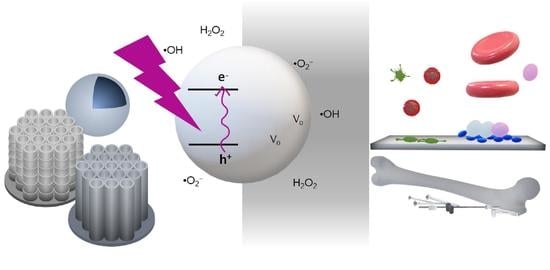
A Review on Nano Ti-Based Oxides for Dark and Photocatalysis: From Photoinduced Processes to Bioimplant Applications
Abstract
:1. Introduction
2. TiO2 Photocatalysis
2.1. Reactive Oxygen Species in TiO2 Photocatalysis
2.2. Nanomorphologies and Structural States of TiO2
2.2.1. Safety of TiO2 Nanoparticles
2.3. Photocatalytic Disinfection Using TiO2 Nanostructures
2.4. Efforts to Improve the Photocatalytic Efficiency of TiO2 Nanomaterials
2.4.1. Enhanced Charge Separation

2.4.2. Enhanced Light Harvesting
2.4.3. Black TiO2
3. Dark Catalysis on Ti-Based Oxides
Microbicidal Effect of TiO2 in the Dark
4. Ti and Ti-Based Oxides for Biomedical Implants
4.1. Safety of Ti-Based Implants and Inflammation
5. Conclusions and Future Perspectives
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Buddee, S.; Wongnawa, S.; Sirimahachai, U.; Puetpaibool, W. Recyclable UV and Visible Light Photocatalytically Active Amorphous TiO2 Doped with M (III) Ions (M = Cr and Fe). Mater. Chem. Phys. 2011, 126, 167–177. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Bogdanoff, P.; Dittrich, T.; Fiechter, S.; Fujishima, A.; Tributsch, H. Observations of P-Type Semiconductivity in Titanium Dioxide at Room Temperature. Mater. Lett. 2010, 64, 928–930. [Google Scholar] [CrossRef]
- Nowotny, J.; Bak, T.; Nowotny, M.K.; Sheppard, L.R. Titanium Dioxide for Solar-Hydrogen II. Defect Chemistry. Int. J. Hydrogen Energy 2007, 32, 2630–2643. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- De Souza, M.L.; Tristao, D.C.; Corio, P. Vibrational Study of Adsorption of Congo Red onto TiO2 and the LSPR Effect on Its Photocatalytic Degradation Process. RSC Adv. 2014, 4, 23351–23358. [Google Scholar] [CrossRef]
- Bhat, V.T.; Duspara, P.A.; Seo, S.; Abu Bakar, N.S.B.; Greaney, M.F. Visible Light Promoted Thiol-Ene Reactions Using Titanium Dioxide. Chem. Commun. 2015, 51, 4383–4385. [Google Scholar] [CrossRef] [Green Version]
- Tayade, R.J.; Surolia, P.K.; Kulkarni, R.G.; Raksh, V.; Tayade, R.J.; Surolia, P.K.; Kulkarni, R.G.; Jasra, R.V. Photocatalytic Degradation of Dyes and Organic Contaminants in Water Using Nanocrystalline Anatase and Rutile TiO2. Sci. Technol. Adv. Mater. 2007, 8, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Bin Mukhlish, M.Z.; Najnin, F.; Rahman, M.M.; Uddin, M.J. Photocatalytic Degradation of Different Dyes Using TiO2 with High Surface Area: A Kinetic Study. J. Sci. Res. 2013, 5, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, S.; Hidalgo, D.; Sacco, A.; Chiodoni, A.; Lamberti, A.; Cauda, V.; Tressoab, E.; Saraccob, G. Comparison of Photocatalytic and Transport Properties of TiO2 and ZnO Nanostructures for Solar-Driven Water Splitting. Phys. Chem. Chem. Phys. 2015, 17, 7775–7786. [Google Scholar] [CrossRef] [Green Version]
- Samsudin, E.M.; Abd Hamid, S.B. Effect of Band Gap Engineering in Anionic-Doped TiO2 Photocatalyst. Appl. Surf. Sci. 2017, 391, 326–336. [Google Scholar] [CrossRef]
- Dozzi, M.V.; D’Andrea, C.; Ohtani, B.; Valentini, G.; Selli, E. Fluorine-Doped TiO2 Materials: Photocatalytic Activity vs Time-Resolved Photoluminescence. J. Phys. Chem. C 2013, 117, 25586–25595. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and Mechanisms of Photocatalytic Dye Degradation on TiO 2 Based Photocatalysts: A Comparative Overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Giovannetti, R.; Amato, C.A.D.; Zannotti, M.; Rommozzi, E.; Gunnella, R.; Minicucci, M.; Di Cicco, A. Visible Light Photoactivity of Polypropylene Coated Nano-TiO2 for Dyes Degradation in Water. Sci. Rep. 2015, 5, 17801. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, P.; Moreira, J.; Gomaa, H.; Ray, A.K. Visible-Solar-Light-Driven Photocatalytic Degradation of Phenol with Dye-Sensitized TiO2: Parametric and Kinetic Study. Ind. Eng. Chem. Res. 2012, 51, 4523–4532. [Google Scholar] [CrossRef]
- Shang, M.; Hou, H.; Gao, F.; Wang, L.; Yang, W. Mesoporous Ag@TiO2 Nanofibers and Their Photocatalytic Activity for Hydrogen Evolution. RSC Adv. 2017, 7, 30051–30059. [Google Scholar] [CrossRef] [Green Version]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Bahnemann, D.W. Photoelectrocatalytic Materials for Environmental Applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Nowotny, J.; Bak, T.; Nowotny, M.K.; Sheppard, L.R. Titanium Dioxide for Solar-Hydrogen I. Functional Properties. Int. J. Hydrogen Energy 2007, 32, 2609–2629. [Google Scholar] [CrossRef]
- Han, X.X.; Chen, L.; Kuhlmann, U.; Schulz, C.; Weidinger, I.M.; Hildebrandt, P. Magnetic Titanium Dioxide Nanocomposites for Surface-Enhanced Resonance Raman Spectroscopic Determination and Degradation of Toxic Anilines and Phenols. Angew. Chem.—Int. Ed. 2014, 53, 2481–2484. [Google Scholar] [CrossRef]
- Zhu, P.; Nair, A.S.; Shengjie, P.; Shengyuan, Y.; Ramakrishna, S. Facile Fabrication of TiO2-Graphene Composite with Enhanced Photovoltaic and Photocatalytic Properties by Electrospinning. ACS Appl. Mater. Interfaces 2012, 4, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Kohtani, S.; Kawashima, A.; Miyabe, H. Reactivity of Trapped and Accumulated Electrons in Titanium Dioxide Photocatalysis. Catalysts 2017, 7, 303. [Google Scholar] [CrossRef] [Green Version]
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge Carrier Trapping, Recombination and Transfer during TiO2 Photocatalysis: An Overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Li, G.; Chen, L.; Graham, M.E.; Gray, K.A. A Comparison of Mixed Phase Titania Photocatalysts Prepared by Physical and Chemical Methods: The Importance of the Solid-Solid Interface. J. Mol. Catal. A Chem. 2007, 275, 30–35. [Google Scholar] [CrossRef]
- Pillai, S.C.; Periyat, P.; George, R.; Mccormack, D.E.; Seery, M.K.; Hayden, H.; Colreavy, J.; Corr, D.; Hinder, S.J. Synthesis of High-Temperature Stable Anatase TiO2 Photocatalyst. J. Phys. Chem. C 2007, 111, 1605–1611. [Google Scholar] [CrossRef] [Green Version]
- Hariharan, A.; Goldberg, P.; Gustmann, T.; Maawad, E.; Pilz, S.; Schell, F.; Kunze, T.; Zwahr, C.; Gebert, A. Designing the Microstructural Constituents of an Additively Manufactured near β Ti Alloy for an Enhanced Mechanical and Corrosion Response. Mater. Des. 2022, 217, 110618. [Google Scholar] [CrossRef]
- Gebert, A.; Oswald, S.; Helth, A.; Voss, A.; Gostin, P.F.; Rohnke, M.; Janek, J.; Calin, M.; Eckert, J. Effect of Indium (In) on Corrosion and Passivity of a Beta-Type Ti-Nb Alloy in Ringer’s Solution. Appl. Surf. Sci. 2015, 335, 213–222. [Google Scholar] [CrossRef]
- Pilz, S.; Gebert, A.; Voss, A.; Oswald, S.; Göttlicher, M.; Hempel, U.; Eckert, J.; Rohnke, M.; Janek, J.; Calin, M. Metal Release and Cell Biological Compatibility of Beta-Type Ti-40Nb Containing Indium. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2018, 106, 1686–1697. [Google Scholar] [CrossRef]
- Sopha, H.; Krbal, M.; Ng, S.; Prikryl, J.; Zazpe, R.; Yam, F.K.; Macak, J.M. Highly Efficient Photoelectrochemical and Photocatalytic Anodic TiO2 Nanotube Layers with Additional TiO2 Coating. Appl. Mater. Today 2017, 9, 104–110. [Google Scholar] [CrossRef]
- Jayaraj, J.; Park, J.M.; Gostin, P.F.; Fleury, E.; Gebert, A.; Schultz, L. Nano-Porous Surface States of Ti-Y-Al-Co Phase Separated Metallic Glass. Intermetallics 2009, 17, 1120–1123. [Google Scholar] [CrossRef]
- Pham, V.T.H.; Truong, V.K.; Orlowska, A.; Ghanaati, S.; Barbeck, M.; Booms, P.; Fulcher, A.J.; Bhadra, C.M.; Buividas, R.; Baulin, V.; et al. Race for the Surface: Eukaryotic Cells Can Win. ACS Appl. Mater. Interfaces 2016, 8, 22025–22031. [Google Scholar] [CrossRef] [Green Version]
- Mehrjou, B.; Mo, S.; Dehghan-Baniani, D.; Wang, G.; Qasim, A.M.; Chu, P.K. Antibacterial and Cytocompatible Nanoengineered Silk-Based Materials for Orthopedic Implants and Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 31605–31614. [Google Scholar] [CrossRef]
- Gallo, J.; Holinka, M.; Moucha, C. Antibacterial Surface Treatment for Orthopaedic Implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [Green Version]
- An, Y.H.; Friedman, R.J. Prevention of Sepsis in Total Joint Arthroplasty. J. Hosp. Infect. 1996, 33, 93–108. [Google Scholar] [CrossRef]
- Dong, J.; Wang, W.; Zhou, W.; Zhang, S.; Li, M.; Li, N.; Pan, G.; Zhang, X.; Bai, J.; Zhu, C. Immunomodulatory Biomaterials for Implant-Associated Infections: From Conventional to Advanced Therapeutic Strategies. Biomater. Res. 2022, 26, 72. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Osseointegration and Foreign Body Reaction: Titanium Implants Activate the Immune System and Suppress Bone Resorption during the First 4 Weeks after Implantation. Clin. Implant Dent. Relat. Res. 2018, 20, 82–91. [Google Scholar] [CrossRef]
- Pérez León, C.; Kador, L.; Peng, B.; Thelakkat, M. Characterization of the Adsorption of Ru-Bpy Dyes on Mesoporous TiO2 Films with UV-Vis, Raman, and FTIR Spectroscopies. J. Phys. Chem. B 2006, 110, 8723–8730. [Google Scholar] [CrossRef]
