The Local and Electronic Structure Study of LuxGd1−xVO4 (0 ≤ x ≤ 1) Solid Solution Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis and Characterization of LuxGd1−xVO4 Nanocrystals
3.1.1. XRD and Rietveld Refinement
3.1.2. EPMA
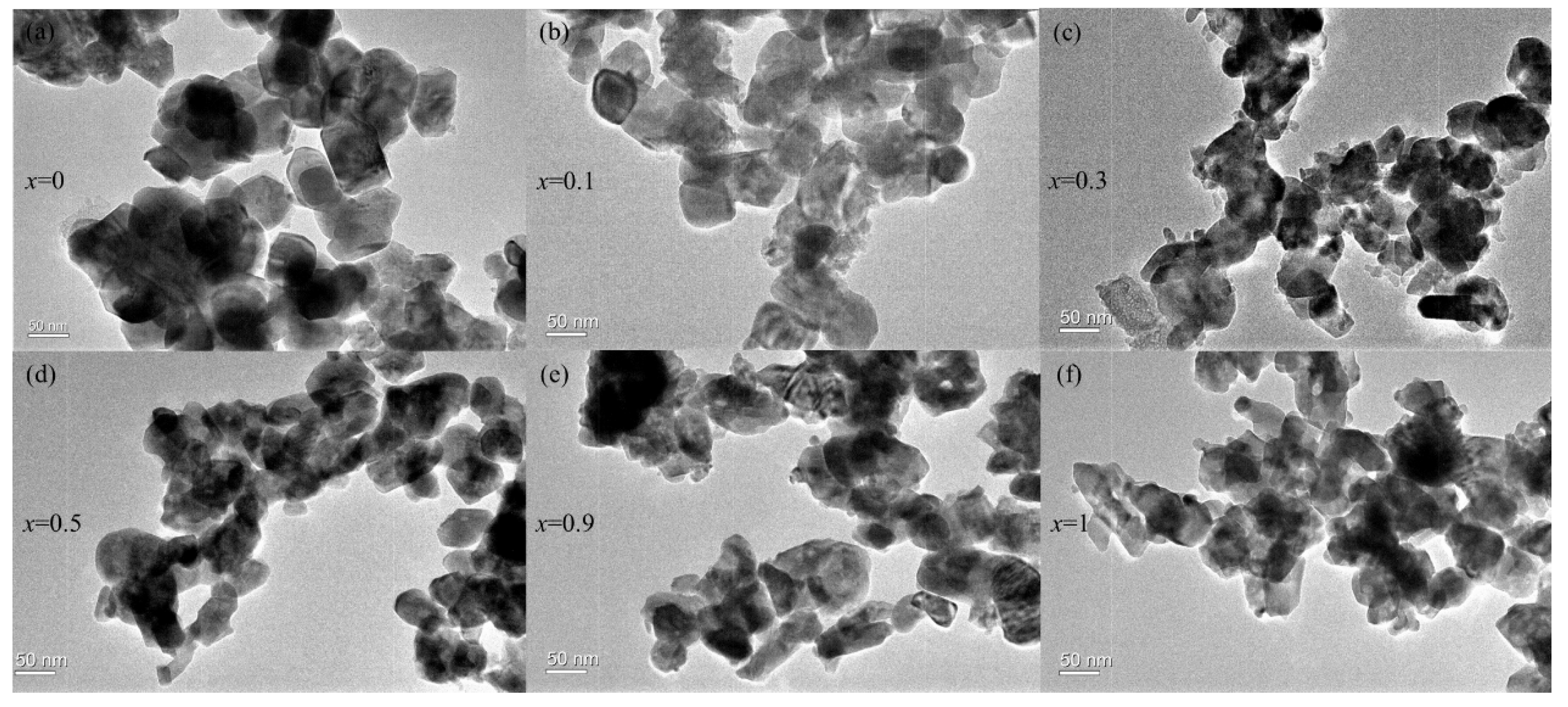
3.1.3. TEM
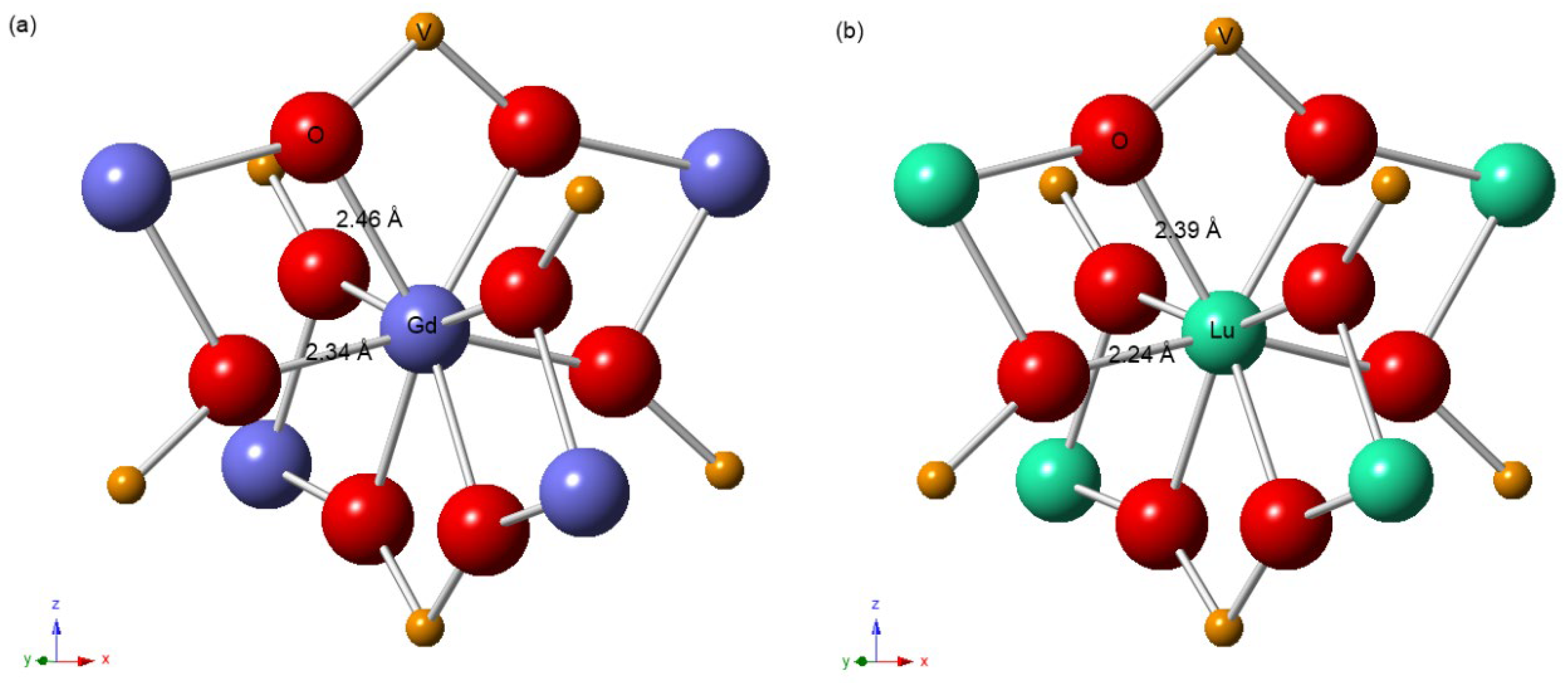
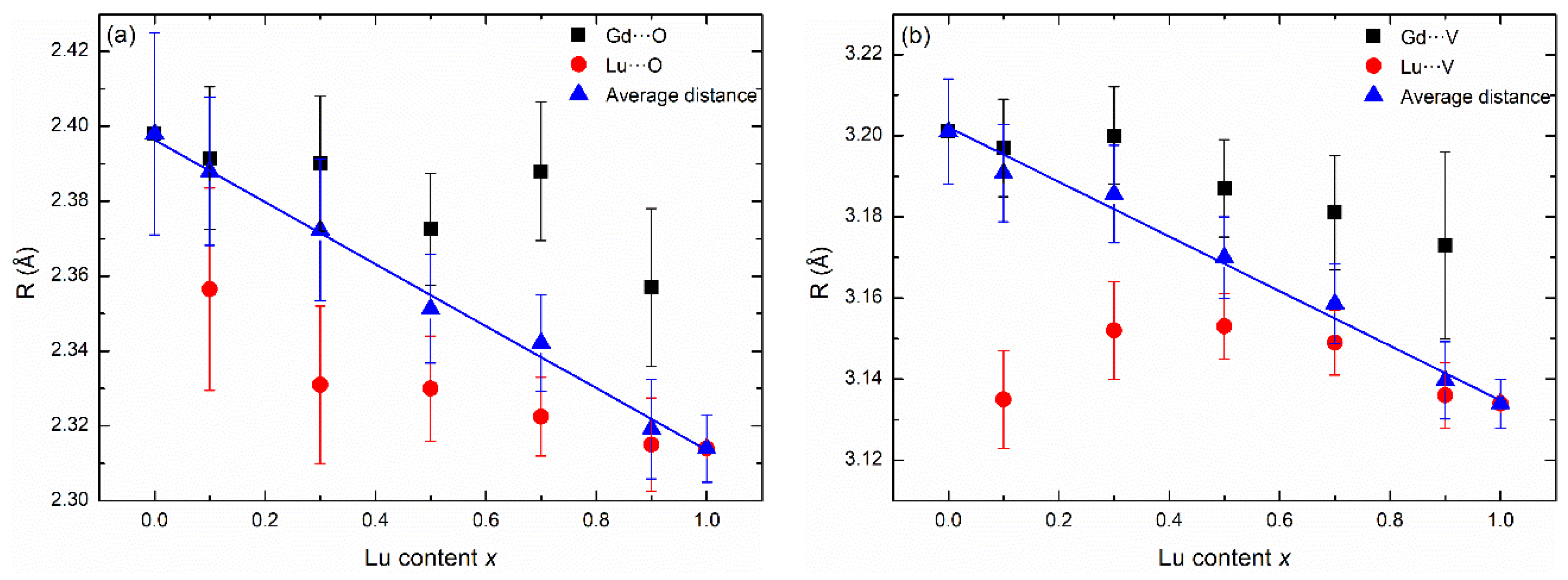
3.2. Local Structure of Lanthanide Atoms in LuxGd1−xVO4 Solid Solutions
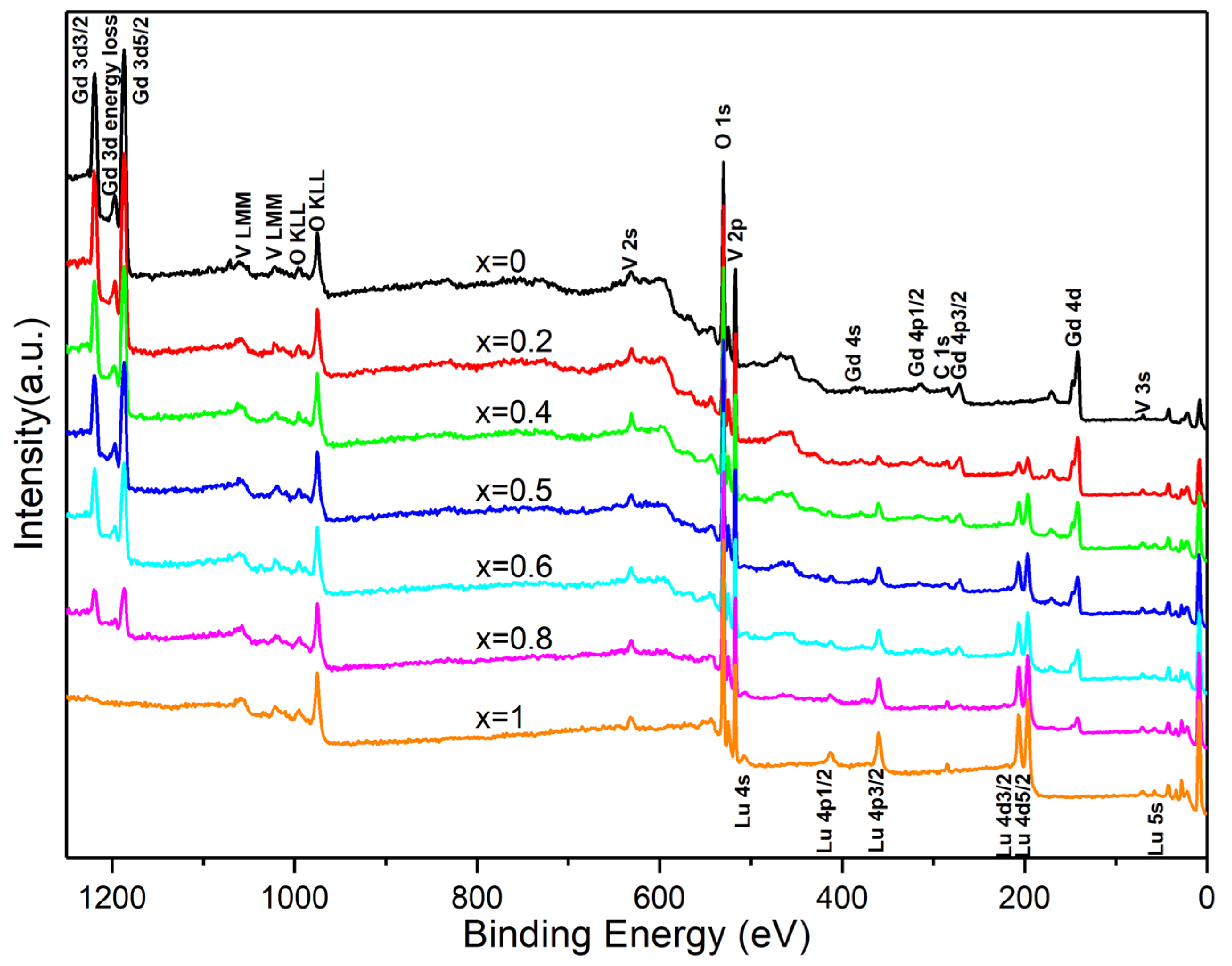
3.3. X-ray Photoelectron Spectroscopy of LuxGd1−xVO4 Solid Solutions
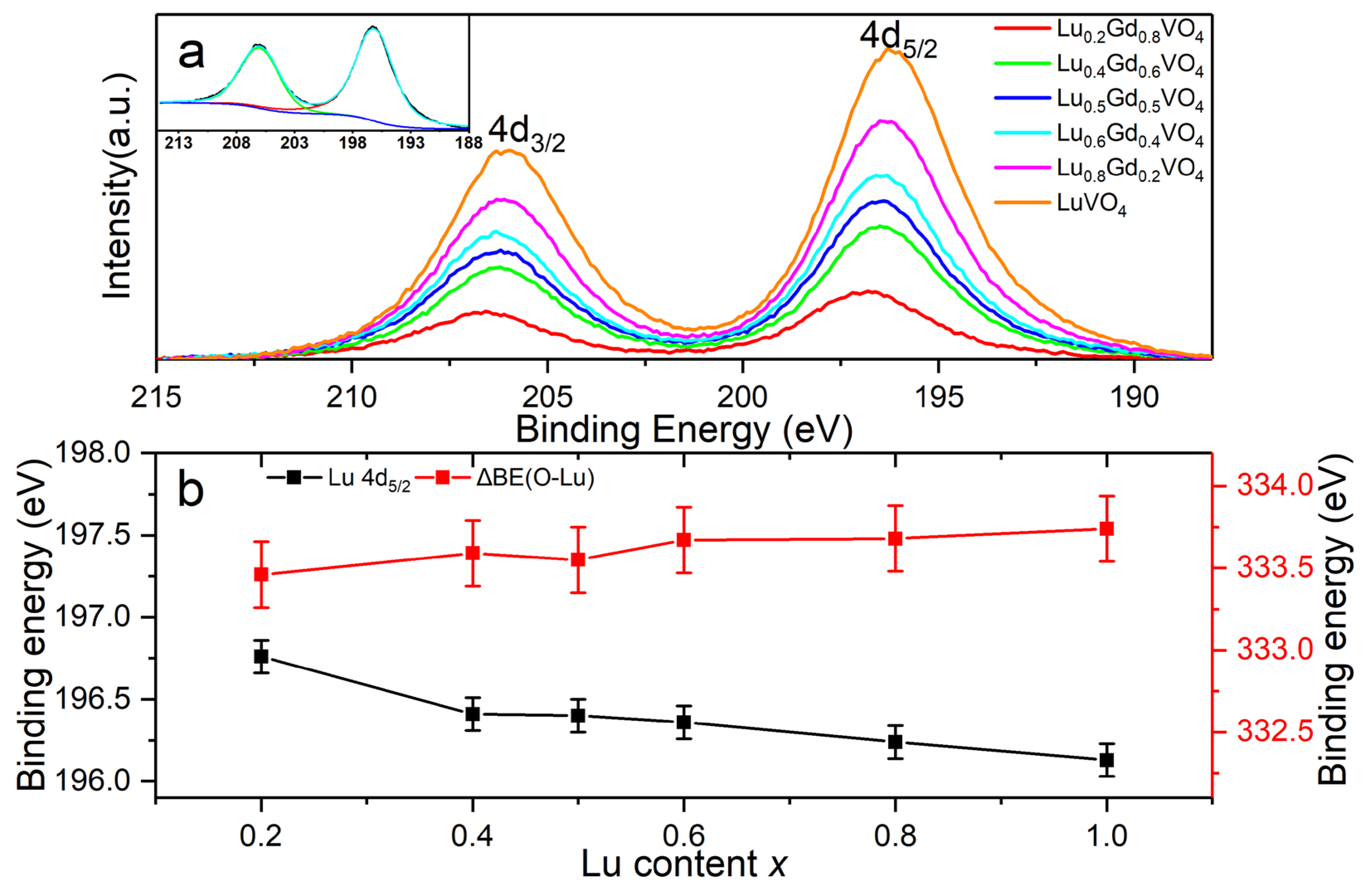
3.3.1. Chemical States of O, V, Lu, and Gd in LuxGd1−xVO4 Solid Solutions
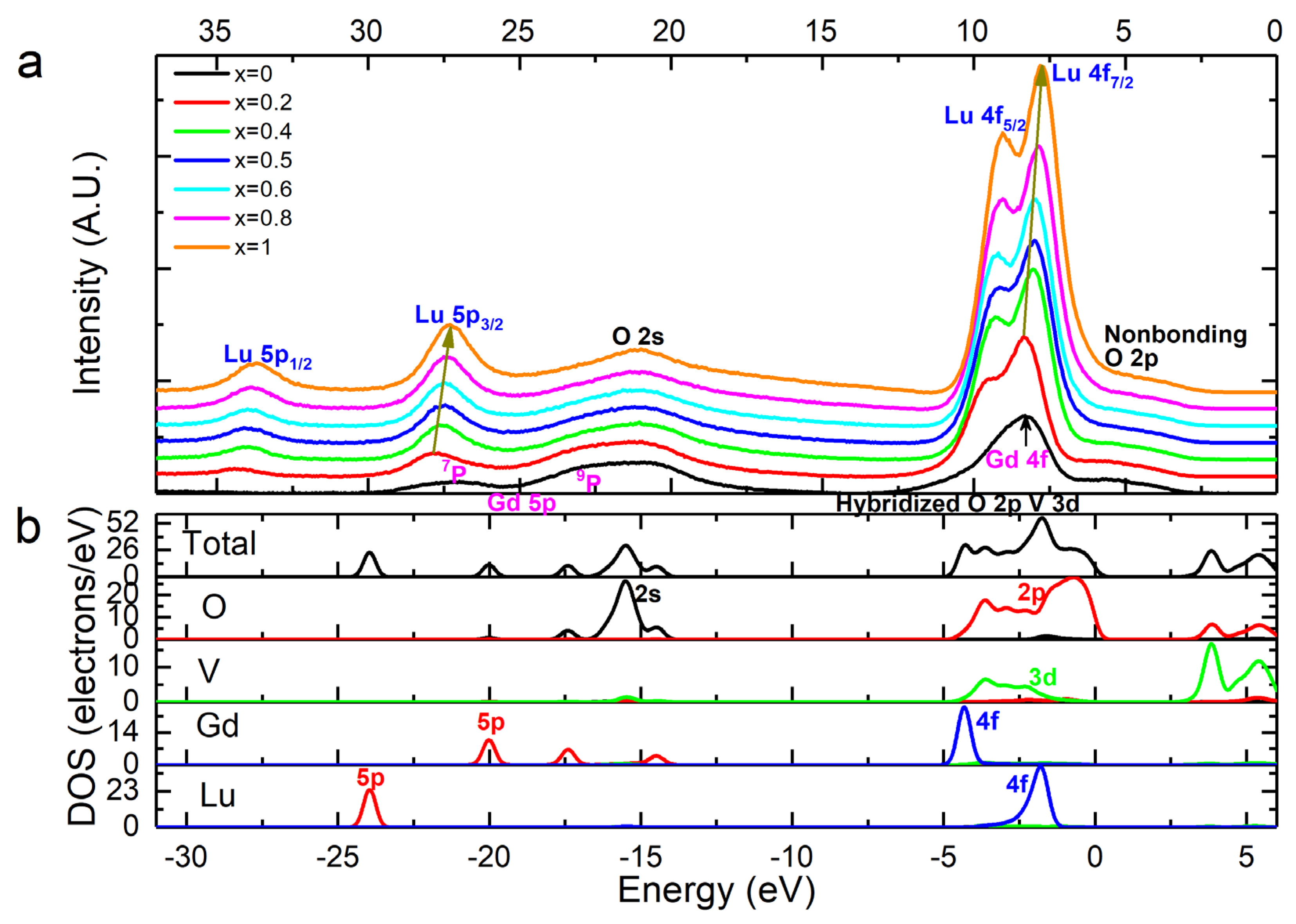
3.3.2. Valence Band Structure Analysis of LuxGd1−xVO4 Solid Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tymiński, A.; Grzyb, T.; Lis, S. REVO4-Based Nanomaterials (RE = Y, La, Gd, and Lu) as Hosts for Yb3+/Ho3+, Yb3+/Er3+, and Yb3+/Tm3+ Ions: Structural and Up-Conversion Luminescence Studies. J. Am. Ceram. Soc. 2016, 99, 3300–3308. [Google Scholar] [CrossRef]
- Yu, H.; Liu, J.; Zhang, H.; Kaminskii, A.A.; Wang, Z.; Wang, J. Advances in vanadate laser crystals at a lasing wavelength of 1 micrometer. Laser Photonics Rev. 2014, 8, 847–864. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Yanagida, T.; Yokota, Y.; Chani, V.; Kochurikhin, V.V.; Yoshikawa, A. Comparative study of optical and scintillation properties of YVO4, (Lu0.5Y0.5)VO4, and LuVO4 single crystals. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 635, 53–56. [Google Scholar] [CrossRef]
- Oshikiri, M.; Ye, J.; Boero, M. Inhomogeneous RVO4 Photocatalyst Systems (R = Y, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu). J. Phys. Chem. C 2014, 118, 8331–8341. [Google Scholar] [CrossRef]
- Bass, M. Correspondence Electrooptic Q Switching of the Nd: YVO4 Laser without an Intracavity Polarizer. IEEE J. Quantum Electron. 1975, 11, 938–939. [Google Scholar] [CrossRef]
- Yu, D.C.; Ye, S.; Peng, M.Y.; Zhang, Q.Y.; Qiu, J.R.; Wang, J.; Wondraczek, L. Efficient near-infrared downconversion in GdVO4:Dy3+ phosphors for enhancing the photo-response of solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 1590–1593. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, R.; Zhang, C.; Zhang, J.; Zhang, T. Low-Temperature-Fired ReVO4 (Re = La, Ce) Microwave Dielectric Ceramics. J. Am. Ceram. Soc. 2015, 98, 1–4. [Google Scholar] [CrossRef]
- Ryu, S.-M.; Nam, C. Effects of annealing on nanocrystalline GdVO4 and its magnetocaloric properties. Appl. Phys. A 2020, 126, 409. [Google Scholar] [CrossRef]
