High-Performance Ethylene Glycol Sensor Based on Imine Covalent Organic Frameworks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
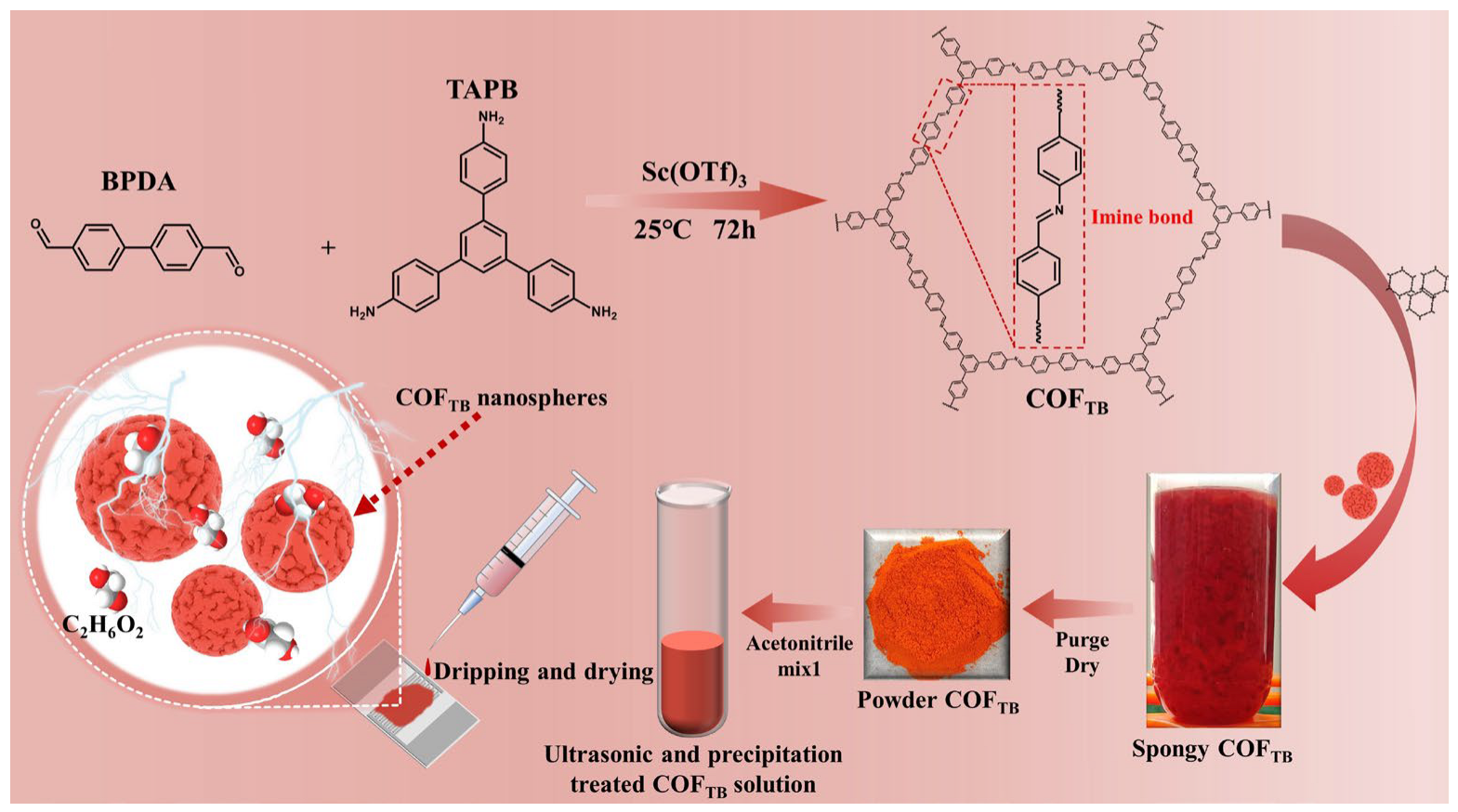
2.2. Preparation of Materials
2.3. Sensor Preparation and Gas-Sensitive Testing
2.4. Characterization of Materials
3. Results and Discussion
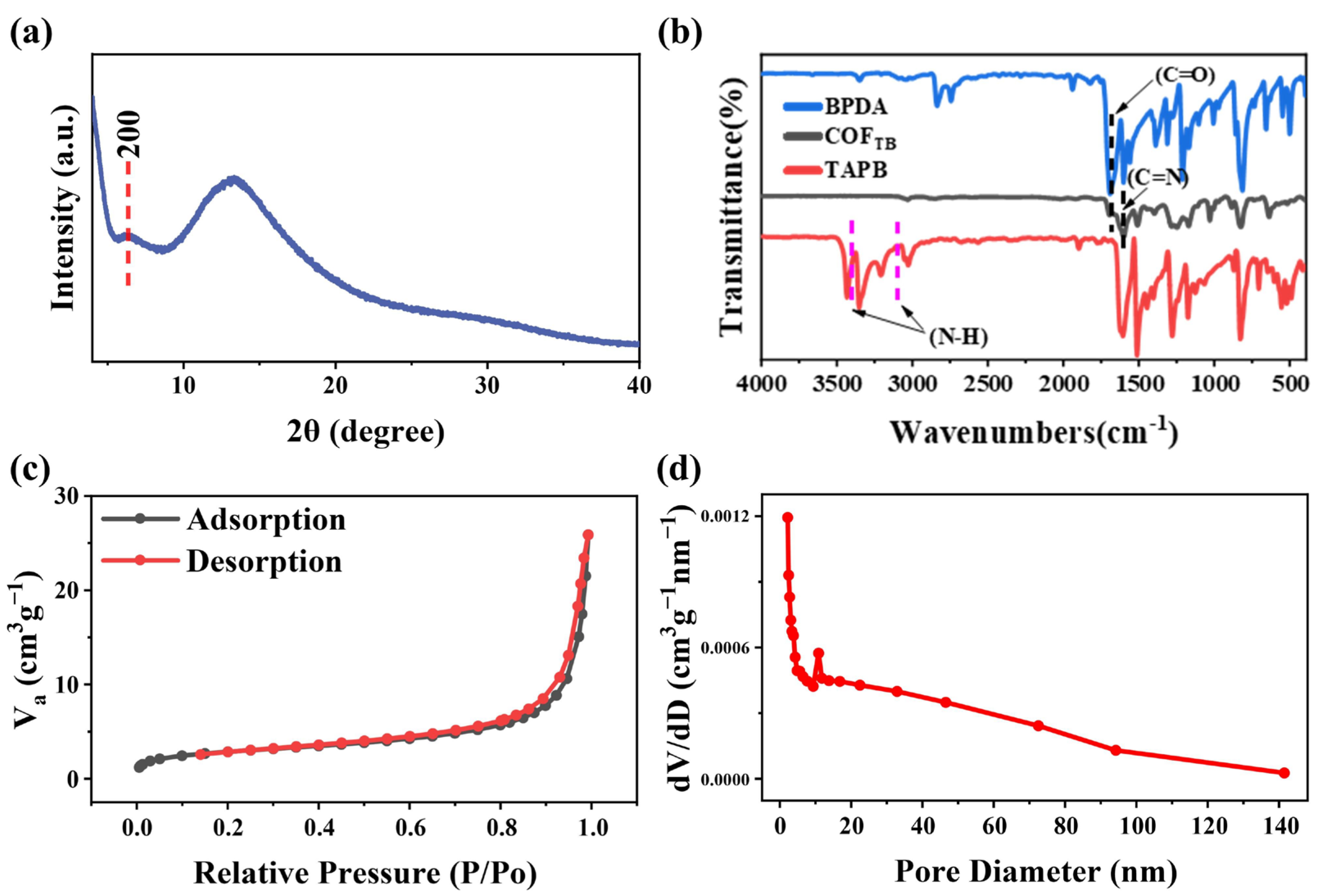
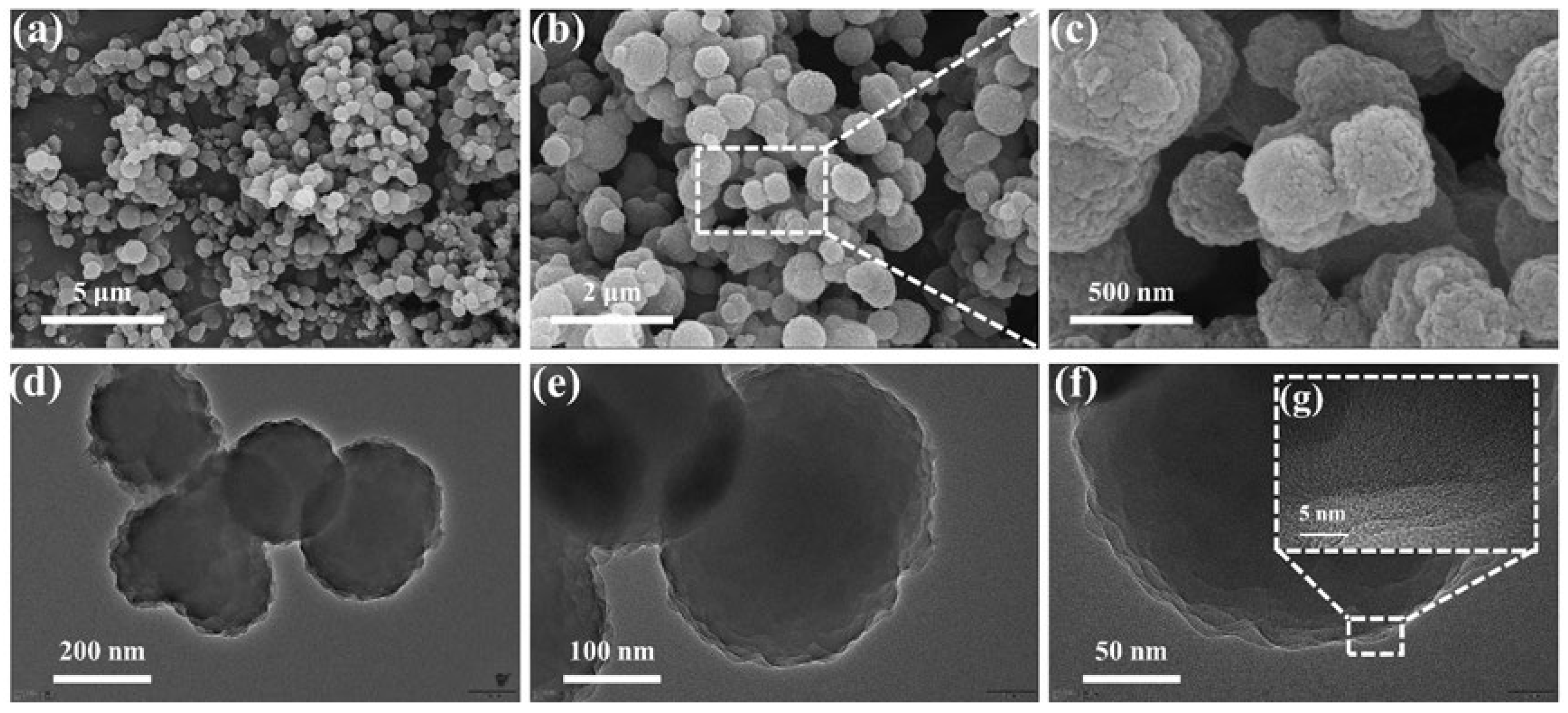
3.1. Structure and Composition of the COFTB Sample
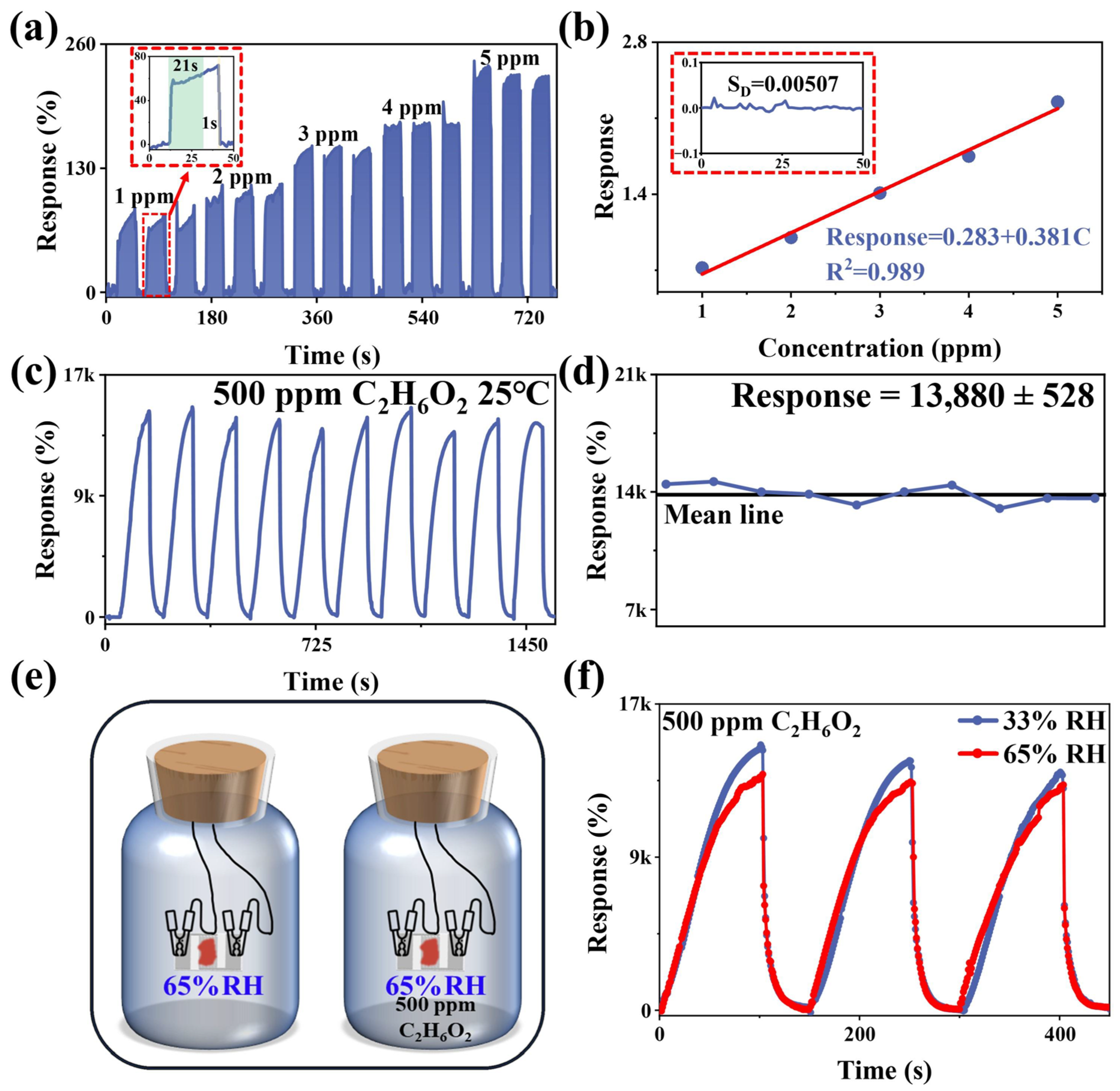
3.2. Gas Sensitivity of COFTB
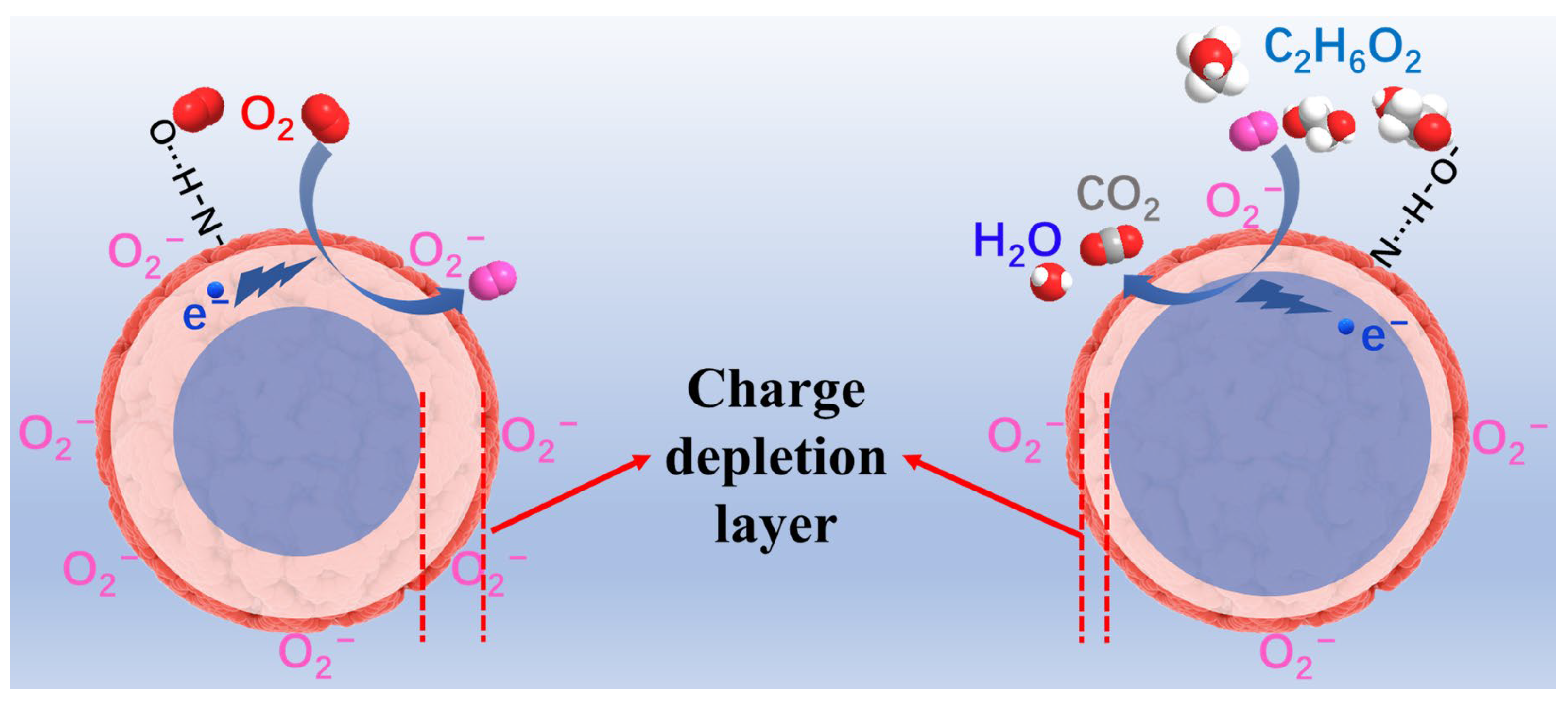
3.3. Analysis of Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Filip, A.B.; Farnsworth, C.W.; Mullins, M.E.; Crews, B.O.; Kraut, J.A. Accuracy of a Glycerol Dehydrogenase Assay for Ethylene Glycol Detection. J. Appl. Toxicol. 2023, 19, 362–367. [Google Scholar] [CrossRef] [PubMed]
- El Menyiy, N.; Al Waili, N.; Bakour, M.; Al-Waili, H.; Lyoussi, B. Protective Effect of Propolis in Proteinuria, Crystaluria, Nephrotoxicity and Hepatotoxicity Induced by Ethylene Glycol Ingestion. Arch. Med. Res. 2016, 47, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.M.; Lea-Henry, T.N. Simplifying the hemodialysis prescription in patients with ethylene glycol poisoning. Kidney Int. Rep. 2017, 92, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, M.; Zhang, Y.; Liu, T.; Ren, C.; Li, P.; Yin, X.; Zhang, L.; Zhang, M.; Wu, W. Boosting Ethylene Glycol Sensing Performance with Dendritic Hierarchical CuO/Co3O4 Heterojunction Nanowire. ACS Appl. Mater. Interfaces 2023, 6, 19249–19256. [Google Scholar] [CrossRef]