- Goff, A.H.; Joiret, S.; Falaras, P.; Curie, M. Raman Resonance Effect in a Monolayer of Polypyridyl Ruthenium (II) Complex Adsorbed on Nanocrystalline TiO2 via Phosphonated Terpyridyl Ligands. J. Phys. Chem. B 1999, 103, 9569–9575. [Google Scholar] [CrossRef]
- Öner, I.H.; Querebillo, C.J.; David, C.; Gernert, U.; Walter, C.; Driess, M.; Leimkühler, S.; Ly, K.H.; Weidinger, I.M.; Leimk, S.; et al. Hohe Elektromagnetische Feldverstärkung in Nanotubularen TiO2-Elektroden. Angew. Chem. 2018, 130, 7344–7348. [Google Scholar] [CrossRef]
- Shoute, L.C.T.; Loppnow, G.R. Excited-State Dynamics of Alizarin-Sensitized TiO2 Nanoparticles from Resonance Raman Spectroscopy. J. Chem. Phys. 2002, 117, 842–850. [Google Scholar] [CrossRef]
- Blackbourn, R.L.; Johnson, C.S.; Hupp, J.T. Surface Intervalence Enhanced Raman Scattering from Fe(CN)6 on Colloidal Titanium Dioxide. A Mode-by-Mode Description of the Franck—Condon Barrier to Interfacial Charge Transfer. J. Am. Chem. Soc. 1991, 113, 1060–1062. [Google Scholar] [CrossRef]
- Finnie, K.S.; Bartlett, J.R.; Woolfrey, J.L. Vibrational Spectroscopic Study of the Coordination of (2,2′-Bipyridyl-4,4′-Dicarboxylic Acid)Ruthenium(II) Complexes to the Surface of Nanocrystalline Titania. Langmuir 1998, 14, 2744–2749. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, X.; Ruan, W.; Zhao, B.; Xu, W.; Lombardi, J.R. Observation of Enhanced Raman Scattering for Molecules Adsorbed on TiO2 Nanoparticles: Charge-Transfer Contribution. J. Phys. Chem. C 2008, 112, 20095–20098. [Google Scholar] [CrossRef]
- Musumeci, A.; Gosztola, D.; Schiller, T.; Dimitrijevic, N.M.; Mujica, V.; Martin, D.; Rajh, T. SERS of Semiconducting Nanoparticles (TiO2 Hybrid Composites). J. Am. Chem. Soc. 2009, 131, 6040–6041. [Google Scholar] [CrossRef]
- Maznichenko, D.; Venkatakrishnan, K.; Tan, B. Stimulating Multiple SERS Mechanisms by a Nanofibrous Three-Dimensional Network Structure of Titanium Dioxide (TiO2). J. Phys. Chem. C 2013, 117, 578–583. [Google Scholar] [CrossRef]
- Han, X.X.; Köhler, C.; Kozuch, J.; Kuhlmann, U.; Paasche, L.; Sivanesan, A.; Weidinger, I.M.; Hildebrandt, P. Potential-Dependent Surface-Enhanced Resonance Raman Spectroscopy at Nanostructured TiO2: A Case Study on Cytochrome b5. Small 2013, 9, 4175–4181. [Google Scholar] [CrossRef]
- Öner, I.H.; Querebillo, C.J.; David, C.; Gernert, U.; Walter, C.; Driess, M.; Leimkühler, S.; Ly, K.H.; Weidinger, I.M. High Electromagnetic Field Enhancement of TiO2 Nanotube Electrodes. Angew. Chem. Int. Ed. 2018, 57, 7225–7229. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional Coatings of Titanium Implants Toward Promoting Osseointegration and Preventing Infection: Recent Developments. Front. Bioeng. Biotechnol. 2021, 9, 783816. [Google Scholar] [CrossRef]
- Xue, T.; Attarilar, S.; Liu, S.; Liu, J.; Song, X.; Li, L.; Zhao, B.; Tang, Y. Surface Modification Techniques of Titanium and Its Alloys to Functionally Optimize Their Biomedical Properties: Thematic Review. Front. Bioeng. Biotechnol. 2020, 8, 603072. [Google Scholar] [CrossRef]
- Sopha, H.; Pohl, D.; Damm, C.; Hromadko, L.; Rellinghaus, B.; Gebert, A.; Macak, J.M. Self-Organized Double-Wall Oxide Nanotube Layers on Glass-Forming Ti-Zr-Si(-Nb) Alloys. Mater. Sci. Eng. C 2017, 70, 258–263. [Google Scholar] [CrossRef]
- Yang, B.; Uchida, M.; Kim, H.M.; Zhang, X.; Kokubo, T. Preparation of Bioactive Titanium Metal via Anodic Oxidation Treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Petronis, S.; Krozer, A.; Jeong, Y.; Wennerberg, A.; Albrektsson, T. Characteristics of the Surface Oxides on Turned and Electrochemically Oxidized Pure Titanium Implants up to Dielectric Breakdown: The Oxide Thickness, Micropore Configurations, Surface Roughness, Crystal Structure and Chemical Composition. Biomaterials 2002, 23, 491–501. [Google Scholar] [CrossRef]
- Herzer, R.; Gebert, A.; Hempel, U.; Hebenstreit, F.; Oswald, S.; Damm, C.; Schmidt, O.G.; Medina-Sánchez, M. Rolled-Up Metal Oxide Microscaffolds to Study Early Bone Formation at Single Cell Resolution. Small 2021, 17, 2005527. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Jedsukontorn, T.; Meeyoo, V.; Saito, N.; Hunsom, M. Effect of Electron Acceptors H2O2 and O2 on the Generated Reactive Oxygen Species 1O2 and OH· in TiO2-Catalyzed Photocatalytic Oxidation of Glycerol. Cuihua Xuebao/Chin. J. Catal. 2016, 37, 1975–1981. [Google Scholar] [CrossRef]
- Gao, R.; Stark, J.; Bahnemann, D.W.; Rabani, J. Quantum Yields of Hydroxyl Radicals in Illuminated TiO2 Nanocrystallite Layers. J. Photochem. Photobiol. A Chem. 2002, 148, 387–391. [Google Scholar] [CrossRef]
- Diesen, V.; Jonsson, M. Formation of H2O2 in TiO2 Photocatalysis of Oxygenated and Deoxygenated Aqueous Systems: A Probe for Photocatalytically Produced Hydroxyl Radicals. J. Phys. Chem. C 2014, 118, 10083–10087. [Google Scholar] [CrossRef]
- Lawless, D.; Serpone, N.; Meisel, D. Role of OH· Radicals and Trapped Holes in Photocatalysis. A Pulse Radiolysis Study. J. Phys. Chem. 1991, 95, 5166–5170. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Mechanism of the OH Radical Generation in Photocatalysis with TiO2 of Different Crystalline Types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar] [CrossRef]
- Liao, H.; Reitberger, T. Generation of Free OHaq Radicals by Black Light Illumination of Degussa (Evonik) P25 TiO2 Aqueous Suspensions. Catalysts 2013, 3, 418–443. [Google Scholar] [CrossRef]
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 Nanocomposite Coatings for Surfaces, Dental and Orthopaedic Implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef]
- Kakuma, Y.; Nosaka, A.Y.; Nosaka, Y. Difference in TiO2 Photocatalytic Mechanism between Rutile and Anatase Studied by the Detection of Active Oxygen and Surface Species in Water. Phys. Chem. Chem. Phys. 2015, 17, 18691–18698. [Google Scholar] [CrossRef]
- Fu, Z.; Liang, Y.; Wang, S.; Zhong, Z. Structural Phase Transition and Mechanical Properties of TiO2 under High Pressure. Phys. Status Solidi Basic Res. 2013, 250, 2206–2214. [Google Scholar] [CrossRef]
- Rich, C.C.; Knorr, F.J.; McHale, J.L. Trap State Photoluminescence of Nanocrystalline and Bulk TiO2: Implications for Carrier Transport. Mater. Res. Soc. Symp. Proc. 2010, 1268, 117–122. [Google Scholar] [CrossRef]
- Kurtz, R.L.; Stock-Bauer, R.; Msdey, T.E.; Román, E.; De Segovia, J.L. Synchrotron Radiation Studies of H2O Adsorption on TiO2(110). Surf. Sci. 1989, 218, 178–200. [Google Scholar] [CrossRef]
- Tôrres, A.R.; Azevedo, E.B.; Resende, N.S.; Dezotti, M. A Comparison between Bulk and Supported TiO2 Photocatalysts in the Degradation of Formic Acid. Braz. J. Chem. Eng. 2007, 24, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Lenz, K.A.; Gao, X.; Li, S.; Wallis, L.K. Comparative Toxicity of a Food Additive TiO2, a Bulk TiO2, and a Nano-Sized P25 to a Model Organism the Nematode C. Elegans. Environ. Sci. Pollut. Res. 2019, 26, 3556–3568. [Google Scholar] [CrossRef]
- Macak, J.M.; Zlamal, M.; Krysa, J.; Schmuki, P. Self-Organized TiO2 Nanotube Layers as Highly Efficient Photocatalysts. Small 2007, 3, 300–304. [Google Scholar] [CrossRef]
- Bai, H.; Liu, L.; Liu, Z.; Sun, D.D. Hierarchical 3D Dendritic TiO2 Nanospheres Building with Ultralong 1D Nanoribbon/Wires for High Performance Concurrent Photocatalytic Membrane Water Purification. Water Res. 2013, 47, 4126–4138. [Google Scholar] [CrossRef]
- En Du, Y.; Niu, X.; Bai, Y.; Qi, H.; Guo, Y.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Synthesis of Anatase TiO2 Nanocrystals with Defined Morphologies from Exfoliated Nanoribbons: Photocatalytic Performance and Application in Dye-Sensitized Solar Cell. ChemistrySelect 2019, 4, 4443–4457. [Google Scholar] [CrossRef]
- Wang, X.; Xia, R.; Muhire, E.; Jiang, S.; Huo, X.; Gao, M. Highly Enhanced Photocatalytic Performance of TiO2 Nanosheets through Constructing TiO2/TiO2 Quantum Dots Homojunction. Appl. Surf. Sci. 2018, 459, 9–15. [Google Scholar] [CrossRef]
- Kang, L.; Liu, X.Y.; Wang, A.; Li, L.; Ren, Y.; Li, X.; Pan, X.; Li, Y.; Zong, X.; Liu, H.; et al. Photo–Thermo Catalytic Oxidation over a TiO2-WO3 -Supported Platinum Catalyst. Angew. Chem. 2020, 132, 13009–13016. [Google Scholar] [CrossRef]
- Panniello, A.; Curri, M.L.; Diso, D.; Licciulli, A.; Locaputo, V.; Agostiano, A.; Comparelli, R.; Mascolo, G. Nanocrystalline TiO2 Based Films onto Fibers for Photocatalytic Degradation of Organic Dye in Aqueous Solution. Appl. Catal. B Environ. 2012, 121–122, 190–197. [Google Scholar] [CrossRef]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic Performance of Fe-Doped TiO2 Nanoparticles under Visible-Light Irradiation. Mater. Res. Express 2017, 4, 015022. [Google Scholar] [CrossRef]
- Zhang, H.; Miao, G.; Ma, X.; Wang, B.; Zheng, H. Enhancing the Photocatalytic Activity of Nanocrystalline TiO2 by Co-Doping with Fluorine and Yttrium. Mater. Res. Bull. 2014, 55, 26–32. [Google Scholar] [CrossRef]
