Solvent Welding-Based Methods Gently and Effectively Enhance the Conductivity of a Silver Nanowire Network
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ag NWs
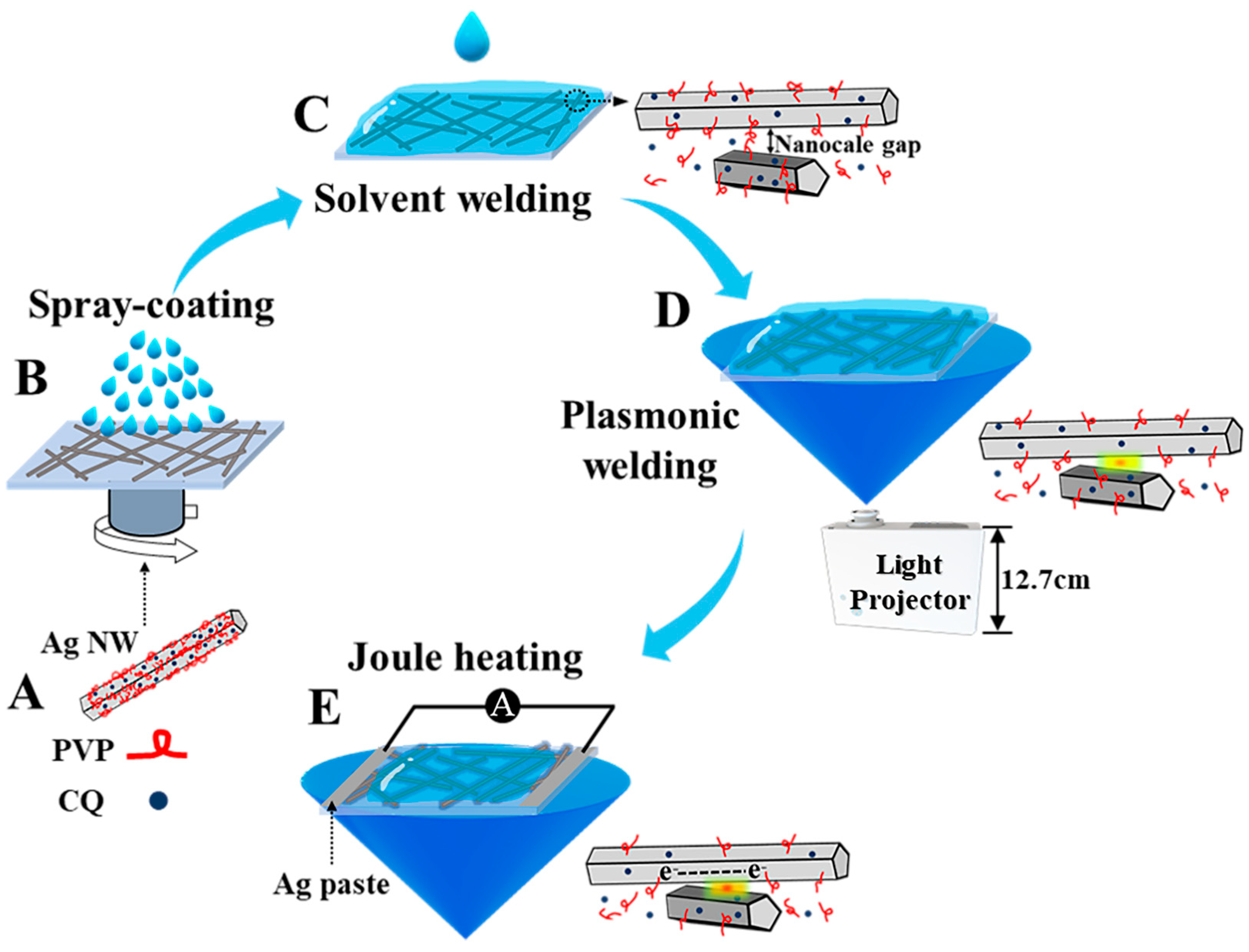
2.3. Fabrication of Ag NW Networks
2.4. Welding of Ag NW Networks
2.5. Characterization
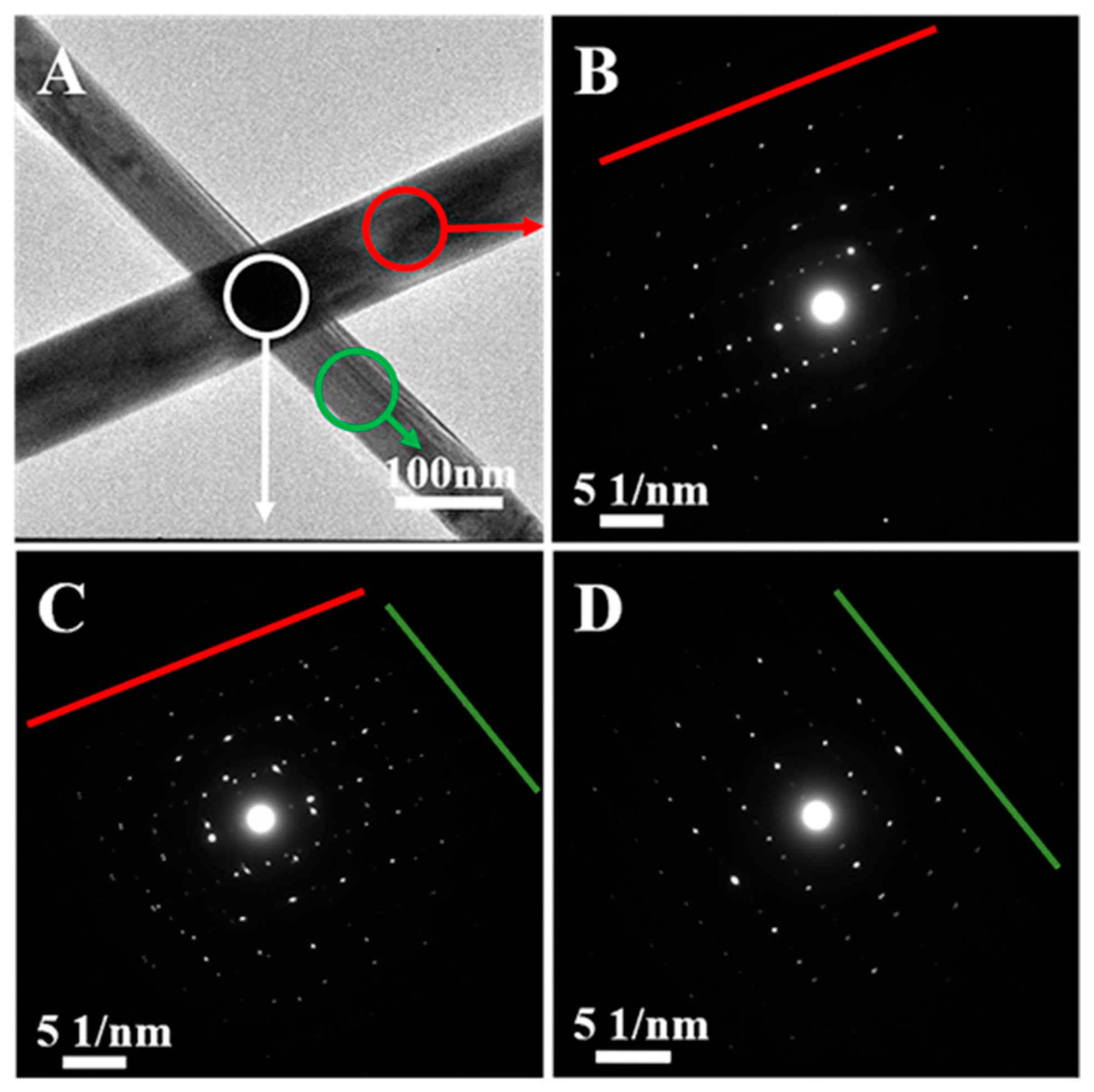
3. Results and Discussion
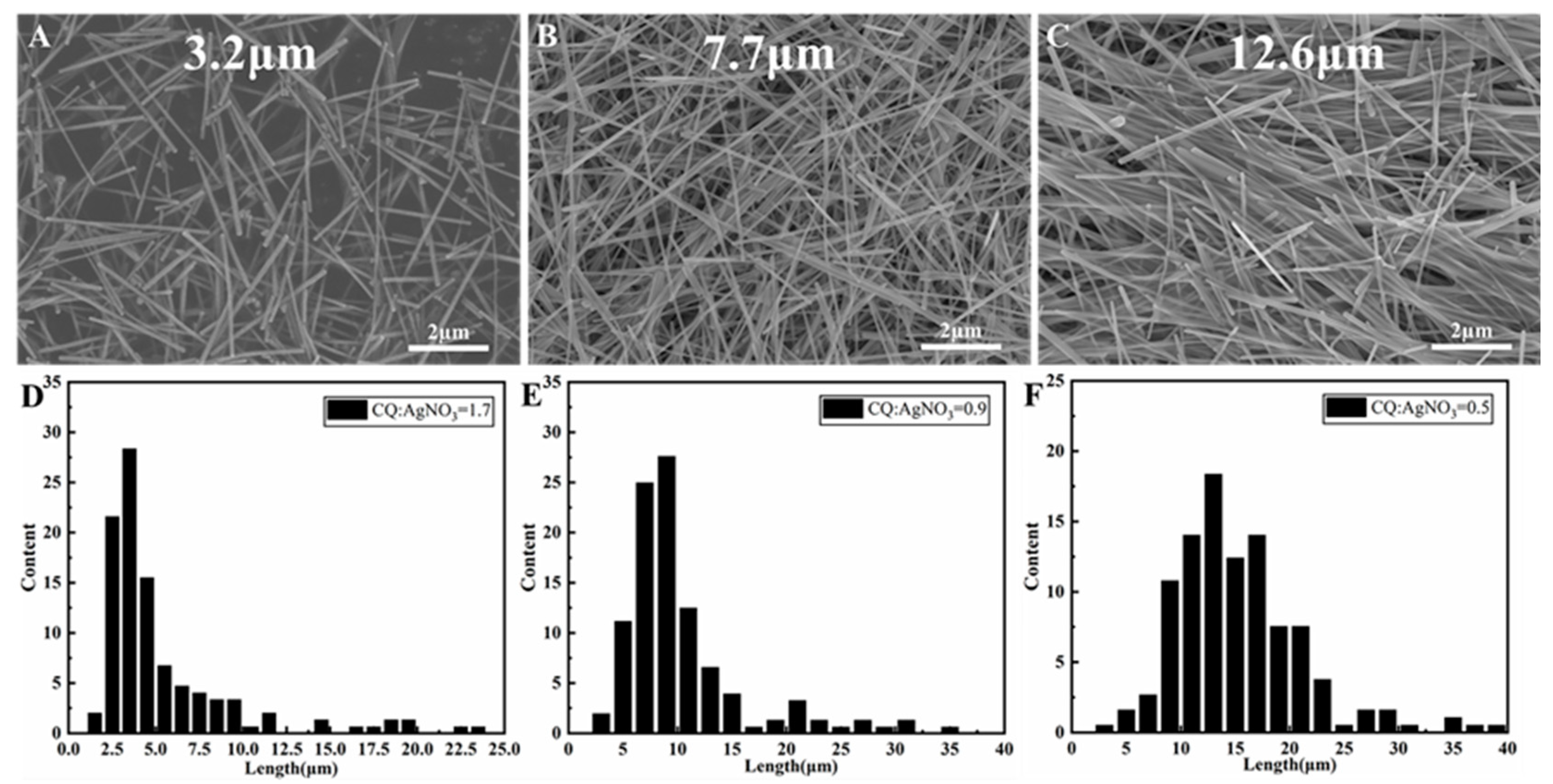
3.1. CQ-Mediated Polyol Synthesis of Ag Nanowires with Different Dimensions
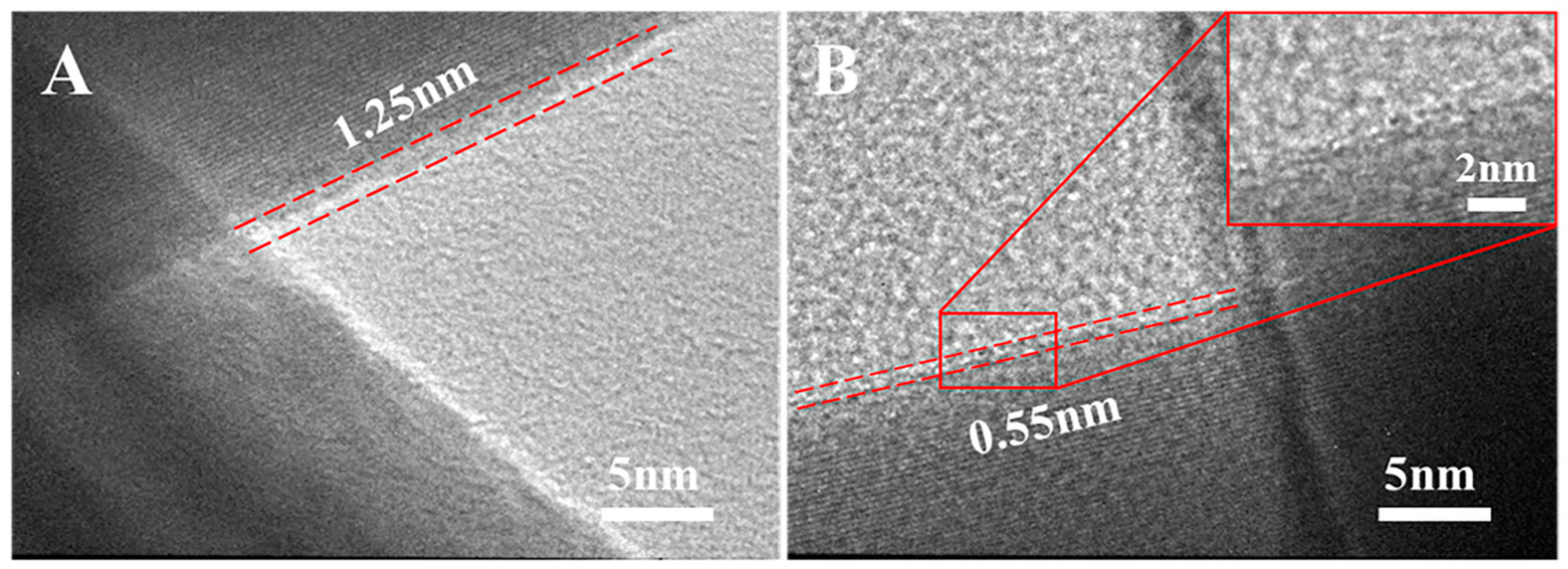
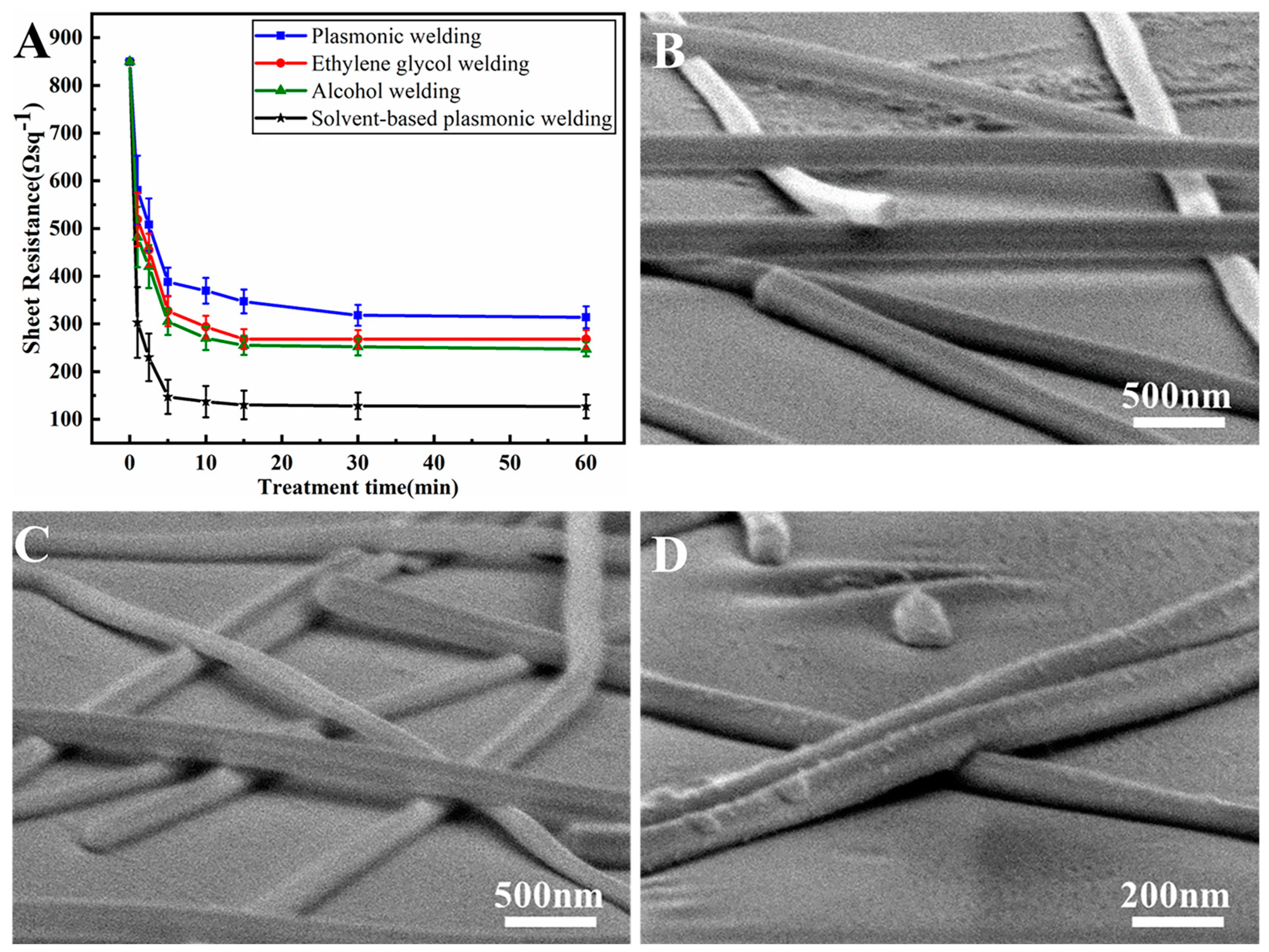
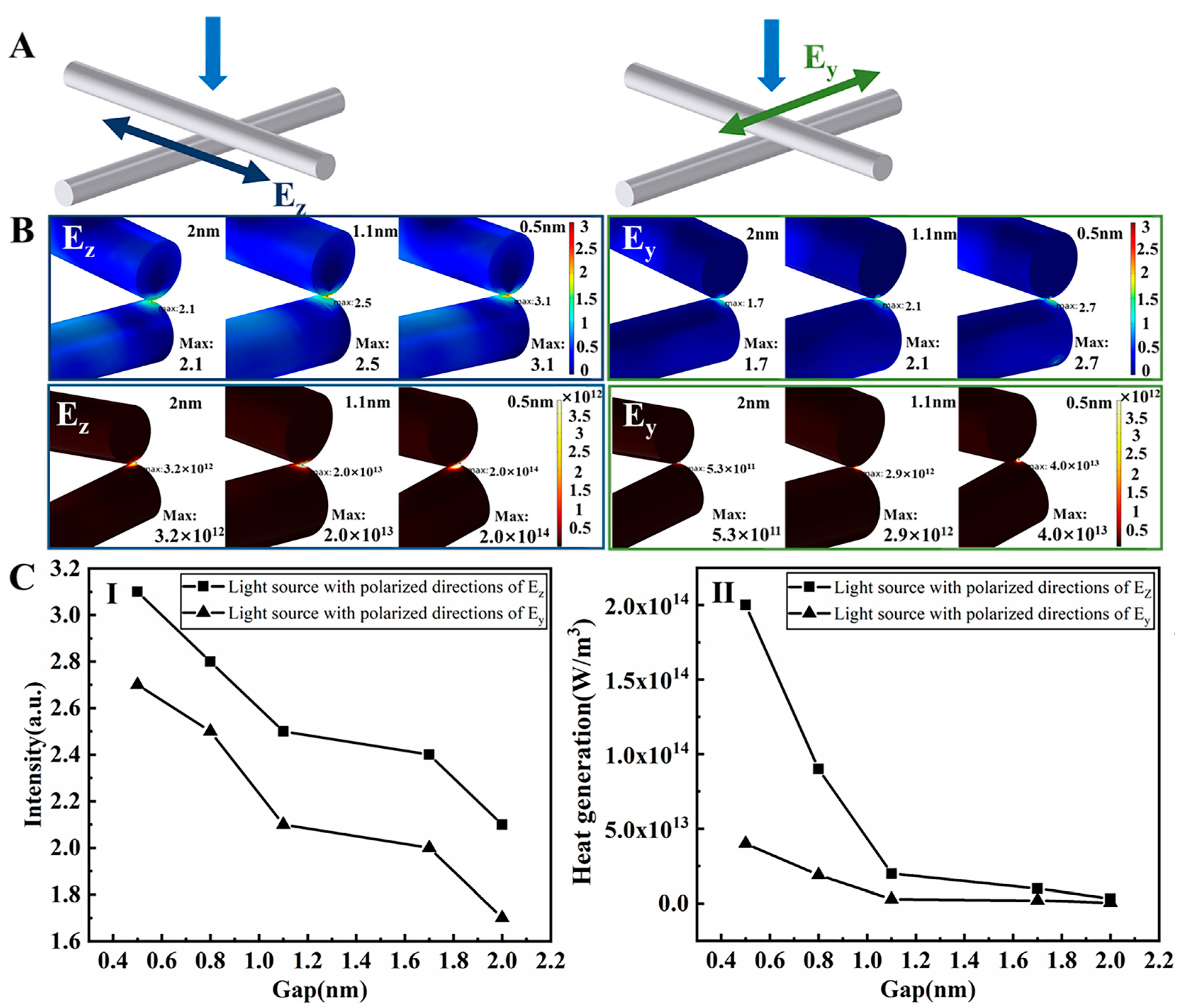
3.2. Solvent-Based Plasmonic Welding of Ag Nanowire Networks
3.3. Combined Solvent-Based Plasmonic and Joule Heating Welding of Ag Nanowire Networks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De, S.; Higgins, T.M.; Lyons, P.E.; Doherty, E.M.; Nirmalraj, P.N.; Blau, W.J.; Boland, J.J.; Coleman, J.N. Silver Nanowire Networks as Flexible, Transparent, Conducting Films: Extremely High DC to Optical Conductivity Ratios. ACS Nano 2009, 3, 1767–1774. [Google Scholar] [CrossRef]
- Kim, H.; Lee, G.; Becker, S.; Kim, J.S.; Kim, H.; Hwang, B. Novel Patterning of Flexible and Transparent Ag Nanowire Electrodes Using Oxygen Plasma Treatment. J. Mater. Chem. C 2018, 6, 9394–9398. [Google Scholar] [CrossRef]