- Robson, J.; Townsend, S.; Bowdler, P.; Honeychurch, K.C. Direct thermal desorption gas chromatographic determination of toxicologically relevant concentrations of ethylene glycol in whole blood. Analyst 2018, 143, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Sankaralingam, A.; Thomas, A.; James, D.R.; Wierzbicki, A.S. Assessment of a semi-quantitative screening method for diagnosis of ethylene glycol poisoning. Ann. Clin. Biochem. 2016, 54, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.T.; Berg, J.D. Five-year review of a UK 24 hour testing service for plasma ethylene glycol and diethylene glycol. Ann. Clin. Biochem. 2015, 53, 459–465. [Google Scholar] [CrossRef]
- Aslani, S.; Armstrong, D.W. High information spectroscopic detection techniques for gas chromatography. J. Chromatogr. A 2022, 1676, 463255. [Google Scholar] [CrossRef]
- Zavahir, J.S.; Nolvachai, Y.; Marriott, P.J. Molecular spectroscopy—Information rich detection for gas chromatography. Trends Analyt. Chem. 2018, 99, 47–65. [Google Scholar] [CrossRef]
- Pereira, P.F.M.; de Sousa Picciani, P.H.; Calado, V.; Tonon, R.V. Electrical gas sensors for meat freshness assessment and quality monitoring: A review. Trends Food Sci. Technol. 2021, 118, 36–44. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Multivariable Sensors for Ubiquitous Monitoring of Gases in the Era of Internet of Things and Industrial Internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef]
- Travlou, N.A.; Bandosz, T.J. Nanoporous carbon-composites as gas sensors: Importance of the specific adsorption forces for ammonia sensing mechanism. Carbon 2017, 121, 114–126. [Google Scholar] [CrossRef]