- del Rosal, B.; Pérez-Delgado, A.; Carrasco, E.; Jovanović, D.J.; Dramićanin, M.D.; Dražić, G.; de la Fuente, Á.J.; Sanz-Rodriguez, F.; Jaque, D. Neodymium-Based Stoichiometric Ultrasmall Nanoparticles for Multifunctional Deep-Tissue Photothermal Therapy. Adv. Opt. Mater. 2016, 4, 782–789. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, Z.; Yu, M.; Quan, Z.; Lin, J. Fabrication and optical properties of core–shell structured spherical SiO2@GdVO4:Eu3+ phosphors via sol–gel process. J. Solid State Chem. 2006, 179, 2698–2706. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B. Radiative return to the ground state: Emission. In Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994; pp. 33–70. [Google Scholar]
- Xu, Z.; Li, C.; Hou, Z.; Peng, C.; Lin, J. Morphological control and luminescence properties of lanthanide orthovanadate LnVO4(Ln = La to Lu) nano-/microcrystals viahydrothermal process. CrystEngComm 2011, 13, 474–482. [Google Scholar] [CrossRef]
- Vairapperumal, T.; Saraswathy, A.; Ramapurath, J.S.; Kalarical Janardhanan, S.; Balachandran Unni, N. Catechin tuned magnetism of Gd-doped orthovanadate through morphology as T1-T2 MRI contrast agents. Sci. Rep. 2016, 6, 34976. [Google Scholar] [CrossRef] [Green Version]