- Hu, D.; Li, R.; Li, M.; Pei, J.; Guo, F.; Zhang, S. Photocatalytic Efficiencies of WO3/TiO2 Nanoparticles for Exhaust Decomposition under UV and Visible Light Irradiation. Mater. Res. Express 2018, 5, 095029. [Google Scholar] [CrossRef]
- Naufal, B.; Ullattil, S.G.; Periyat, P. A Dual Function Nanocrystalline TiO2 Platform for Solar Photocatalysis and Self Cleaning Application. Sol. Energy 2017, 155, 1380–1388. [Google Scholar] [CrossRef]
- Dong, G.; Wang, Y.; Lei, H.; Tian, G.; Qi, S. Hierarchical Mesoporous Titania Nanoshell Encapsulated on Polyimide Nano Fiber as Flexible, Highly Reactive, Energy Saving and Recyclable Photocatalyst for Water Purification. J. Clean. Prod. 2020, 253, 120021. [Google Scholar] [CrossRef]
- Xie, J.; Wen, W.; Jin, Q.; Xiang, X.B.; Wu, J.M. TiO2 Nanotrees for the Photocatalytic and Photoelectrocatalytic Phenol Degradation. New J. Chem. 2019, 43, 11050–11056. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wu, W.; Dai, P.; Yu, X.; Wu, M.; Li, G. Hydrothermal Growth of TiO2 Nanowire Membranes Sensitized with CdS Quantum Dots for the Enhancement of Photocatalytic Performance. Nanoscale Res. Lett. 2014, 9, 270. [Google Scholar] [CrossRef] [Green Version]
- Luan, S.; Qu, D.; An, L.; Jiang, W.; Gao, X.; Hua, S.; Miao, X.; Wen, Y.; Sun, Z. Enhancing Photocatalytic Performance by Constructing Ultrafine TiO2 Nanorods/g-C3N4 Nanosheets Heterojunction for Water Treatment. Sci. Bull. 2018, 63, 683–690. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, M. Photocatalytic Activity of TiO2 Nanofibers: The Surface Crystalline Phase Matters. Nanomaterials 2019, 9, 535. [Google Scholar] [CrossRef] [Green Version]
- Teodorescu-Soare, C.T.; Catrinescu, C.; Dobromir, M.; Stoian, G.; Arvinte, A.; Luca, D. Growth and Characterization of TiO2 Nanotube Arrays under Dynamic Anodization. Photocatalytic Activity. J. Electroanal. Chem. 2018, 823, 388–396. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Hu, J.; Li, J.; Wang, Y. Photocatalytic Performance of TiO2 Nanotube Structure Based on TiN Coating Doped with Ag and Cu. Ceram. Int. 2021, 47, 7233–7240. [Google Scholar] [CrossRef]
- Ariyanti, D.; Mo’Ungatonga, S.; Li, Y.; Gao, W. Formation of TiO2 Based Nanoribbons and the Effect of Post-Annealing on Its Photocatalytic Activity. IOP Conf. Ser. Mater. Sci. Eng. 2018, 348, 012002. [Google Scholar] [CrossRef]
- Shaban, M.; Ashraf, A.M.; Abukhadra, M.R. TiO2 Nanoribbons/Carbon Nanotubes Composite with Enhanced Photocatalytic Activity; Fabrication, Characterization, and Application. Sci. Rep. 2018, 8, 781. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Wang, J.; Wang, X.; Xu, H.; Yuan, S.; Zhang, Q.; Zhang, M. Preparation of Inverse Opal Titanium Dioxide for Photocatalytic Performance Research. Opt. Mater. 2019, 96, 109287. [Google Scholar] [CrossRef]
- Albu, S.P.; Kim, D.; Schmuki, P. Growth of Aligned TiO2 Bamboo-Type Nanotubes and Highly Ordered Nanolace. Angew. Chem.—Int. Ed. 2008, 47, 1916–1919. [Google Scholar] [CrossRef]
- Chahrour, K.M.; Yam, F.K.; Eid, A.M.; Nazeer, A.A. Enhanced Photoelectrochemical Properties of Hierarchical Black TiO2-x Nanolaces for Cr (VI) Photocatalytic Reduction. Int. J. Hydrogen Energy 2020, 45, 22674–22690. [Google Scholar] [CrossRef]
- Harris, J.; Silk, R.; Smith, M.; Dong, Y.; Chen, W.T.; Waterhouse, G.I.N. Hierarchical TiO2 Nanoflower Photocatalysts with Remarkable Activity for Aqueous Methylene Blue Photo-Oxidation. ACS Omega 2020, 5, 18919–18934. [Google Scholar] [CrossRef]
- Wen, W.; Hai, J.; Yao, J.; Gu, Y.J.; Kobayashi, H.; Tian, H.; Sun, T.; Chen, Q.; Yang, P.; Geng, C.; et al. Univariate Lattice Parameter Modulation of Single-Crystal-like Anatase TiO2 Hierarchical Nanowire Arrays to Improve Photoactivity. Chem. Mater. 2021, 33, 1489–1497. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.Y.; Liu, J.B.; Wu, J.M. UV and Visible Light Photocatalytic Activity of Au/TiO2 Nanoforests with Anatase/Rutile Phase Junctions and Controlled Au Locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Wen, G.; Liu, H.; Han, S.; Chen, S.; Kong, Q.; Feng, W. One-Step Hydrothermal Synthesis and Characterization of Cu-Doped TiO2 Nanoparticles/Nanobucks/Nanorods with Enhanced Photocatalytic Performance under Simulated Solar Light. J. Mater. Sci. Mater. Electron. 2019, 30, 13826–13834. [Google Scholar] [CrossRef]
- Hosseinnia, A.; Keyanpour-Rad, M.; Pazouki, M. Photo-Catalytic Degradation of Organic Dyes with Different Chromophores by Synthesized Nanosize TiO2 Particles. World Appl. Sci. J. 2010, 8, 1327–1332. [Google Scholar]
- Shrivastava, V.S. Photocatalytic Degradation of Methylene Blue Dye and Chromium Metal from Wastewater Using Nanocrystalline TiO2 Semiconductor. Arch. Appl. Sci. Res. 2012, 4, 1244–1254. [Google Scholar]
- Joshi, K.M.; Shrivastava, V.S. Degradation of Alizarine Red-S (A Textiles Dye) by Photocatalysis Using ZnO and TiO2 as Photocatalyst. Int. J. Environ. Sci. 2011, 2, 8–21. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, T.; Ito, S.; Kuwabata, S.; Yoneyama, H. Effects of Adsorbents Used as Supports for Titanium Dioxide Loading on Photocatalytic Degradation of Propyzamide. Environ. Sci. Technol. 1996, 30, 1275–1281. [Google Scholar] [CrossRef]
- Fox, M.A.; Doan, K.E.; Dulay, M.T. The Effect of the “Inert” Support on Relative Photocatalytic Activity in the Oxidative Decomposition of Alcohols on Irradiated Titanium Dioxide Composites. Res. Chem. Intermed. 1994, 20, 711–721. [Google Scholar] [CrossRef]
- Rachel, A.; Subrahmanyam, M.; Boule, P. Comparison of Photocatalytic Efficiencies of TiO2 in Suspended and Immobilised Form for the Photocatalytic Degradation of Nitrobenzenesulfonic Acids. Appl. Catal. B Environ. 2002, 37, 301–308. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Zhao, J. Enhanced Photocatalytic Activity of Mesoporous and Ordinary TiO2 thin Films by Sulfuric Acid Treatment. Appl. Catal. B Environ. 2002, 36, 31–43. [Google Scholar] [CrossRef]
- Wang, J.A.; Limas-Ballesteros, R.; López, T.; Moreno, A.; Gómez, R.; Novaro, O.; Bokhimi, X. Quantitative Determination of Titanium Lattice Defects and Solid-State Reaction Mechanism in Iron-Doped TiO2 Photocatalysts. J. Phys. Chem. B 2001, 105, 9692–9698. [Google Scholar] [CrossRef]
- Yan, J.; Wu, G.; Guan, N.; Li, L.; Li, Z.; Cao, X. Understanding the Effect of Surface/Bulk Defects on the Photocatalytic Activity of TiO2: Anatase versus Rutile. Phys. Chem. Chem. Phys. 2013, 15, 10978–10988. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Ophus, E.M.; Rode, L.; Gylseth, B.; Nicholson, D.G.; Saeed, K. Analysis of Titanium Pigments in Human Lung Tissue. Scand. J. Work. Environ. Health 1979, 5, 290–296. [Google Scholar] [CrossRef] [Green Version]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini-Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef]
- Lu, N.; Chen, Z.; Song, J.; Weng, Y.; Yang, G.; Liu, Q.; Yang, K.; Lu, X.; Liu, Y. Size Effect of TiO2 Nanoparticles as Food Additive and Potential Toxicity. Food Biophys. 2022, 17, 75–83. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.Y.; Lowry, G.V.; Jolivet, J.P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General Review of Titanium Toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.J.; Sanderson, B.J.S.; Wang, H. Cyto-and Genotoxicity of Ultrafine TiO2 Particles in Cultured Human Lymphoblastoid Cells. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 2007, 628, 99–106. [Google Scholar] [CrossRef]
- Uchino, T.; Tokunaga, H.; Ando, M.; Utsumi, H. Quantitative Determination of OH Radical Generation and Its Cytotoxicity Induced by TiO2-UVA Treatment. Toxicol. Vitr. 2002, 16, 629–635. [Google Scholar] [CrossRef]
- Dodd, N.J.F.; Jha, A.N. Titanium Dioxide Induced Cell Damage: A Proposed Role of the Carboxyl Radical. Mutat. Res.—Fundam. Mol. Mech. Mutagen. 2009, 660, 79–82. [Google Scholar] [CrossRef]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium Dioxide in Our Everyday Life; Is It Safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical Sterilization of Microbial Cells by Semiconductor Powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Maness, P.C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal Activity of Photocatalytic TiO2 Reaction: Toward an Understanding of Its Killing Mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of Photocatalytic Bactericidal Action of Powdered Semiconductor TiO2 on Mutans Streptococci. J. Photochem. Photobiol. B Biol. 1992, 14, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Robertson, P.K.J.; Robertson, J.M.C.; Bahnemann, D.W. Removal of Microorganisms and Their Chemical Metabolites from Water Using Semiconductor Photocatalysis. J. Hazard. Mater. 2012, 211–212, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Lin, W.Y.; Zainal, Z.; Williams, N.E.; Zhu, K.; Kruzlc, A.P.; Smith, R.L.; Rajeshwar, K. Bactericidal Activity of TiO2 Photocatalyst in Aqueous Media: Toward a Solar-Assisted Water Disinfection System. Environ. Sci. Technol. 1994, 28, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Hegde, P.B.; Avasthi, S.; Sen, P. Scalable Hybrid Antibacterial Surfaces: TiO2 Nanoparticles with Black Silicon. ACS Omega 2022, 7, 7816–7824. [Google Scholar] [CrossRef]