- Liu, M.M.; Ma, S.Y.; Cai, Y.H.; Ma, N.N.; Wang, L.; Sheng, H. ZnO/ZnCo2O4 composite prepared by one-step hydrothermal method for high-performance ethylene glycol sensor. Ceram. Int. 2022, 48, 22305–22312. [Google Scholar] [CrossRef]
- Ding, J.; Dai, H.; Chen, H.; Jin, Y.; Fu, H.; Xiao, B. Highly sensitive ethylene glycol gas sensor based on ZnO/rGO nanosheets. Sens. Actuators B Chem. 2022, 372, 132655. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Wang, X.; Li, X.; Sui, X.; Jiang, H.; Liu, G.; Li, B.; Sheng, Y.; Zhou, J.; et al. Heterostructure engineering of NiO foam /In2S3 film for high-performance ethylene glycol gas sensors. Sens. Actuators B Chem. 2023, 392, 134110. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Covalent organic frameworks (COFs) for environmental applications. Coord. Chem. Rev. 2019, 400, 213046. [Google Scholar] [CrossRef]
- Meng, Z.; Mirica, K.A. Covalent organic frameworks as multifunctional materials for chemical detection. Chem. Soc. Rev. 2021, 50, 13498–13558. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Qin, Y.; Wang, X.; Zhang, Q. Recent Progress in External-Stimulus-Responsive 2D Covalent Organic Frameworks. Adv. Mater. 2021, 34, 2101175. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mei, A.; Chen, W.; Wang, Z.; Guo, H.; Liu, Y. Control of morphology of covalent organic framework-5 for chemiresistive low-temperature ammonia sensors. Sens. Actuators B Chem. 2023, 392, 134051. [Google Scholar] [CrossRef]
- Choi, J.; Kim, T.; Li, H.; Jung, H.-T.; Zhao, D. Gas Sensors with Two-Dimensional rGO@COF Composite Materials for Fast NO2 Detection under Room Temperature. ACS Appl. Mater. 2023, 15, 44119–44126. [Google Scholar] [CrossRef]