- Cho, S.; Kang, S.; Pandya, A.; Shanker, R.; Khan, Z.; Lee, Y.; Park, J.; Craig, S.L.; Ko, H. Large-Area Cross-Aligned Silver Nanowire Electrodes for Flexible, Transparent, and Force-Sensitive Mechanochromic Touch Screens. ACS Nano 2017, 11, 4346–4357. [Google Scholar] [CrossRef]
- Lagrange, M.; Langley, D.P.; Giusti, G.; Jiménez, C.; Bréchet, Y.; Bellet, D. Optimization of Silver Nanowire-Based Transparent Electrodes: Effects of Density, Size and Thermal Annealing. Nanoscale 2015, 7, 17410–17423. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Pan, Y.; Xie, F.; Zhang, Y.; Zhao, J.; Wen, S.; Gao, F. Performance Enhancement of Silver Nanowire-Based Transparent Electrodes by Ultraviolet Irradiation. Nanomaterials 2022, 12, 2956. [Google Scholar] [CrossRef]
- Zhan, K.; Su, R.; Bai, S.; Yu, Z.; Cheng, N.; Wang, C.; Xu, S.; Liu, W.; Guo, S.; Zhao, X.Z. One-Pot Stirring-Free Synthesis of Silver Nanowires with Tunable Lengths and Diameters via Fe 3+ & Cl− Co-Mediated Polyol Method and Their Application as Transparent Conductive Films. Nanoscale 2016, 8, 18121–18133. [Google Scholar] [CrossRef]
- Amiri Zarandi, A.; Khosravi, A.; Dehghani, M.; Tajabadi, F.; Taghavinia, N. The Role of Mixed Reaction Promoters in Polyol Synthesis of High Aspect Ratio Ag Nanowires for Transparent Conducting Electrodes. J. Electron. Mater. 2020, 49, 4822–4829. [Google Scholar] [CrossRef]