- Kalelkar, P.P.; Riddick, M.; García, A.J. Biomaterial-Based Antimicrobial Therapies for the Treatment of Bacterial Infections. Nat. Rev. Mater. 2022, 7, 39–54. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Nagay, B.E.; Dini, C.; Cordeiro, J.M.; Ricomini-Filho, A.P.; De Avila, E.D.; Rangel, E.C.; Da Cruz, N.C.; Barão, V.A.R. Visible-Light-Induced Photocatalytic and Antibacterial Activity of TiO2 Codoped with Nitrogen and Bismuth: New Perspectives to Control Implant-Biofilm-Related Diseases. ACS Appl. Mater. Interfaces 2019, 11, 18186–18202. [Google Scholar] [CrossRef]
- Han, X.; Zhang, G.; Chai, M.; Zhang, X. Light-Assisted Therapy for Biofilm Infected Micro-Arc Oxidation TiO2 Coating on Bone Implants. Biomed. Mater. 2021, 16, 025018. [Google Scholar] [CrossRef]
- Wang, R.; Shi, M.; Xu, F.; Qiu, Y.; Zhang, P.; Shen, K.; Zhao, Q.; Yu, J.; Zhang, Y. Graphdiyne-Modified TiO2 Nanofibers with Osteoinductive and Enhanced Photocatalytic Antibacterial Activities to Prevent Implant Infection. Nat. Commun. 2020, 11, 4465. [Google Scholar] [CrossRef]
- Horváth, E.; Rossi, L.; Mercier, C.; Lehmann, C.; Sienkiewicz, A.; Forró, L. Photocatalytic Nanowires-Based Air Filter: Towards Reusable Protective Masks. Adv. Funct. Mater. 2020, 30, 2004615. [Google Scholar] [CrossRef]
- Khaiboullina, S.; Uppal, T.; Dhabarde, N.; Subramanian, V.R.; Verma, S.C. Inactivation of Human Coronavirus by Titania Nanoparticle Coatings and Uvc Radiation: Throwing Light on Sars-CoV-2. Viruses 2021, 13, 19. [Google Scholar] [CrossRef]
- Yoshizawa, N.; Ishihara, R.; Omiya, D.; Ishitsuka, M.; Hirano, S.; Suzuki, T. Application of a Photocatalyst as an Inactivator of Bovine Coronavirus. Viruses 2020, 12, 1372. [Google Scholar] [CrossRef]
- He, H.; Liu, C.; Dubois, K.D.; Jin, T.; Louis, M.E.; Li, G. Enhanced Charge Separation in Nanostructured TiO2 Materials for Photocatalytic and Photovoltaic Applications. Ind. Eng. Chem. Res. 2012, 51, 11841–11849. [Google Scholar] [CrossRef]
- Kočí, K.; Obalová, L.; Matějová, L.; Plachá, D.; Lacný, Z.; Jirkovský, J.; Šolcová, O. Effect of TiO2 Particle Size on the Photocatalytic Reduction of CO2. Appl. Catal. B Environ. 2009, 89, 494–502. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Satoh, N.; Nakashima, T.; Kamikura, K.; Yamamoto, K. Quantum Size Effect in TiO2 Nanoparticles Prepared by Finely Controlled Metal Assembly on Dendrimer Templates. Nat. Nanotechnol. 2008, 3, 106–111. [Google Scholar] [CrossRef]
- Li, W.; Ni, C.; Lin, H.; Huang, C.P.; Shah, S.I. Size Dependence of Thermal Stability of TiO2 Nanoparticles. J. Appl. Phys. 2004, 96, 6663–6668. [Google Scholar] [CrossRef] [Green Version]
- Jansson, I.; Suárez, S.; Garcia-Garcia, F.J.; Sánchez, B. Zeolite-TiO2 Hybrid Composites for Pollutant Degradation in Gas Phase. Appl. Catal. B Environ. 2015, 178, 100–107. [Google Scholar] [CrossRef]
- Wang, C.Y.; Böttcher, C.; Bahnemann, D.W.; Dohrmann, J.K. A Comparative Study of Nanometer Sized Fe(III)-Doped TiO2 Photocatalysts: Synthesis, Characterization and Activity. J. Mater. Chem. 2003, 13, 2322–2329. [Google Scholar] [CrossRef]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced Charge-Collection Efficiencies and Light Scattering in Dye-Sensitized Solar Cells Using Oriented TiO2 Nanotubes Arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Vanmaekelbergh, D. Trap-Limited Electronic Transport in Assemblies of Nanometer-Size TiO2 Particles. Phys. Rev. Lett. 1996, 77, 3427–3430. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Haque, S.A.; Klug, D.R.; Durrant, J.R. Trap-Limited Recombination in Dye-Sensitized Nanocrystalline Metal Oxide Electrodes. Phys. Rev. B 2001, 63, 205321-1–205321-29. [Google Scholar] [CrossRef]
- Cao, F.; Oskam, G.; Meyer, G.J.; Searson, P.C. Electron Transport in Porous Nanocrystalline TiO2 Photoelectrochemical Cells. J. Phys. Chem. 1996, 100, 17021–17027. [Google Scholar] [CrossRef]
- Dloczik, L.; Ileperuma, O.; Lauermann, I.; Peter, L.M.; Ponomarev, E.A.; Redmond, G.; Shaw, N.J.; Uhlendorf, I. Dynamic Response of Dye-Sensitized Nanocrystalline Solar Cells: Characterization by Intensity-Modulated Photocurrent Spectroscopy. J. Phys. Chem. B 1997, 5647, 10281–10289. [Google Scholar] [CrossRef]
- Park, N.G.; Frank, A.J. Evaluation of the Charge-Collection Efficiency of Dye-Sensitized Nanocrystalline TiO2 Solar Cells. J. Phys. Chem. B 1999, 103, 782–791. [Google Scholar] [CrossRef]
- Paulose, M.; Shankar, K.; Yoriya, S.; Prakasam, H.E.; Varghese, O.K.; Mor, G.K.; Latempa, T.A.; Fitzgerald, A.; Grimes, C.A. Anodic Growth of Highly Ordered TiO2 Nanotube Arrays to 134 µm in Length. J. Phys. Chem. B 2006, 110, 16179–16184. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Masaki, N.; Kitamura, T.; Wada, Y.; Okamoto, T.; Sekino, T.; Niihara, K.; Yanagida, S. Dye-Sensitized TiO2 Nanotube Solar Cells: Fabrication and Electronic Characterization. Phys. Chem. Chem. Phys. 2005, 7, 4157–4163. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of Highly-Ordered TiO2 Nanotube Arrays in Dye-Sensitized Solar Cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
- Bahnemann, D.W. Current Challenges in Photo Catalysis: Improved Photocatalysts and Appropriate Photoreactor Engineering. Res. Chem. Intermed. 2000, 26, 207–220. [Google Scholar] [CrossRef]
- Qin, Y.; Deng, L.; Wei, S.; Bai, H.; Gao, W.; Jiao, W.; Yu, T. An Effective Strategy for Improving Charge Separation Efficiency and Photocatalytic Degradation Performance Using a Facilely Synthesized Oxidative TiO2 Catalyst. Dalt. Trans. 2022, 51, 6899–6907. [Google Scholar] [CrossRef]
- Furube, A.; Asahi, T.; Masuhara, H.; Yamashita, H.; Anpo, M. Direct Observation of a Picosecond Charge Separation Process in Photoexcited Platinum-Loaded TiO2 Particles by Femtosecond Diffuse Reflectance Spectroscopy. Chem. Phys. Lett. 2001, 336, 424–430. [Google Scholar] [CrossRef]
- Yang, L.; Gao, P.; Lu, J.; Guo, W.; Zhuang, Z.; Wang, Q.; Li, W.; Feng, Z. Mechanism Analysis of Au, Ru Noble Metal Clusters Modified on TiO2(101) to Intensify Overall Photocatalytic Water Splitting. RSC Adv. 2020, 10, 20654–20664. [Google Scholar] [CrossRef]
- Kmetykó, Á.; Szániel, Á.; Tsakiroglou, C.; Dombi, A.; Hernádi, K. Enhanced Photocatalytic H2 Generation on Noble Metal Modified TiO2 Catalysts Excited with Visible Light Irradiation. React. Kinet. Mech. Catal. 2016, 117, 379–390. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen Production by Photocatalytic Steam Reforming of Methanol on Noble Metal-Modified TiO2. J. Catal. 2010, 273, 182–190. [Google Scholar] [CrossRef]
- Nie, J.; Schneider, J.; Sieland, F.; Zhou, L.; Xia, S.; Bahnemann, D.W. New Insights into the Surface Plasmon Resonance (SPR) Driven Photocatalytic H2 Production of Au-TiO2. RSC Adv. 2018, 8, 25881–25887. [Google Scholar] [CrossRef]
- Singh, Y.; Raghuwanshi, S.K. Titanium Dioxide (TiO2) Coated Optical Fiber-Based SPR Sensor in near-Infrared Region with Bimetallic Structure for Enhanced Sensitivity. Optik 2021, 226, 165842. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, X.; Liu, J.; Tian, Z.; Dai, L.; He, B.; Han, C.; Wu, Y.; Zeng, Z.; Hu, Z. On the Role of Localized Surface Plasmon Resonance in UV-Vis Light Irradiated Au/TiO2 Photocatalysis Systems: Pros and Cons. Nanoscale 2015, 7, 4114–4123. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef]
- Wu, L.; Ma, S.; Chen, P.; Li, X. The Mechanism of Enhanced Charge Separation and Photocatalytic Activity for Au@TiO2 Core-Shell Nanocomposite. Int. J. Environ. Anal. Chem. 2023, 103, 201–211. [Google Scholar] [CrossRef]
- Lan, D.; Pang, F.; Ge, J. Enhanced Charge Separation in NiO and PdCo-Modified TiO2 Photocatalysts for Efficient and Selective Photoreduction of CO2. ACS Appl. Energy Mater. 2021, 4, 6324–6332. [Google Scholar] [CrossRef]
- Cao, F.; Xiong, J.; Wu, F.; Liu, Q.; Shi, Z.; Yu, Y.; Wang, X.; Li, L. Enhanced Photoelectrochemical Performance from Rationally Designed Anatase/Rutile TiO2 Heterostructures. ACS Appl. Mater. Interfaces 2016, 8, 12239–12245. [Google Scholar] [CrossRef] [PubMed]
- Bickley, R.I.; Gonzalez-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. A Structural Investigation of Titanium Dioxide Photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Nair, R.G.; Paul, S.; Samdarshi, S.K. High UV/Visible Light Activity of Mixed Phase Titania: A Generic Mechanism. Sol. Energy Mater. Sol. Cells 2011, 95, 1901–1907. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; An, X.; Guo, Z.X.; Tang, J. Fe2O3-TiO2 Nanocomposites for Enhanced Charge Separation and Photocatalytic Activity. Chem.—A Eur. J. 2014, 20, 15571–15579. [Google Scholar] [CrossRef]