- Krishnaveni, V.; Esclance Dmello, M.; Sahoo, P.; Thokala, N.; Bakuru, V.R.; Vankayala, K.; Basavaiah, K.; Kalidindi, S.B. Palladium-Nanoparticle-Decorated Covalent Organic Framework Nanosheets for Effective Hydrogen Gas Sensors. ACS Appl. Nano Mater. 2023, 6, 10960–10966. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Z.; Cao, Y.; Guo, J.; Long, M.; Duan, H.; Jia, D. Chemiresistive sensor arrays based on noncovalently functionalized multi-walled carbon nanotubes for ozone detection. Sens. Actuators B Chem. 2019, 297, 126689. [Google Scholar] [CrossRef]
- Matsumoto, M.; Dasari, R.R.; Ji, W.; Feriante, C.H.; Parker, T.C.; Marder, S.R.; Dichtel, W.R. Rapid, Low Temperature Formation of Imine-Linked Covalent Organic Frameworks Catalyzed by Metal Triflates. J. Am. Chem. Soc. 2017, 139, 4999–5002. [Google Scholar] [CrossRef]
- Giuseppone, N.; Schmitt, J.L.; Lehn, J.M. Generation of Dynamic Constitutional Diversity and Driven Evolution in Helical Molecular Strands under Lewis Acid Catalyzed Component Exchange. Angew. Chem. Int. Ed. 2004, 43, 4902–4906. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Shao, Z.-W.; Zhu, J.-L.; Tao, L.-M.; Ding, Y. Structural evolution of imine-linked covalent organic frameworks and their NH3 sensing performance. J. Mater. Chem. C 2021, 9, 8562–8569. [Google Scholar] [CrossRef]
- Khalil, S.; Meyer, M.D.; Alazmi, A.; Samani, M.H.K.; Huang, P.-C.; Barnes, M.; Marciel, A.B.; Verduzco, R. Enabling Solution Processable COFs through Suppression of Precipitation during Solvothermal Synthesis. ACS Nano 2022, 16, 20964–20974. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Song, S.; Xiao, D.; Gan, L.; Wang, Y. Easily Constructed Imine-Bonded COFs for Iodine Capture at Ambient Temperature. ACS Omega 2020, 5, 24262–24271. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ren, X.; Guo, M.; Li, W.; Li, H.; Yang, Q. Highly active ultrafine Pd NPs confined in imine-linked COFs for nitrobenzene hydrogenation. Catal. Sci. Technol. 2021, 11, 3873–3879. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Z.; Qin, Z.; Chen, X.; Zhang, C.; Cao, B.; Duan, H.; Zhang, J. A dog nose-inspired high-performance NH3 gas sensor of biomass carbon materials with a pleated structure derived from rose tea. J. Mater. Chem. A 2022, 10, 14326–14335. [Google Scholar] [CrossRef]
- Srinivasan, P.; Samanta, S.; Krishnakumar, A.; Rayappan, J.B.B.; Kailasam, K. Insights into g-C3N4 as a chemi-resistive gas sensor for VOCs and humidity—A review of the state of the art and recent advancements. J. Mater. Chem. A 2021, 9, 10612–10651. [Google Scholar] [CrossRef]
- Singh, H.; Tomer, V.K.; Jena, N.; Bala, I.; Sharma, N.; Nepak, D.; De Sarkar, A.; Kailasam, K.; Pal, S.K. A porous, crystalline truxene-based covalent organic framework and its application in humidity sensing. J. Mater. Chem. A 2017, 5, 21820–21827. [Google Scholar] [CrossRef]
- Wei, J.S.; Ma, S.Y.; Cai, Y.H.; Xu, C.Y.; Liu, J.M.; Jiang, H.T. A high-performance ethylene glycol sensor based on fibrous ErFeO3 prepared by electrostatic spinning. Ceram. Int. 2023, 49, 32611–32618. [Google Scholar] [CrossRef]
- Qin, Z.; Wu, Z.; Sun, Q.; Sun, J.; Zhang, M.; Shaymurat, T.; Lv, C.; Duan, H. Biomimetic gas sensor derived from disposable bamboo chopsticks for highly sensitive and selective detection of NH3. Chem. Eng. J. 2023, 462, 142203. [Google Scholar] [CrossRef]