- Jeon, K.H.; Jeon, S.J. Synthesis of Size-Controlled Ag Nanowires via a Seed-Mediated Growth Method. Korean J. Chem. Eng. 2020, 37, 1251–1257. [Google Scholar] [CrossRef]
- Lee, J.Y.; Connor, S.T.; Cui, Y.; Peumans, P. Solution-Processed Metal Nanowire Mesh Transparent Electrodes. Nano Lett. 2008, 8, 689–692. [Google Scholar] [CrossRef]
- Lu, J.; Liu, D.; Dai, J. Preparation of Highly Conductive Silver Nanowires for Electrically Conductive Adhesives. J. Mater. Sci. Mater. Electron. 2019, 30, 15786–15794. [Google Scholar] [CrossRef]
- Li, Q.; Chen, S.; Yu, H.; Chen, J.; Yan, X.; Li, L.; Xu, M. Overcoming the Conductivity Limit of Insulator through Tunneling-Current Junction Welding: Ag@PVP Core-Shell Nanowire for High-Performance Transparent Electrode. J. Mater. Chem. C 2021, 9, 3957–3968. [Google Scholar] [CrossRef]
- Lee, J.; Lee, I.; Kim, T.S.; Lee, J.Y. Efficient Welding of Silver Nanowire Networks without Post-Processing. Small 2013, 9, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.P.A. Controlling the Rayleigh Instability of Nanowires. Appl. Phys. Lett. 2013, 102, 143108. [Google Scholar] [CrossRef]