- Jia, C.-J.; Sun, L.-D.; You, L.-P.; Jiang, X.-C.; Luo, F.; Pang, Y.-C.; Yan, C.-H. Selective Synthesis of Monazite- and Zircon-type LaVO4 Nanocrystals. J. Phys. Chem. B 2005, 109, 3284–3290. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y. General synthesis of colloidal rare earth orthovanadate nanocrystals. J. Mater. Chem. 2007, 17, 1797–1803. [Google Scholar] [CrossRef]
- Qian, L.; Zhu, J.; Chen, Z.; Gui, Y.; Gong, Q.; Yuan, Y.; Zai, J.; Qian, X. Self-Assembled Heavy Lanthanide Orthovanadate Architecture with Controlled Dimensionality and Morphology. Chem.—A Eur. J. 2009, 15, 1233–1240. [Google Scholar] [CrossRef]
- Robinson, M.D.M.; Oropeza, F.E.; Cui, M.; Zhang, K.H.L.; Hohmann, M.V.; Payne, D.J.; Egdell, R.G.; Regoutz, A. Electronic Structure of Lanthanide-Doped Bismuth Vanadates: A Systematic Study by X-ray Photoelectron and Optical Spectroscopies. J. Phys. Chem. C 2019, 123, 8484–8499. [Google Scholar] [CrossRef] [Green Version]
- Kang, F.; Zhang, H.; Wondraczek, L.; Yang, X.; Zhang, Y.; Lei, D.Y.; Peng, M. Band-Gap Modulation in Single Bi3+-Doped Yttrium–Scandium–Niobium Vanadates for Color Tuning over the Whole Visible Spectrum. Chem. Mater. 2016, 28, 2692–2703. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Z.; Yu, H.; Hu, D.; Zhuang, S.; Chen, L.; Zhao, Y.; Sun, X.; Xu, X. Thermal, spectroscopic, and laser characterization of Nd:LuxY1−xVO4 series crystals. AIP Adv. 2011, 1, 042143. [Google Scholar] [CrossRef]