- Haase, F.; Lotsch, B.V. Solving the COF trilemma: Towards crystalline, stable and functional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 8469–8500. [Google Scholar] [CrossRef]
- Chen, X.; Kong, L.; Mehrez, J.A.-A.; Fan, C.; Quan, W.; Zhang, Y.; Zeng, M.; Yang, J.; Hu, N.; Su, Y.; et al. Outstanding Humidity Chemiresistors Based on Imine-Linked Covalent Organic Framework Films for Human Respiration Monitoring. Nanomicro Lett. 2023, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Liu, M.; Chen, J.; Huang, X.; Li, Q.-H.; Ye, X.-L.; Wang, G.-E.; Xu, G. Dangling bond formation on COF nanosheets for enhancing sensing performances. Chem. Sci. 2023, 14, 4824–4831. [Google Scholar] [CrossRef]
- Han, T.; Ma, S.Y.; Xu, X.L.; Xu, X.H.; Pei, S.T.; Tie, Y.; Cao, P.F.; Liu, W.W.; Wang, B.J.; Zhang, R.; et al. Rough SmFeO3 nanofibers as an optimization ethylene glycol gas sensor prepared by electrospinning. Mater. Lett. 2020, 268, 127575. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Wang, X.; Li, X.; Sui, X.; Liu, G.; Li, B.; Zhou, J.; Xie, E.; Zhang, Z. Facile fabrication of NiO foam@Sn-doped In2O3 nanowire heterosturctures for highly sensitive ethylene glycol gas sensors at low temperatures. Sens. Actuators B Chem. 2023, 377, 132991. [Google Scholar] [CrossRef]
- Su, C.; Zhang, L.; Han, Y.; Ren, C.; Li, B.; Wang, T.; Zeng, M.; Su, Y.; Hu, N.; Zhou, Z.; et al. Glucose-assisted synthesis of hierarchical NiO-ZnO heterostructure with enhanced glycol gas sensing performance. Sens. Actuators B Chem. 2021, 329, 129167. [Google Scholar] [CrossRef]
