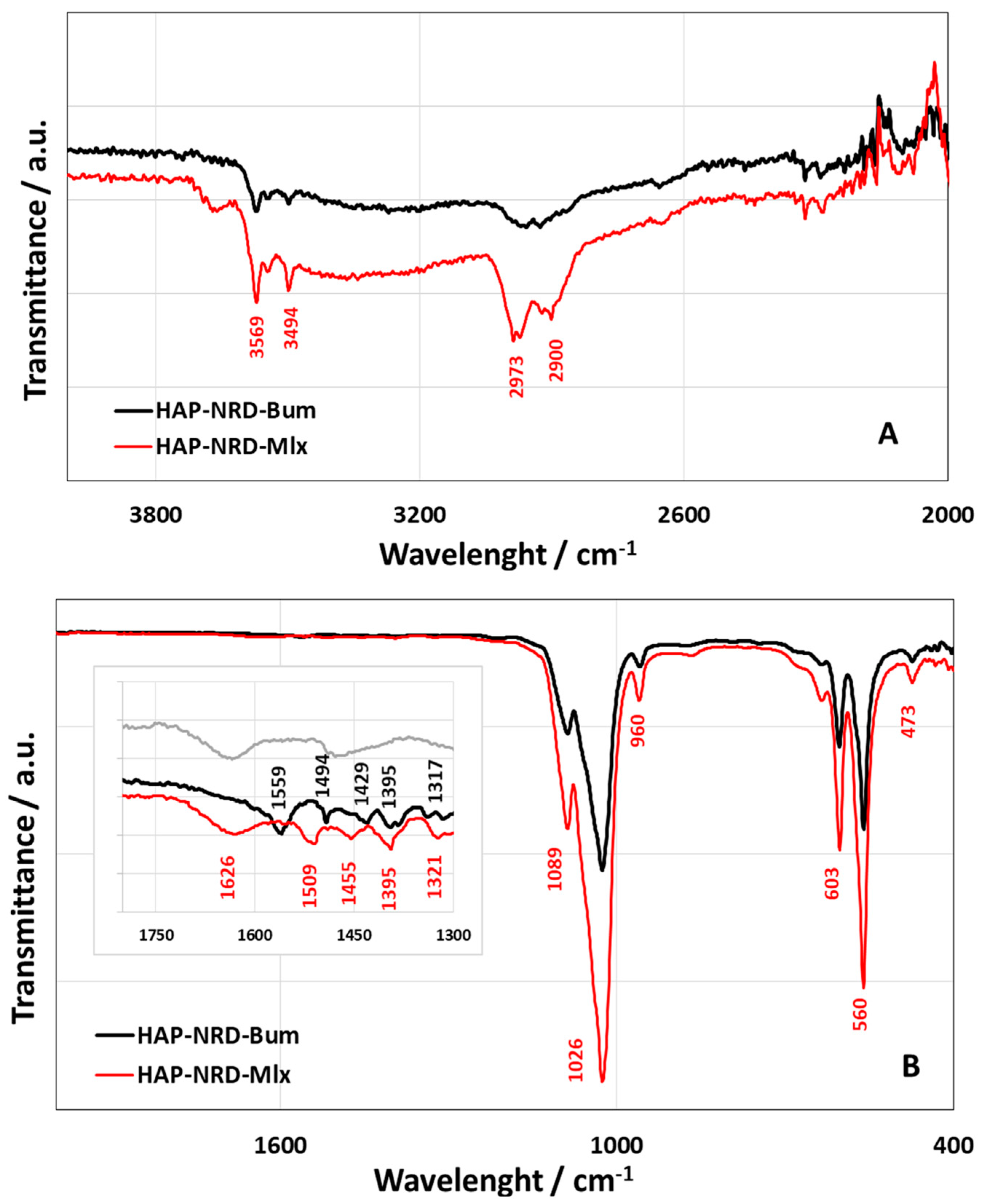
3.1. Physical–Chemical Characterization
3.2. Pharmaceutical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- La Rocca, M.; Rinaldi, A.; Bruni, G.; Friuli, V.; Maggi, L.; Bini, M. New Emerging Inorganic–Organic Systems for Drug-Delivery: Hydroxyapatite@Furosemide Hybrids. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2249–2259. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, M.; Yibang, Z.; Raza, F.; Hasnat, M.; Cao, J.; Qi, X.; Hussain, A.; Liu, D. Hollow Mesoporous Silica Nanoparticles for Dual Chemo-starvation Therapy of Hepatocellular Carcinoma. Pharm. Res. 2023, 40, 2215–2228. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Margel, S. Containers for Drug Delivery. In Micro- and Nano-containers for Smart Applications. Composites Science and Technology; Parameswaranpillai, J., Salim, N.V., Pulikkalparambil, H., Mavinkere Rangappa, S., Suchart Siengchin, I.H., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Chandel, A.K.S. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, C. A Study on Solubility Enhancement Methods for Poorly Water Soluble Drugs. Am. J. Pharmacol. Sci. 2013, 1, 67–73. [Google Scholar] [CrossRef]
- Malkawi, R.; Malkawi, W.I.; Al-Mahmoud, Y.; Tawalbeh, J. Current Trends on Solid Dispersions: Past, Present, and Future. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 5916013. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, Z. Hyaluronic acid-based nanoparticles to deliver drugs to the ocular posterior segment. Drug Deliv. 2023, 30, 2204206. [Google Scholar] [CrossRef]
- Liu, R.; Luo, C.; Pang, Z.; Zhang, J.; Ruan, S.; Wu, M.; Wang, L.; Sun, T.; Li, N.; Han, L.; et al. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chin. Chem. Lett. 2023, 34, 107518. [Google Scholar] [CrossRef]
- Obada, D.O.; Osseni, S.A.; Sina, H.; Oyedeji, A.N.; Salami, K.A.; Okafor, E.; Csaki, S.; Abolade, S.A.; Akande, A.; Dauda, M.; et al. Hydroxyapatite materials-synthesis routes, mechanical behavior, theoretical insights, and artificial intelligence models: A review. J. Aust. Ceram. Soc. 2023, 59, 565–596. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Martynková, G.S.; Hundáková, M.; Kratošová, G.; Holešová, S.; Kupková, J.; Pazourková, L.; Majewska, J. Alkali-Treated Alumina and Zirconia Powders Decorated with Hydroxyapatite for Prospective Biomedical Applications. Materials 2022, 15, 1390. [Google Scholar] [CrossRef]
- Lopes Gama e Silva, G.; Sato de Souza de Bustamante Monteiro, M.; de Abreu Garofalo, D.; Lopes Dias, M.; Malta Rossi, A.; Mavropoulos Oliveira Tude, E.; da Silva Cardoso, V.; Vermelho, A.B.; dos Santos Matos, A.P.; Santos-Oliveira, R.; et al. Nanofibers containing vancomycin for the treatment of bone infections: Development, characterization, efficacy and safety tests in cell cultures. J. Drug Deliv. Sci. Technol. 2023, 87, 104780. [Google Scholar] [CrossRef]