- Kaikanov, M.; Kemelbay, A.; Amanzhulov, B.; Demeuova, G.; Akhtanova, G.; Bozheyev, F.; Tikhonov, A. Electrical Conductivity Enhancement of Transparent Silver Nanowire Films on Temperature-Sensitive Flexible Substrates Using Intense Pulsed Ion Beam. Nanotechnology 2021, 32, 145706. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Hong, S.; Jang, J.; Kim, H.-K. Blue Laser Annealing of Ag Nanowire Electrodes for Flexible Thin Film Heaters To. ECS J. Solid State Sci. Technol. 2017, 6, 746–751. [Google Scholar] [CrossRef]
- Garnett, E.C.; Cai, W.; Cha, J.J.; Mahmood, F.; Connor, S.T.; Greyson Christoforo, M.; Cui, Y.; McGehee, M.D.; Brongersma, M.L. Self-Limited Plasmonic Welding of Silver Nanowire Junctions. Nat. Mater. 2012, 11, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Woo, J.Y.; Kim, J.T.; Lee, B.Y.; Han, C.S. Synergistically Enhanced Stability of Highly Flexible Silver Nanowire/Carbon Nanotube Hybrid Transparent Electrodes by Plasmonic Welding. ACS Appl. Mater. Interfaces 2014, 6, 10974–10980. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.R.; Chung, W.H.; Hwang, Y.T.; Hwang, H.J.; Kim, S.H.; Kim, H.S. Selective Wavelength Plasmonic Flash Light Welding of Silver Nanowires for Transparent Electrodes with High Conductivity. ACS Appl. Mater. Interfaces 2018, 10, 24099–24107. [Google Scholar] [CrossRef]
- Park, J.H.; Hwang, G.T.; Kim, S.; Seo, J.; Park, H.J.; Yu, K.; Kim, T.S.; Lee, K.J. Flash-Induced Self-Limited Plasmonic Welding of Silver Nanowire Network for Transparent Flexible Energy Harvester. Adv. Mater. 2017, 29, 1603473. [Google Scholar] [CrossRef]