- Al Mayyahi, A.; Everhart, B.M.; Shrestha, T.B.; Back, T.C.; Amama, P.B. Enhanced Charge Separation in TiO2/Nanocarbon Hybrid Photocatalysts through Coupling with Short Carbon Nanotubes. RSC Adv. 2021, 11, 11702–11713. [Google Scholar] [CrossRef]
- Du, X.; Hu, J.; Xie, J.; Hao, A.; Lu, Z.; Cao, Y. Simultaneously Tailor Band Structure and Accelerate Charge Separation by Constructing Novel In(OH)3-TiO2 Heterojunction for Enhanced Photocatalytic Water Reduction. Appl. Surf. Sci. 2022, 593, 153305. [Google Scholar] [CrossRef]
- Ge, Z.; Wang, C.; Chen, Z.; Wang, T.; Chen, T.; Shi, R.; Yu, S.; Liu, J. Investigation of the TiO2 Nanoparticles Aggregation with High Light Harvesting for High-Efficiency Dye-Sensitized Solar Cells. Mater. Res. Bull. 2021, 135, 111148. [Google Scholar] [CrossRef]
- Ram, S.K.; Rizzoli, R.; Desta, D.; Jeppesen, B.R.; Bellettato, M.; Samatov, I.; Tsao, Y.C.; Johannsen, S.R.; Neuvonen, P.T.; Pedersen, T.G.; et al. Directly Patterned TiO2 Nanostructures for Efficient Light Harvesting in Thin Film Solar Cells. J. Phys. D Appl. Phys. 2015, 48, 365101. [Google Scholar] [CrossRef]
- Zada, I.; Zhang, W.; Zheng, W.; Zhu, Y.; Zhang, Z.; Zhang, J.; Imtiaz, M.; Abbas, W.; Zhang, D. The Highly Efficient Photocatalytic and Light Harvesting Property of Ag-TiO2 with Negative Nano-Holes Structure Inspired from Cicada Wings. Sci. Rep. 2017, 7, 17277. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.; Hwang, S.H.; Jang, J. Fabrication of Au@Ag Core/Shell Nanoparticles Decorated TiO2 Hollow Structure for Efficient Light-Harvesting in Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 2055–2063. [Google Scholar] [CrossRef]
- Yang, H.Y.; Rho, W.Y.; Lee, S.K.; Kim, S.H.; Hahn, Y.B. TiO2 Nanoparticles/Nanotubes for Efficient Light Harvesting in Perovskite Solar Cells. Nanomaterials 2019, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, J.R.; Birke, R.L. Theory of Surface-Enhanced Raman Scattering in Semiconductors. J. Phys. Chem. C 2014, 118, 11120–11130. [Google Scholar] [CrossRef]
- Hayashi, S.; Koh, R.; Ichiyama, Y.; Yamamoto, K. Evidence for Surface-Enhanced Raman Scattering on Nonmetallic Surfaces: Copper Phthalocyanine Molecules on GaP Small Particles. Phys. Rev. Lett. 1988, 60, 1085–1089. [Google Scholar] [CrossRef]
- Xue, X.; Ji, W.; Mao, Z.; Mao, H.; Wang, Y.; Wang, X.; Ruan, W.; Zhao, B.; Lombardi, J.R. Raman Investigation of Nanosized TiO2: Effect of Crystallite Size and Quantum Confinement. J. Phys. Chem. C 2012, 116, 8792–8797. [Google Scholar] [CrossRef]
- Querebillo, C.J.; Öner, H.I.; Hildebrandt, P.; Ly, K.H.; Weidinger, I.M. Accelerated Photo-Induced Degradation of Benzidine-p- Aminothiophenolate Immobilized at Light-Enhancing TiO2 Nanotube Electrodes. Chem. Eur. J. 2019, 25, 16048–16053. [Google Scholar] [CrossRef] [Green Version]
- Öner, I.H.; David, C.; Querebillo, C.J.; Weidinger, I.M.; Ly, K.H. Electromagnetic Field Enhancement of Nanostructured TiN Electrodes Probed with Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 487. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, L.; Zhang, J.; Chen, Q.; Bao, J.; Fang, B. A Novel CdS/S-TiO2 Nanotubes Photocatalyst with High Visible Light Activity. Sep. Purif. Technol. 2009, 66, 417–421. [Google Scholar] [CrossRef]
- Shin, S.W.; Lee, J.Y.; Ahn, K.S.; Kang, S.H.; Kim, J.H. Visible Light Absorbing TiO2 Nanotube Arrays by Sulfur Treatment for Photoelectrochemical Water Splitting. J. Phys. Chem. C 2015, 119, 13375–13383. [Google Scholar] [CrossRef]
- Shen, J.; Meng, Y.; Xin, G. CdS/TiO2 Nanotubes Hybrid as Visible Light Driven Photocatalyst for Water Splitting. Rare Met. 2011, 30, 280–283. [Google Scholar] [CrossRef]
- Qi, D.; Lu, L.; Wang, L.; Zhang, J. Improved SERS Sensitivity on Plasmon-Free TiO2 Photonic Microarray by Enhancing Light-Matter Coupling. J. Am. Chem. Soc. 2014, 136, 9886–9889. [Google Scholar] [CrossRef] [PubMed]
- Joannopoulos, J.D.; Pierre, R.; Villeneuve, S.F. Photonic Crystals:Putting a New Twist on Light. Nature 1997, 386, 7. [Google Scholar] [CrossRef]
- Al-Haddad, A.; Wang, Z.; Xu, R.; Qi, H.; Vellacheri, R.; Kaiser, U.; Lei, Y. Dimensional Dependence of the Optical Absorption Band Edge of TiO2 Nanotube Arrays beyond the Quantum Effect. J. Phys. Chem. C 2015, 119, 16331–16337. [Google Scholar] [CrossRef]
- Yip, C.T.; Huang, H.; Zhou, L.; Xie, K.; Wang, Y.; Feng, T.; Li, J.; Tam, W.Y. Direct and Seamless Coupling of TiO2 Nanotube Photonic Crystal to Dye-Sensitized Solar Cell: A Single-Step Approach. Adv. Mater. 2011, 23, 5624–5628. [Google Scholar] [CrossRef]
- Gesesse, G.D.; Li, C.; Paineau, E.; Habibi, Y.; Remita, H.; Colbeau-Justin, C.; Ghazzal, M.N. Enhanced Photogenerated Charge Carriers and Photocatalytic Activity of Biotemplated Mesoporous TiO2 Films with a Chiral Nematic Structure. Chem. Mater. 2019, 31, 4851–4863. [Google Scholar] [CrossRef]
- Chen, J.I.L.; Loso, E.; Ebrahim, N.; Ozin, G.A. Synergy of Slow Photon and Chemically Amplified Photochemistry in Platinum Nanocluster-Loaded Inverse Titania Opals. J. Am. Chem. Soc. 2008, 130, 5420–5421. [Google Scholar] [CrossRef]
- Zhang, X.; John, S. Enhanced Photocatalysis by Light-Trapping Optimization in Inverse Opals. J. Mater. Chem. A 2020, 8, 18974–18986. [Google Scholar] [CrossRef]
- Huo, J.; Yuan, C.; Wang, Y. Nanocomposites of Three-Dimensionally Ordered Porous TiO2 Decorated with Pt and Reduced Graphene Oxide for the Visible-Light Photocatalytic Degradation of Waterborne Pollutants. ACS Appl. Nano Mater. 2019, 2, 2713–2724. [Google Scholar] [CrossRef]
- Chen, J.I.L.; Von Freymann, G.; Choi, S.Y.; Kitaev, V.; Ozin, G.A. Slow Photons in the Fast Lane in Chemistry. J. Mater. Chem. 2008, 18, 369–373. [Google Scholar] [CrossRef]
- Sordello, F.; Duca, C.; Maurino, V.; Minero, C. Photocatalytic Metamaterials: TiO2 Inverse Opals. Chem. Commun. 2011, 47, 6147–6149. [Google Scholar] [CrossRef]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A Review of Its Properties and Conflicting Trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, Š.; Zbořil, R.; Schmuki, P. Photocatalysis with Reduced TiO2: From Black TiO2 to Cocatalyst-Free Hydrogen Production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, L.; Huang, F. Black Titanium Dioxide (TiO2) Nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Niu, M.; Mao, C.; Cao, D.; Cheng, D.; Feng, P.; Sun, Z. A Facile and Versatile Method for Preparation of Colored TiO2 with Enhanced Solar-Driven Photocatalytic Activity. Nanoscale 2014, 6, 10216–10223. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, H.; Han, H.; Fu, Y.; Ma, C.; Yu, Z.; Dong, X. Black TiO2 Nanotube Arrays Fabricated by Electrochemical Self-Doping and Their Photoelectrochemical Performance. RSC Adv. 2018, 8, 18992–19000. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Zhou, W.; Zhang, K.; Zhang, X.; Wang, L.; Jiang, B.; Tian, G.; Zhao, D.; Fu, H. Facile Strategy for Controllable Synthesis of Stable Mesoporous Black TiO2 Hollow Spheres with Efficient Solar-Driven Photocatalytic Hydrogen Evolution. J. Mater. Chem. A 2016, 4, 7495–7502. [Google Scholar] [CrossRef]
- Zhou, W.; Li, W.; Wang, J.Q.; Qu, Y.; Yang, Y.; Xie, Y.; Zhang, K.; Wang, L.; Fu, H.; Zhao, D. Ordered Mesoporous Black TiO2 as Highly Efficient Hydrogen Evolution Photocatalyst. J. Am. Chem. Soc. 2014, 136, 9280–9283. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, H.; Yang, C.; Cui, H.; Wang, Z.; Xu, J.; Lin, T.; Huang, F. Black Titania for Superior Photocatalytic Hydrogen Production and Photoelectrochemical Water Splitting. ChemCatChem 2015, 7, 2614–2619. [Google Scholar] [CrossRef]
- Dong, J.; Han, J.; Liu, Y.; Nakajima, A.; Matsushita, S.; Wei, S.; Gao, W. Defective Black TiO2 Synthesized via Anodization for Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 1385–1388. [Google Scholar] [CrossRef]
- Fan, C.; Chen, C.; Wang, J.; Fu, X.; Ren, Z.; Qian, G.; Wang, Z. Black Hydroxylated Titanium Dioxide Prepared via Ultrasonication with Enhanced Photocatalytic Activity. Sci. Rep. 2015, 5, 11712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.Z.; Reed, A.; Nagpure, S.; Wanninayake, N.; Browning, J.F.; Strzalka, J.; Kim, D.Y.; Rankin, S.E. Hydrogen Incorporation by Plasma Treatment Gives Mesoporous Black TiO2 Thin Films with Visible Photoelectrochemical Water Oxidation Activity. Microporous Mesoporous Mater. 2018, 261, 35–43. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Periyat, P. A “one Pot” Gel Combustion Strategy towards Ti3+ Self-Doped “Black” Anatase TiO2−x Solar Photocatalyst. J. Mater. Chem. A 2016, 4, 5854–5858. [Google Scholar] [CrossRef]