- Zhao, G.; Li, M.; Li, H.; Ping, Z.; Wang, P.; Wu, Y.; Li, L. La-doped micro-angular cube ZnSnO3 with nano-La2O3 decoration for enhanced ethylene glycol sensing performance at low temperature. Sens. Actuator A Phys. 2023, 362, 114649. [Google Scholar] [CrossRef]
- Xu, X.L.; Jiang, H.T.; Wang, X.P.; Wang, S.Y.; Liu, W.W.; Ma, W.; Wang, M.Y.; Ma, S.Y. A novel glycol sensor of silkworm excrement based microporous carbons (SEMCs)/SnO2 nanoparticles. Vacuum 2023, 209, 11754. [Google Scholar] [CrossRef]
- Chi, Y.; Han, J.; Zheng, J.; Yang, J.; Cao, Z.; Ghasemian, M.B.; Rahim, M.A.; Kalantar-Zadeh, K.; Kumar, P.; Tang, J. Insights into the Interfacial Contact and Charge Transport of Gas-Sensing Liquid Metal Marbles. ACS Appl. Mater. Interfaces 2022, 14, 30112–30123. [Google Scholar] [CrossRef]
- Liu, K.; Luo, P.; Han, W.; Yang, S.; Zhou, S.; Li, H.; Zhai, T. Approaching ohmic contact to two-dimensional semiconductors. Sci. Bull. 2019, 64, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B Chem. 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, Z.; Cheng, X.; Sui, L.; Gao, S.; Zhang, X.; Xu, Y.; Zhao, H.; Huo, L. Ionic liquid-assisted synthesis of α-Fe2O3 mesoporous nanorod arrays and their excellent trimethylamine gas-sensing properties for monitoring fish freshness. J. Mater. Chem. A 2017, 5, 19846–19856. [Google Scholar] [CrossRef]
- Niavol, S.S.; Khatibani, A.B.; Hashemi Karouei, S.F.; Hejazi Juybari, S.A.; Moghaddam, H.M. Mesoporous Zn2SnO4 for efficient sensing of ethylene glycol vapor. Mater. Chem. Phys. 2023, 303, 127799. [Google Scholar] [CrossRef]