- Milligan, W.O.; Vernon, L.W. Crystal Structure of Heavy Metal Orthovanadates. J. Phys. Chem. 1952, 56, 145–147. [Google Scholar] [CrossRef]
- Chumha, N.; Kittiwachana, S.; Thongtem, T.; Thongtem, S.; Kaowphong, S. Synthesis and characterization of GdVO4 nanoparticles by a malic acid-assisted sol–gel method. Mater. Lett. 2014, 136, 18–21. [Google Scholar] [CrossRef]
- Pan, B.; Tang, P.; Gao, S.; Shen, W.; Chen, H. Characterization and Photocatalytic Activity of Nanoparticulate LuVO4 Prepared by Sol-Gel Method. Integr. Ferroelectr. 2020, 206, 17–23. [Google Scholar] [CrossRef]
- Yin, W.; Zhou, L.; Gu, Z.; Tian, G.; Jin, S.; Yan, L.; Liu, X.; Xing, G.; Ren, W.; Liu, F.; et al. Lanthanide-doped GdVO4 upconversion nanophosphors with tunable emissions and their applications for biomedical imaging. J. Mater. Chem. 2012, 22, 6974. [Google Scholar] [CrossRef]
- Nunez, N.O.; Rivera, S.; Alcantara, D.; de la Fuente, J.M.; Garcia-Sevillano, J.; Ocana, M. Surface modified Eu:GdVO4 nanocrystals for optical and MRI imaging. Dalton Trans. 2013, 42, 10725–10734. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Yu, Y.; Zhang, H.; Wang, Z.; Wang, J.; Cheng, X.; Shao, Z.; Jiang, M. Growth and laser characterization of mixed Nd:LuxGd1−xVO4 laser crystals. J. Cryst. Growth 2006, 293, 394–397. [Google Scholar] [CrossRef]
- Yu, H.H.; Zhang, H.J.; Wang, Z.P.; Wang, J.Y.; Yu, Y.G.; Cheng, X.F.; Shao, Z.S.; Jiang, M.H.; Ling, Z.C.; Xia, H.R. Characterization of mixed Nd:LuxGd1−xVO4 laser crystals. J. Appl. Phys. 2007, 101, 113109. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Wang, Z.; Wang, J.; Yu, Y.; Shao, Z.; Jiang, M. Enhancement of passive Q-switching performance with mixed Nd:LuxGd1−xVO4 laser crystals. Opt. Lett. 2007, 32, 2152–2154. [Google Scholar] [CrossRef]
- Kutzler, F.W.; Ellis, D.E.; Lam, D.J.; Veal, B.W.; Paulikas, A.P.; Aldred, A.T.; Gubanov, V.A. Electronic structure of rare-earth orthovanadates and its relation to photoelectron and optical spectra. Phys. Rev. B 1984, 29, 1008–1021. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 1991, 44, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Ceperley, D.M.; Alder, B.J. Ground State of the Electron Gas by a Stochastic Method. Phys. Rev. Lett. 1980, 45, 566–569. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.L.; Seal, S. Nature of the use of adventitious carbon as a binding energy standard. J. Vac. Sci. Technol. A 1995, 13, 1239–1246. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Silversmit, G.; Poelman, H.; Depla, D.; Barrett, N.; Marin, G.B.; De Gryse, R. A comparative XPS and UPS study of VOx layers on mineral TiO2(001)-anatase supports. Surf. Interface Anal. 2006, 38, 1257–1265. [Google Scholar] [CrossRef]
- Bondarenka, V.; Grebinskij, S.; Kaciulis, S.; Mattogno, G.; Mickevicius, S.; Tvardauskas, H.; Volkov, V.; Zakharova, G. XPS study of vanadium–yttrium hydrates. J. Electron Spectrosc. Relat. Phenom. 2001, 120, 131–135. [Google Scholar] [CrossRef]
- Lu, A.; Liu, J.; Zhao, D.; Guo, Y.; Li, Q.; Li, N. Photocatalysis of V-bearing rutile on degradation of halohydrocarbons. Catal. Today 2004, 90, 337–342. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corp. 1992, 40, 221. [Google Scholar]
- Demeter, M.; Neumann, M.; Reichelt, W. Mixed-valence vanadium oxides studied by XPS. Surf. Sci. 2000, 454–456, 41–44. [Google Scholar] [CrossRef]
- Lang, W.C.; Padalia, B.D.; Watson, L.M.; Fabian, D.J.; Norris, P.R. Multiplet structure in X-ray photoelectron spectra of rare earth elements and their surface oxides. Faraday Discuss. Chem. Soc. 1975, 60, 37–43. [Google Scholar] [CrossRef]