- Sun, L.; Xie, J.; Li, Q.; Wang, F.; Xi, X.; Li, L.; Wu, J.; Shao, R.; Chen, Z. Facile Synthesis of Thin Black TiO2−x Nanosheets with Enhanced Lithium-Storage Capacity and Visible Light Photocatalytic Hydrogen Production. J. Solid State Electrochem. 2019, 23, 803–810. [Google Scholar] [CrossRef]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 Nanotube Arrays for Photoelectrochemical Water Splitting. J. Mater. Chem. A 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Zhang, M.; Pei, Q.; Chen, W.; Liu, L.; He, T.; Chen, P. Room Temperature Synthesis of Reduced TiO2 and Its Application as a Support for Catalytic Hydrogenation. RSC Adv. 2017, 7, 4306–4311. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Ji, J.; Liu, B.; Huang, H. Reduced TiO2 with Tunable Oxygen Vacancies for Catalytic Oxidation of Formaldehyde at Room Temperature. Appl. Surf. Sci. 2019, 473, 934–942. [Google Scholar] [CrossRef]
- Will, J.; Wierzbicka, E.; Wu, M.; Götz, K.; Yokosawa, T.; Liu, N.; Tesler, A.B.; Stiller, M.; Unruh, T.; Altomare, M.; et al. Hydrogenated Anatase TiO2 Single Crystals: Defects Formation and Structural Changes as Microscopic Origin of Co-Catalyst Free Photocatalytic H2 evolution Activity. J. Mater. Chem. A 2021, 9, 24932–24942. [Google Scholar] [CrossRef]
- Katal, R.; Salehi, M.; Davood Abadi Farahani, M.H.; Masudy-Panah, S.; Ong, S.L.; Hu, J. Preparation of a New Type of Black TiO2 under a Vacuum Atmosphere for Sunlight Photocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 35316–35326. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, X.; Nguyen, N.T.; Peters, K.; Zoller, F.; Hwang, I.; Schneider, C.; Miehlich, M.E.; Freitag, D.; Meyer, K.; et al. Black Magic in Gray Titania: Noble-Metal-Free Photocatalytic H2 Evolution from Hydrogenated Anatase. ChemSusChem 2017, 10, 62–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Z.; Liu, X.; Li, Z.; Wu, X.; Jiang, J.; Li, M.; Zhu, Q.; Zhou, W. Ti3+ Self-Doped Blue TiO2(B) Single-Crystalline Nanorods for Efficient Solar-Driven Photocatalytic Performance. ACS Appl. Mater. Interfaces 2016, 8, 26851–26859. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Liu, Z.; Marcus, M.A.; Wang, W.C.; Oyler, N.A.; Grass, M.E.; Mao, B.; Glans, P.A.; Yu, P.Y.; et al. Properties of Disorder-Engineered Black Titanium Dioxide Nanoparticles through Hydrogenation. Sci. Rep. 2013, 3, 1510. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Zhang, Z.; Li, Y.; Xiao, C.; Zhang, H.; Li, W.; Zhan, L.; Liang, G.; Chang, Y.; Ning, C.; et al. In Situ Construction of Black Titanium Oxide with a Multilevel Structure on a Titanium Alloy for Photothermal Antibacterial Therapy. ACS Biomater. Sci. Eng. 2022, 8, 2419–2427. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.; Li, K.; Zhao, J.; Ma, H.; Wu, C.; Zhang, C.; Xie, Y.; Yang, F.; Zheng, X. A Hydrogenated Black TiO2 Coating with Excellent Effects for Photothermal Therapy of Bone Tumor and Bone Regeneration. Mater. Sci. Eng. C 2019, 102, 458–470. [Google Scholar] [CrossRef]
- Janczarek, M.; Endo-Kimura, M.; Wang, K.; Wei, Z.; Akanda, M.M.A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Is Black Titania a Promising Photocatalyst? Catalysts 2022, 12, 1320. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, N.; Yang, J.; Zhang, Z. Photoelectrochemical Antibacterial Platform Based on Rationally Designed Black TiO2−x Nanowires for Efficient Inactivation against Bacteria. ACS Appl. Bio Mater. 2022, 5, 1341–1347. [Google Scholar] [CrossRef]
- Campbell, L.; Nguyen, S.H.; Webb, H.K.; Eldridge, D.S. Photocatalytic Disinfection of S. aureus Using Black TiO2−x under Visible Light. Catal. Sci. Technol. 2022, 13, 62–71. [Google Scholar] [CrossRef]
- Tengvall, P.; Lundström, I.; Sjöqvist, L.; Elwing, H.; Bjursten, L.M. Titanium-Hydrogen Peroxide Interaction: Model Studies of the Influence of the Inflammatory Response on Titanium Implants. Biomaterials 1989, 10, 166–175. [Google Scholar] [CrossRef]
- Tengvall, P.; Elwing, H.; Sjöqvist, L.; Lundström, I.; Bjursten, L.M. Interaction between Hydrogen Peroxide and Titanium: A Possible Role in the Biocompatibility of Titanium. Biomaterials 1989, 10, 118–120. [Google Scholar] [CrossRef]
- Randorn, C.; Wongnawa, S.; Boonsin, P. Bleaching of Methylene Blue by Hydrated Titanium Dioxide. ScienceAsia 2004, 30, 149–156. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, K.; Cao, S.; Yao, W.; Piao, L. Synergetic Catalysis Enhancement between H2O2 and TiO2 with Single-Electron-Trapped Oxygen Vacancy. Nano Res. 2020, 13, 551–556. [Google Scholar] [CrossRef]
- Zou, J.; Gao, J.; Xie, F. An Amorphous TiO2 Sol Sensitized with H2O2 with the Enhancement of Photocatalytic Activity. J. Alloys Compd. 2010, 497, 420–427. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, D.; Wei, W.; Chen, X.; Han, Q.; Yao, W.; Ma, X.; Zhu, Y. Ultrathin TiO2(B) Nanosheets as the Inductive Agent for Transfrering H2O2 into Superoxide Radicals. ACS Appl. Mater. Interfaces 2017, 9, 15533–15540. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.D.; Taxt-Lamolle, S.F.M.; Hole, E.O.; Krivokapić, A.; Sagstuen, E.; Haugen, H.J. TiO2 Suspension Exposed to H2O2 in Ambient Light or Darkness: Degradation of Methylene Blue and EPR Evidence for Radical Oxygen Species. Appl. Catal. B Environ. 2013, 142–143, 662–667. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Lin, T.; He, Y.Y.; Mou, Y.X. Heterogeneous Activation of H2O2 by Defect-Engineered TiO2−X Single Crystals for Refractory Pollutants Degradation: A Fenton-like Mechanism. J. Hazard. Mater. 2016, 311, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Wiedmer, D.; Sagstuen, E.; Welch, K.; Haugen, H.J.; Tiainen, H. Oxidative Power of Aqueous Non-Irradiated TiO2-H2O2 Suspensions: Methylene Blue Degradation and the Role of Reactive Oxygen Species. Appl. Catal. B Environ. 2016, 198, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Luo, J.; Chen, Q.; Jiang, Y.; Dong, X.; Cui, F. Titanate Nanosheets as Highly Efficient Non-Light-Driven Catalysts for Degradation of Organic Dyes. Chem. Commun. 2015, 51, 10847–10849. [Google Scholar] [CrossRef]
- Krishnan, P.; Liu, M.; Itty, P.A.; Liu, Z.; Rheinheimer, V.; Zhang, M.H.; Monteiro, P.J.M.; Yu, L.E. Characterization of Photocatalytic TiO2 Powder under Varied Environments Using near Ambient Pressure X-Ray Photoelectron Spectroscopy. Sci. Rep. 2017, 7, 43298. [Google Scholar] [CrossRef] [Green Version]
- Vorontsov, A.V.; Valdés, H.; Smirniotis, P.G.; Paz, Y. Recent Advancements in the Understanding of the Surface Chemistry in TiO2 Photocatalysis. Surfaces 2020, 3, 72–92. [Google Scholar] [CrossRef] [Green Version]
- Jose, M.; Haridas, M.P.; Shukla, S. Predicting Dye-Adsorption Capacity of Hydrogen Titanate Nanotubes via One-Step Dye-Removal Method of Novel Chemically-Activated Catalytic Process Conducted in Dark. J. Environ. Chem. Eng. 2014, 2, 1980–1988. [Google Scholar] [CrossRef]
- Xiong, F.; Yin, L.-L.; Wang, Z.; Jin, Y.; Sun, G.; Gong, X.-Q.; Huang, W. Surface Reconstruction-Induced Site-Specific Charge Separation and Photocatalytic Reaction on Anatase TiO2 (001) Surface. J. Phys. Chem. C 2017, 121, 9991–9999. [Google Scholar] [CrossRef]
- Petković, J.; Küzma, T.; Rade, K.; Novak, S.; Filipič, M. Pre-Irradiation of Anatase TiO2 Particles with UV Enhances Their Cytotoxic and Genotoxic Potential in Human Hepatoma HepG2 Cells. J. Hazard. Mater. 2011, 196, 145–152. [Google Scholar] [CrossRef]
- Humphreys, H. Surgical Site Infection, Ultraclean Ventilated Operating Theatres and Prosthetic Joint Surgery: Where Now? J. Hosp. Infect. 2012, 81, 71–72. [Google Scholar] [CrossRef]
- Van Hengel, I.A.J.; Tierolf, M.W.A.M.; Fratila-apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Antibacterial Titanium Implants Biofunctionalized by Plasma Electrolytic Oxidation with Silver, Zinc, and Copper: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 3800. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial Titanium Surfaces for Medical Implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, M.; Zhang, L.; Wei, H. Antibacterial Adhesion Strategy for Dental Titanium Implant Surfaces: From Mechanisms to Application. J. Funct. Biomater. 2022, 13, 169. [Google Scholar] [CrossRef]
- Akshaya, S.; Rowlo, P.K.; Dukle, A.; Nathanael, A.J. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics 2022, 11, 1719. [Google Scholar] [CrossRef]
- Rincón, A.G.; Pulgarin, C. Absence of E. Coli Regrowth after Fe3+ and TiO2 Solar Photoassisted Disinfection of Water in CPC Solar Photoreactor. Catal. Today 2007, 124, 204–214. [Google Scholar] [CrossRef]
- Delanois, R.E.; Mistry, J.B.; Gwam, C.U.; Mohamed, N.S.; Choksi, U.S.; Mont, M.A. Current Epidemiology of Revision Total Knee Arthroplasty in the United States. J. Arthroplast. 2017, 32, 2663–2668. [Google Scholar] [CrossRef]
- Savio, D.; Bagno, A. When the Total Hip Replacement Fails: A Review on the Stress-Shielding Effect. Processes 2022, 10, 612. [Google Scholar] [CrossRef]
- Kwak, J.M.; Koh, K.H.; Jeon, I.H. Total Elbow Arthroplasty: Clinical Outcomes, Complications, and Revision Surgery. CiOS Clin. Orthop. Surg. 2019, 11, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Shukla, P. A Review of Titanium Based Orthopaedic Implants (Part-I): Physical Characteristics, Problems and the Need for Surface Modification. Int. J. Peen. Sci. Technol. 2020, 1, 301–332. [Google Scholar]