- Smith, B.J.; Overholts, A.C.; Hwang, N.; Dichtel, W.R. Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks. ChemComm 2016, 52, 3690–3693. [Google Scholar] [CrossRef]

| Materials | Concentration (ppm) | Response | Preparation Method | LoD (ppb) | Temp (°C) |
|---|---|---|---|---|---|
| ErFeO3 [32] | 100 | 15.8 b | Electrostatic spinning | 35 | 230 |
| ZnO/ZnCo2O4 [13] | 100 | 15.63 b | Hydrothermal method | 1590 | 160 |
| ZnO/rGO [14] | 100 | 277 b | Hydrothermal method | 1000 | 200 |
| CuO/Co3O4 [4] | 100 | 6.3 b | Hydrothermal method | - | 130 |
| SmFeO3 [37] | 100 | 18.19 b | Electrostatic spinning and calcination | - | 240 |
| NTO [38] | 100 | 160.72 b | Chemical vapor deposition | 472 | 125 |
| G-NiO-ZnO [39] | 100 | 142 b | Hydrothermal method | - | 140 |
| La-doped ZnSnO3 [40] | 100 | 1488.79 b | Hydrothermal method | 200 | 140 |
| (SEMCs)/SnO2 [41] | 100 | 132 b | Carbonization and activation | 4.8 | 160 |
| This work | 500 | 13,880 a | Normal temperature catalyst synthesis | 40 | RT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, G.; Zhang, W.; Tian, N.; Sun, Q.; Wu, Z. High-Performance Ethylene Glycol Sensor Based on Imine Covalent Organic Frameworks. Nanomaterials 2023, 13, 3103. https://doi.org/10.3390/nano13243103
Liu S, Zhang G, Zhang W, Tian N, Sun Q, Wu Z. High-Performance Ethylene Glycol Sensor Based on Imine Covalent Organic Frameworks. Nanomaterials. 2023; 13(24):3103. https://doi.org/10.3390/nano13243103
Chicago/Turabian StyleLiu, Shiwei, Guojie Zhang, Weiyu Zhang, Ning Tian, Qihua Sun, and Zhaofeng Wu. 2023. "High-Performance Ethylene Glycol Sensor Based on Imine Covalent Organic Frameworks" Nanomaterials 13, no. 24: 3103. https://doi.org/10.3390/nano13243103