- Ranjbar, E.; Namazi, H. Ultrasound-assisted synthesis layered double hydroxide@hydroxyapatite-doxorubicin coated magnetic PEG nanocomposite: A biocompatible pH-sensitive nanocarrier for anticancer drug delivery. FlatChem 2023, 42, 100571. [Google Scholar] [CrossRef]
- Demirel, M.; Aslan, N.; Aksakal, B.; Arslan, M.E. Fabrication of hydroxyapatite-based nano-gold and nano-silver-doped bioceramic bone grafts: Enhanced mechanostructure, cell viability, and nuclear abnormality properties. J. Biomed. Mater. Res. 2023, 111, 1386–1397. [Google Scholar] [CrossRef]
- Siva Prasad, P.; Marupalli, B.C.G.; Das, S.; Das, K. Surfactant-assisted synthesis of hydroxyapatite particles: A comprehensive review. J. Mater. Sci. 2023, 58, 6076–6105. [Google Scholar] [CrossRef]
- Ochoa, S.L.; Ortega-Lara, W.; Guerrero-Beltrán, C.E. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics 2021, 13, 1642. [Google Scholar] [CrossRef]
- Maisons, V.; Joseph-Delaffon, K. Le bumétanide, un diurétique de l’ombre. Actual. Pharm. 2021, 609, 45–47. [Google Scholar] [CrossRef]
- Flamenbaum, W.; Friedman, R. Pharmacology, therapeutic efficacy, and adverse effects of bumetanide, a new “loop” diuretic. Pharmacotherapy 1982, 2, 213–222. [Google Scholar] [CrossRef]
- Eades, S.K.; Christensen, M.L. The clinical pharmacology of loop diuretics in the pediatric patient. Pediatr. Nephrol. 1998, 12, 603–616. [Google Scholar] [CrossRef]
- Pentikäinen, P.J.; Penttilä, A.; Neuvonen, P.J.; Gothoni, G. Fate of [14C]-bumetanide in man. Br. J. Clin. Pharmacol. 1977, 4, 39–44. [Google Scholar] [CrossRef]
- Bruni, G.; Maietta, M.; Berbenni, V.; Mustarelli, P.; Ferrara, C.; Freccero, M.; Grande, V.; Maggi, L.; Milanese, C.; Girella, A.; et al. Mechanochemical Synthesis of Bumetanide−4-Aminobenzoic Acid Molecular Cocrystals: A Facile and Green Approach to Drug Optimization. J. Phys. Chem. B 2014, 118, 9180–9190. [Google Scholar] [CrossRef]
- Luger, P.; Daneck, K.; Engel, W.; Trummhtz, G.; Wagner, K. Structure and physicochemical properties of meloxicam, a new NSAID. Eur. J. Pharm. Sci. 1996, 4, 175–187. [Google Scholar] [CrossRef]
- Weyna, D.R.; Cheney, M.L.; Shan, N.; Hanna, M.; Zaworotko, M.J.; Sava, V.; Song, S.; Sanchez-Ramos, J.R. Improving solubility and pharmacokinetics of meloxicam via multiple-component crystal formation. Mol. Pharm. 2012, 9, 2094–2102. [Google Scholar] [CrossRef]
- Wu, X.Q.; Tang, P.X.; Li, S.S.; Zhang, L.L.; Li, H. X-ray powder diffraction data for meloxicam, C14H13N3O4S2. Powder Diffr. 2014, 29, 196–198. [Google Scholar] [CrossRef]