- Prestat, M.; Thierry, D. Corrosion of Titanium under Simulated Inflammation Conditions: Clinical Context and in Vitro Investigations. Acta Biomater. 2021, 136, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Cossey, A.; Tong, J. Stress Shielding in Periprosthetic Bone Following a Total Knee Replacement: Effects of Implant Material, Design and Alignment. Med. Eng. Phys. 2016, 38, 1481–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Thouas, G.A. Metallic Implant Biomaterials. Mater. Sci. Eng. R Reports 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [Green Version]
- Rack, H.J.; Qazi, J.I. Titanium Alloys for Biomedical Applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Hansen, D.C. Metal Corrosion in the Human Body: The Ultimate Bio-Corrosion Scenario. Electrochem. Soc. Interface 2008, 17, 31–34. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova; Kralj-Iglic, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium Nanostructures for Biomedical Applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef]
- Ventre, M.; Coppola, V.; Iannone, M.; Netti, P.A.; Tekko, I.; Larrañeta, E.; Rodgers, A.M.; Scott, C.J.; Kissenpfennig, A.; Donnelly, R.F.; et al. Nanotechnologies for Tissue Engineering and Regeneration. In Nanotechnologies in Preventive and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 93–206. [Google Scholar]
- Wu, J.M. Nanostructured TiO2 Layers on Ti for Bone Bonding; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780081029992. [Google Scholar]
- Li, Y.; Xu, J. Is Niobium More Corrosion-Resistant than Commercially Pure Titanium in Fluoride-Containing Artificial Saliva? Electrochim. Acta 2017, 233, 151–166. [Google Scholar] [CrossRef]
- Reeves, J.F.; Davies, S.J.; Dodd, N.J.F.; Jha, A.N. Hydroxyl Radicals (•OH) Are Associated with Titanium Dioxide (TiO2) Nanoparticle-Induced Cytotoxicity and Oxidative DNA Damage in Fish Cells. Mutat. Res.—Fundam. Mol. Mech. Mutagen. 2008, 640, 113–122. [Google Scholar] [CrossRef]
- Eger, M.; Hiram-Bab, S.; Liron, T.; Sterer, N.; Carmi, Y.; Kohavi, D.; Gabet, Y. Mechanism and Prevention of Titanium Particle-Induced Inflammation and Osteolysis. Front. Immunol. 2018, 9, 2963. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical and XPS Studies of Titanium for Biomaterial Applications with Respect to the Effect of Hydrogen Peroxide. J. Biomed. Mater. Res. 1994, 28, 113–122. [Google Scholar] [CrossRef]
- Sundgren, J.-E.; Bodö, P.; Lundström, I. Auger Electron Spectroscopic Studies of the Interface between Human Tissue and Implants of Titanium and Stainless Steel. J. Colloid Interface Sci. 1986, 110, 9–20. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Mali, S.; Urban, R.M.; Silverton, C.D.; Jacobs, J.J. In Vivo Oxide-Induced Stress Corrosion Cracking of Ti-6Al-4V in a Neck-Stem Modular Taper: Emergent Behavior in a New Mechanism of in Vivo Corrosion. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2012, 100 B, 584–594. [Google Scholar] [CrossRef]
- Haynes, D.R.; Rogers, S.D.; Hay, S.; Pearcy, M.J.; Howie, D.W. The Differences in Toxicity and Release of Bone-Resorbing Mediators Induced by Titanium and Cobalt-Chromium-Alloy Wear Particles. J. Bone Jt. Surg. 1993, 75, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gilbert, J.L. The Effect of Simulated Inflammatory Conditions and Fenton Chemistry on the Electrochemistry of CoCrMo Alloy. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2018, 106, 209–220. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [Green Version]
- Väänänen, H.K.; Zhao, H.; Mulari, M.; Halleen, J.M. The Cell Biology of Osteoclast Function. J. Cell Sci. 2000, 113, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Berglund, F.; Carlmark, B. Titanium, Sinusitis, and the Yellow Nail Syndrome. Biol. Trace Elem. Res. 2011, 143, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decker, A.; Daly, D.; Scher, R.K. Role of Titanium in the Development of Yellow Nail Syndrome. Ski. Appendage Disord. 2015, 1, 28–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ataya, A.; Kline, K.P.; Cope, J.; Alnuaimat, H. Titanium Exposure and Yellow Nail Syndrome. Respir. Med. Case Rep. 2015, 16, 146–147. [Google Scholar] [CrossRef] [Green Version]









| Morphology | Material Dimension | Crystal State | Synthetic Procedure | Photocatalytic Performance § | Targeted Applications | Ref. |
|---|---|---|---|---|---|---|
| Commercially available Degussa P25 nanopowder | 30–40 nm | anatase + rutile | either deposited as film or used as dispersion/colloid | Pseudo-first-order rate constant, k = 0.0085–0.012 min−1 AO7 [72,73], RhB [73,74] degradation; UV light, 100–200 mW HeCd laser with I = 60 or 100 mW cm−2, 254~325 nm | Dye-sensitized solar cells, etc. | [72,73,74] |
| Quantum dots | ~4.7 nm | anatase | autoclave method (+heating) | ~18% MO photodegradation* (Xe lamp with a glass filter, 400 nm cut off, 100 mW cm−2) [75]; *estimated k = 0.0033 min−1 | Energy and environmental applications | [75,76] |
| Nanocrystals/nanopowder/nanoparticles (NPs)/ nanospheres | particle size: 8–10.2 nm [77,78], 14–18 nm [79,80], 19–23 nm [81] | anatase | sol-gel method + heat treatment | k = 0.002−0.036 min−1 (MB degradation) [78,79,81], k = 0.090–0.105 min−1 (MR degradation) [77] (UV light 250–625 W; max. ~250–368 nm); 20% (NOx degradation after 60 min; UV light-20 W, 287.5 nm) [80], *estimated k~0.0037 min−1 UV light 250–625 W; max. ~250–368 nm) | Pollutant degradation and self-cleaning [77,78,79,81], exhaust gas decomposition [80], energy storage [78] | [77,78,79,80,81] |
| Nanoporous shell (polyimide support) | 2.7–2.8 nm pore size, 12~20 nm shell thickness, | anatase | in situ complexation hydrolysis | ~80−95% degradation rate* (365 nm, 30-W UV lamp) *estimated k = 0.04–0.07 min−1 | Air purification and water disinfection | [82] |
| Nanospindles | ~190 nm in length; growth direction along [001] | anatase | hydrothermal synthesis | k = 0.0306 min−1 (RhB degradation; UV illum.; sim. sunlight AM 1.5 G filter, (100 mW/cm2)) | Dye-sensitized solar cells, etc. | [74] |
| Nanowires | 100 nm diameter × 800 µm | anatase | hydrothermal reaction, proton exchangem calcination | k = 0.0154 h−1 (0 0.000256 min−1) (phenol degradation in water; LED UV lamp (18 W); ca. 12 mW cm−2) | Photocatalysis, gas sensors, etc. | [83] |
| Nanowires/ nanobelts | 15 nm diameter | anatase | hydrothermal growth + heat treatment | 51.96%* (MO degradation; 350-W Xe lamp); *estimated k~0.0066 min−1 | Organic pollutants degradation | [84] |
| Nanobelts | 800 µm × 400 nm × 20 nm | anatase | hydrothermal reaction, proton exchange, calcination | k = 0.0256 h−1 (0.000426 min) (phenol degradation in water; LED UV lamp (18 W); ca. 12 mW cm−2) | Photocatalysis, gas sensors, etc. | [83] |
| Nanorods (NRs) | ~1.5 nm diameter and ~8.3 nm in length | rutile | solvothermal reaction | k~0.068 min−1 (RhB degradation, 300 W Xe lamp; full spectrum) | Organic pollutant degradation | [85] |
| Nanofibers/core-shell nanofibers | diameter < 100 nm | anatase, rutile, anatase + rutile | electrospinning/hydrolysis or alkoxide method | Estimated k = 0.027~0.1118 min−1 (lower values for rutile, higher values for anatase, mixed/core-shell have values in between, with values closer to the shell structure) (RhB; UV irradiation | Energy and environmental applications | [86] |
| Nanotubes (bamboo-type) | 2.5 µm in length, 100 nm diameter | amorphous | anodization | k = 0.0045 min−1 (AO7 degradation), 0.0187 min−1 (MB degradation) (UV light, 200 mW HeCd laser with I = 60 mWcm−2, 325 nm) | Dye-sensitized solar cells, etc. | [72] |
| 20–60 nm diameter, 0.5–0.9 µm in length, and ~60 nm interpore distances | anatase + rutile | dynamic anodization + heat treatment | k = 0.0007−0.0013 min−1 (MB degradation, 9W UV black-light lamp (λ = 365 nm), 1 mW/cm2 radiation at surface) | Devices; energy and environmental applications | [87] | |
| Nanotubes (smooth) | 4.5 µm length, 45 nm diameter | anatase | anodization + heat treatment | k = 0.0158 min−1 (AO7 degradation), 0.0213 min−1 (MB degradation) (UV light, 200 mW HeCd laser with I = 60 mWcm−2, 325 nm) | Dye-sensitized solar cells, etc. | [72] |
| 50–60 nm diameter, 1.7–2.2 µm in length, and ~60 nm interpore distances | anatase + rutile | dynamic anodization + heat treatment | k = 0.0024–0.0049 min−1 (MB degradation, 9 W UV black-light lamp (λ = 365 nm), 1 mW/cm2 radiation at surface) | Devices; energy and environmental applications | [87] | |
| Nanotubes (grown from Ti-6Al-4V) | diameter increases with anodizing potential; thickness ~280 nm | anatase (+rutile) | anodization + heat treatment | ~8–42% photodegradation efficiency (depending on the anodization voltage or tube diameter); estimated k = 0.0005–0.003 min−1 (MB degradation; UV-A lamp, 0.39 W/cm2) | Organic pollutant degradation | [88] |