- Kou, P.; Yang, L.; Chang, C.; He, S. Improved Flexible Transparent Conductive Electrodes Based on Silver Nanowire Networks by a Simple Sunlight Illumination Approach. Sci. Rep. 2017, 7, 42052. [Google Scholar] [CrossRef]
- Halas, N.J.; Lal, S.; Chang, W.S.; Link, S.; Nordlander, P. Plasmons in Strongly Coupled Metallic Nanostructures. Chem. Rev. 2011, 111, 3913–3961. [Google Scholar] [CrossRef] [PubMed]
- Song, T.B.; Chen, Y.; Chung, C.H.; Yang, Y.; Bob, B.; Duan, H.S.; Li, G.; Tu, K.N.; Huang, Y. Nanoscale Joule Heating and Electromigration Enhanced Ripening of Silver Nanowire Contacts. ACS Nano 2014, 8, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Khaligh, H.H.; Xu, L.; Khosropour, A.; Madeira, A.; Romano, M.; Pradére, C.; Tréguer-Delapierre, M.; Servant, L.; Pope, M.A.; Goldthorpe, I.A. The Joule Heating Problem in Silver Nanowire Transparent Electrodes. Nanotechnology 2017, 28, 425703. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, Y.; Cheon, S.; Yi, G.R.; Cho, J.H. Halide Welding for Silver Nanowire Network Electrode. ACS Appl. Mater. Interfaces 2017, 9, 30779–30785. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, D.; Cheng, J.; Liu, J.; Mao, J.; Choy, W.C.H. Locally Welded Silver Nano-Network Transparent Electrodes with High Operational Stability by a Simple Alcohol-Based Chemical Approach. Adv. Funct. Mater. 2015, 25, 4211–4218. [Google Scholar] [CrossRef]