- Seedher, N.; Bhatia, S. Solubility enhancement of cox-2 inhibitors using various solvent systems. AAPS PharmSciTech 2003, 4, 36–44. [Google Scholar] [CrossRef]
- Meloxicam Tablets, official monograph. In The United States Pharmacopeia (USP41-NF36); United States Pharmacopeia Convention, Inc.: Rockville, MD, USA, 2018; pp. 2558–2563.
- Bumetanide Tablets, official monograph. In The United States Pharmacopeia (USP43-NF38); United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2023; p. 608.
- Reagents: Solutions/Buffer Solutions. In The United States Pharmacopeia (USP43-NF38); United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2023; p. 6223.
- Diulus, S.C.; Kaduk, J.A.; Gindhart, A.M.; Blanton, T.N. Crystal structure of bumetanide, C17H20N2O5S. Powder Diffr. 2019, 34, 189–195. [Google Scholar] [CrossRef]
- Monteforte, F.; Bruni, G.; Quinzeni, I.; Friuli, V.; Maggi, L.; Capsoni, D.; Bini, M. Meloxicam-LDH Hybrid Compound: A Successful Strategy to Improve Solubility. J. Inorg. Organomet. Polym. Mater. 2020, 30, 637–648. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, L.; Li, Y.; Shi, T. Preparation of irregular mesoporous hydroxyapatite. Mat. Res. Bull. 2008, 43, 1607–1614. [Google Scholar] [CrossRef]
- Fowler, B.O. Infrared studies of apatites. I. Vibrational assignments for calcium, strontium, and barium hydroxyapatites utilizing isotopic substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Su, G.; Yang, C.; Zhu, J.J. Fabrication of Gold Nanorods with Tunable Longitudinal Surface Plasmon Resonance Peaks by Reductive Dopamine. Langmuir 2015, 31, 817–823. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Huang, S.; Hou, Z.; Cheng, Z.; Yang, P.; Peng, C.; Lin, J. Self-activated luminescent and mesoporous strontium hydroxyapatite nanorods for drug delivery. Biomaterials 2010, 31, 3374–3383. [Google Scholar] [CrossRef]
- Murugan, N.; Ramakrishna, S. Production of ultra-fine bioresorbable carbonated hydroxyapatite. Acta Biomater. 2006, 2, 201–206. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friuli, V.; Maggi, L.; Bruni, G.; Caso, F.; Bini, M. Hydroxyapatite Nanorods Based Drug Delivery Systems for Bumetanide and Meloxicam, Poorly Water Soluble Active Principles. Nanomaterials 2024, 14, 113. https://doi.org/10.3390/nano14010113
Friuli V, Maggi L, Bruni G, Caso F, Bini M. Hydroxyapatite Nanorods Based Drug Delivery Systems for Bumetanide and Meloxicam, Poorly Water Soluble Active Principles. Nanomaterials. 2024; 14(1):113. https://doi.org/10.3390/nano14010113
Chicago/Turabian StyleFriuli, Valeria, Lauretta Maggi, Giovanna Bruni, Francesca Caso, and Marcella Bini. 2024. "Hydroxyapatite Nanorods Based Drug Delivery Systems for Bumetanide and Meloxicam, Poorly Water Soluble Active Principles" Nanomaterials 14, no. 1: 113. https://doi.org/10.3390/nano14010113