| Nanoribbons | 200–300 nm in width; several microns in length | anatase (+rutile) | alkaline hydrothermal treatment | k~0.05342–0.08164 min−1 (RhB degradation; simulated sunlight, 300 W 230 V E27) | Organic pollutants degradation | [89] |
| Nanoribbons (nanopitted) | width 20~200 nm, length of 1 µm–few µm with pits of dia. 5–15 nm | TiO2-B | alkaline hydrothermal treatment | k ~ 0.0024–0.011 min−1 (MB degradation) (natural sunlight) | Dye degradation | [90] |
| 2D nanogrid/ nanolaces/ inverse-opal-like structure | 230–610 nm diameter of holes | anatase | opal-templated sol-gel-based synthesis + heat treatment | k~0.022–0.058 min−1 (RhB degradation; 500 W Xe arc lamp, 400 nm cutoff, 25 mW cm−2) | Degradation of various environmental contaminants | [91] |
| ~150 nm diameter holes, lace thickness 10~20 nm | anatase (+rutile) | alternating-voltage anodization + heat treatment/ 2-step anodization | k = 0.003 min−1 (Cr (VI) photocatalytic reduction; simulated sunlight, 300 W xenon lamp with 100 W cm−2 irradiation) | Heavy metal (Cr(VI)) removal from wastewater | [92,93] | |
| ~150 nm diameter holes, lace thickness 10~20 nm | black TiO2 | 2-step anodization process + heat-treat. in reducing atmosphere | k = 0.0657 min−1 (Cr (VI) photocatalytic reduction; simulated sunlight, 300 W xenon lamp with 100 W cm−2 irradiation) | Heavy metal (Cr(VI)) removal from wastewater | [93] | |
| Nanosheets | thickness < 7 nm | anatase | hydrothermal process | k = 0.013 min−1 (RhB degradation, mercury lamp (300 W, as UV light source), 2.62 mW/cm2) | Renewable energy; environment | [75] |
| Nanoflowers | 2–6 nm diameter with ~10 nm thin petals | anatase + rutile | hydrothermal + calcination | k = 0.03–0.12 min−1 (MB degradation at diff. pH; highest k at pH 4; UV lamp (100 W, 365 nm, 6.5 mW cm−2)) | Effluent treatment | [94] |
| Dendritic nanospheres | 2–3 µm diameter of entire structure; nanowire/nanoribbon spikes: 500 nm–1.5 µm long and dia. in nm | rutile | low-temperature hydrothermal method | k~0.018–0.024 min−1 (A07/RhB degradation; UVP Mineralight lamp (254 nm, 40 mW cm−2)) | Photocatalytic membrane water purification | [73] |
| Nanotrees | 130–180 nm nanowire diameter; 10–20 nm nanoparticle size | anatase | hydrothermal method, (1) proton exchange + calcination, (2) TiO2 NP decoration; sequence variation has an effect | k = 0.021, 0.071 min−1 (depending on the lattice parameter) (toluene gas decomposition; UV lamp (PL-L 18W/10/4P, Philips; 365 nm; 8.7 mW cm−2) | Photocatalysis, gas sensors, etc. | [95] |
| Sheet-on-belt (SOB): 800 µm × 400 nm × 20 nm (trunk), up to 100 nm long (branches). Sheet-on-wire (SOW): 100 nm diameter × 800 µm (trunk) with branches up to 200 nm. Branch thickness: a few nm | mostly anatase (+a bit of rutile) | hydrothermal reaction, proton exchange, calcination, solution combustion synthesis + calcination | Up to k = 0.346 h−1 (or 0.00576 min−1) (SOB photocatalytic); up to k = 0.40 h−1 (or 0.0067 min−1) (SOW photo-electrocatalytic) (phenol degradation in water) (LED UV lamp (18 W); ca. 12 mW cm−2) | Environmental remediation, energy storage, green energy production | [83] | |
| branched nanowire; 1 µm thick, branch: 10 nm thick, 45 nm long | anatase + rutile | H2O2 oxidation, intermediate calcination, and H2SO4 treatment | k = 0.0007–0.0086 min−1 (UV + various organic compounds); 0.0057 min−1 (visible + RhB) (18 W UV lamp, 5 mW/cm2; 500 W Xe-lamp, 420 nm cut off, 200 mW/cm2) | Photocatalytic water-splitting, environmental remediation | [96] | |
| Nanosheet + quantum dots (QDs) | nanosheet: thickness < 7 nm quantum dot: 3–5.6 nm | anatase | nanosheet, hydrothermal; QD, autoclave (+heating) homojunction: grinding | k = 0.027–0.064 min−1 (RhB degradation, mercury lamp (300 W, as UV light source), 2.62 mW/cm2) | Renewable energy technologies, environmental protection | [75] |
| Nanoparticles, nanobucks, and nanorods | 15–25 nm NPs; 100–150 nm nanobuck; 15 nm × 150 nm nanorod | rutile + anatase | (one-step) hydrothermal synthesis | k = 0.033 min−1 (RhB degradation) (250 W Xe lamp–simulated solar light source) | Wastewater treatment | [97] |
| Nanorhombus nanocuboids | nanorhombus: 55~80 nm × 35~40 nm; nanocuboids: 20~30 nm × 30~50 nm | anatase | hydrothermal synthesis | k = 0.0134−0.0318 min−1 (RhB degradation; UV; simulated sunlight AM 1.5 G filter, (100 mW/cm2)) | Dye-sensitized solar cells, etc. | [74] |
| Material | Degradation/Removal of Organics | H2 Generation | Reference Material/Comparison | Ref. |
|---|---|---|---|---|
| Black TiO2 nanocrystals/NPs | ~7.5× faster MB degradation, solar illumination | 0.1 ± 0.02 mmol h−1 g−1 (2 orders higher (solar simulator or visible IR light)) | Degradation: pristine TiO2 nanocrystals H2 generation: most semiconductor photocatalysts | [194] |
| Up to k = 0.68 min−1 MO degradation, 2.4× faster (simulated sunlight) | Up to 5.2 mmol h−1g−1, 1.7× faster (simulated sunlight) | Pristine P25 degradation: k = 0.28 min−1 H2 generation: 5.2 mmol h−1 g−1 | [199] | |
| Up to apparent k (kapp) = 0.998 h−1 or 0.0166 min−1 acetaminophen removal, 1.9× faster than P25 and 4.9× faster than sintered P25 (solar illum. AM 1.5G) | P25: k = 0.527 h−1 or 0.00878 min−1 Sintered P25: 0.203 h−1 or 0.00338 min−1 | [209] | ||
| Estimate: ~15 µmol h−1 g−1 ~5−7× higher (AM 1.5 illum.; 100 mW cm−2) | Anatase nanopowders; estimate: ~2−3 µmol h−1 g−1 | [210] | ||
| Anatase; ~1.5× faster, MB degradation finished in 18 min (solar illumination) | Degussa-P25: MB degradation finished in 18 min. (solar illumination) | [203] | ||
| Black hydroxylated TiO2 (ultrasonic.) | 5.8× (solar illumination) and 7.2× (visible light) faster acid fuchsin decomposition; amorphous state | Original sol TiO2 (non-ultrasonically processed) | [201] | |
| Black TiO2 nanotube array (TNA) | Estimate: 10~15% (3–4×) better photocatalytic degradation (brilliant blue KN-R dye; 175 W Xe lamp) | Pristine TNA | [196] | |
| Ordered mesoporous black TiO2 | 136.2 µmol h−1, ~2× higher (solar) | Pristine mesoporous TiO2: 76 µmol h−1 | [198] | |
| Mesoporous black TiO2 hollow spheres | 241 µmol h−1 (0.1) g−1, ~2× higher (solar) | Black TiO2 NPs: 118 µmol h−1 (0.1) g−1 Mesoporous TiO2 hollow spheres: 81 µmol h−1 (0.1) g−1 | [197] | |
| Defective black TiO2 (dimpled morphology, anodization) | High oxygen vacancy concentration (CVo): up to ~80% RhB degradation (after 4 h), ~1.3× better than low CVo and ~4× better than TNA. | Low CVo: up to 60% RhB degradation (after 4 h) TNA: ~20% RhB degradation (after 4 h) | [200] | |
| Grey TiO2 nanoparticles (flow furnace) | Estimate: ~75 µmol h−1 g−1 ~25−37× higher (AM 1.5 illum.; 100 mW cm−2) | Anatase nanopowders: estimate: ~2−3 µmol h−1 g−1 | [210] | |
| Grey TiO2 nanoparticles (hydrogen. at high P) | Estimate: ~80−85 µmol h−1 g−1 ~27−42× higher (AM 1.5 illum.; 100 mW cm−2) | Anatase nanopowders: estimate: ~2−3 µmol h−1 g−1 | [210] | |
| Colored TiO2 (dark blue) | Up to C/C0 = 0.14 (estimated k = 0.197 min−1), 1.4× faster MO degradation (300 W, Xe lamp UV–vis light) | Up to max. prod. of 6.5 mmol h−1 g−1, 7.2× higher (UV-vis light); ~180 µmol h−1 g−1 (vis-IR) | Pristine P25 Degradation: C/C0 = 0.24 (estimated k = 0.143 min−1) H2 generation: 0.9 mmol h−1 g−1 | [195] |
| Blue TiO2(B) single-crystal nanorods | k = 0.0146 min−1; 97.01% RhB degradation (after 150 min), 6.9× and 2.1× better than TiO2 NPs and TiO2 NRs, respectively (vis light); 98.56% deg. RhB (solar light), 99.12% deg. Phenol (solar); reaction constant (krxn) = 0.0250 (RhB) and 0.0366 (phenol), 8.8× higher than TiO2 NPs | Up to 149. µmol h−1 g−1 (AM 1.5 illumination), ~26.6× higher than TiO2 NPs | Degradation: TiO2 NPs: k = 0.0016 min−1; 14.06% RhB degradation (after 150 min, vis light) TiO2 NRs: k = 0.0053 min−1; 46.44% RhB degradation (after 150 min, vis light) H2 evolution: TiO2 NPs: 5.6 µmol h−1 g−1 (AM 1.5 illumination) TiO2 NRs: 40.8 µmol h−1 g−1 (AM 1.5 illumination) | [211] |
| Reaction * | |
|---|---|
| 1 | H2O2 + Ti–OH → Ti–OOH + H2O |
| 2 | + Ti–OOH → Ti–·OOH |
| 3 | + Ti–·OOH → Ti–OH + ½ O2 |
| 4 | + O2 → ·O2− |
| 5 | + H2O2 → ·OH + OH− |
| 6 | H2O2 + ·OH → ·OOH + H2O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Querebillo, C.J. A Review on Nano Ti-Based Oxides for Dark and Photocatalysis: From Photoinduced Processes to Bioimplant Applications. Nanomaterials 2023, 13, 982. https://doi.org/10.3390/nano13060982
Querebillo CJ. A Review on Nano Ti-Based Oxides for Dark and Photocatalysis: From Photoinduced Processes to Bioimplant Applications. Nanomaterials. 2023; 13(6):982. https://doi.org/10.3390/nano13060982
Chicago/Turabian StyleQuerebillo, Christine Joy. 2023. "A Review on Nano Ti-Based Oxides for Dark and Photocatalysis: From Photoinduced Processes to Bioimplant Applications" Nanomaterials 13, no. 6: 982. https://doi.org/10.3390/nano13060982