- Zeng, G.; Chen, W.; Chen, X.; Hu, Y.; Chen, Y.; Zhang, B.; Chen, H.; Sun, W.; Shen, Y.; Li, Y.; et al. Realizing 17.5% Efficiency Flexible Organic Solar Cells via Atomic-Level Chemical Welding of Silver Nanowire Electrodes. J. Am. Chem. Soc. 2022, 144, 8658–8668. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Qian, Y.; Cao, H.; Huang, T.; Ren, Z.; Dai, X.; Zhang, S.; Qiu, Y.; Si, R.; Yang, L.; et al. Chemically Welding Silver Nanowires toward Transferable and Flexible Transparent Electrodes in Heaters and Double-Sided Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 15, 13307–13318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Gao, H.; Wang, Y.; Liu, Q.; Huang, S.; Guo, C.F.; Ren, Z. Capillary-Force-Induced Cold Welding in Silver-Nanowire-Based Flexible Transparent Electrodes. Nano Lett. 2017, 17, 1090–1096. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Fang, Y.; Luo, B.; Zhang, Y.; Li, Y.; Zhou, J.; Hu, B. Unraveling the Solvent Induced Welding of Silver Nanowires for High Performance Flexible Transparent Electrodes. Nanoscale 2018, 10, 12981–12990. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.O.; Hu, H.; Zhang, J.; Wei, B.I.N.; Yang, L. Pseudo-Biological Highly Performance Transparent Electrodes Based on Capillary Force-Welded Hybrid AgNW Network. IEEE Access 2019, 7, 177944–177953. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, X.; Yu, H.; Zhou, W.; Wang, Y.; Han, J.; Guo, F. Improved Polyol Syntheses of Silver Right Bipyramids and Nanorods: Camphorquinone-Mediated Morphology Control Methods. Cryst. Growth Des. 2023, 23, 1455–1465. [Google Scholar] [CrossRef]
- Cunha, J.; Guo, T.L.; Della Valle, G.; Koya, A.N.; Proietti Zaccaria, R.; Alabastri, A. Controlling Light, Heat, and Vibrations in Plasmonics and Phononics. Adv. Opt. Mater. 2020, 8, 2001225. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Yan, M.; Qiu, M. Nanosecond Photothermal Effects in Plasmonic Nanostructures. ACS Nano 2012, 6, 2550–2557. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Y.; Li, Z.; Liu, G. Nanoscale Thermoplasmonic Welding. iScience 2022, 25, 104422. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, Y.W.; Kim, J.E.; Yoon, S.W.; Kim, T.Y.; Noh, J.S.; Suh, K.S. Synthesis of Dimension-Controlled Silver Nanowires for Highly Conductive and Transparent Nanowire Films. Acta Mater. 2015, 83, 84–90. [Google Scholar] [CrossRef]
- Bergin, S.M.; Chen, Y.H.; Rathmell, A.R.; Charbonneau, P.; Li, Z.Y.; Wiley, B.J. The Effect of Nanowire Length and Diameter on the Properties of Transparent, Conducting Nanowire Films. Nanoscale 2012, 4, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, Z.; Ye, Q.; He, J.; Nie, X.; He, G.; Song, C.; Shang, W.; Wu, J.; Tao, P.; et al. Substrateless Welding of Self-Assembled Silver Nanowires at Air/Water Interface. ACS Appl. Mater. Interfaces 2016, 8, 20483–20490. [Google Scholar] [CrossRef] [PubMed]
- Nian, Q.; Saei, M.; Xu, Y.; Sabyasachi, G.; Deng, B.; Chen, Y.P.; Cheng, G.J. Crystalline Nanojoining Silver Nanowire Percolated Networks on Flexible Substrate. ACS Nano 2015, 9, 10018–10031. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Wang, X.; Li, D.; Yu, H.; Li, X.; Guo, F. Solvent Welding-Based Methods Gently and Effectively Enhance the Conductivity of a Silver Nanowire Network. Nanomaterials 2023, 13, 2865. https://doi.org/10.3390/nano13212865
Zhu Z, Wang X, Li D, Yu H, Li X, Guo F. Solvent Welding-Based Methods Gently and Effectively Enhance the Conductivity of a Silver Nanowire Network. Nanomaterials. 2023; 13(21):2865. https://doi.org/10.3390/nano13212865
Chicago/Turabian StyleZhu, Zhaoxi, Xiaolu Wang, Dan Li, Haiyang Yu, Xuefei Li, and Fu Guo. 2023. "Solvent Welding-Based Methods Gently and Effectively Enhance the Conductivity of a Silver Nanowire Network" Nanomaterials 13, no. 21: 2865. https://doi.org/10.3390/nano13212865