- Fadley, C.S.; Shirley, D.A. Multiplet Splitting of Metal-Atom Electron Binding Energies. Phys. Rev. A 1970, 2, 1109–1120. [Google Scholar] [CrossRef] [Green Version]
- Szade, J.; Lachnitt, J.; Neumann, M. High-resolution Gd 4d photoemission from different intermetallic compounds. Phys. Rev. B 1997, 55, 1430–1434. [Google Scholar] [CrossRef]
- Kowalczyk, S.P.; Edelstein, N.; McFeely, F.R.; Ley, L.; Shirley, D.A. X-ray photoemission spectra of the 4d levels in rare-earth metals. Chem. Phys. Lett. 1974, 29, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, H.; Kotani, A.; Thole, B.T. Lifetime effect on the multiplet structure of 4d x-ray-photoemission spectra in heavy rare-earth elements. Phys. Rev. B 1994, 50, 12332–12341. [Google Scholar] [CrossRef] [PubMed]
- Atuchin, V.V.; Kesler, V.G.; Meng, G.; Lin, Z.S. The electronic structure of RbTiOPO4 and the effects of the A-site cation substitution in KTiOPO4-family crystals. J. Phys. Condens. Matter 2012, 24, 405503. [Google Scholar] [CrossRef]
- Bagus, P.S.; Illas, F.; Pacchioni, G.; Parmigiani, F. Mechanisms responsible for chemical shifts of core-level binding energies and their relationship to chemical bonding. J. Electron Spectrosc. Relat. Phenom. 1999, 100, 215–236. [Google Scholar] [CrossRef]
- Liu, J.; Duan, X.; Zhang, Y.; Li, Z.; Yu, F.; Jiang, H. Growth, electronic structure and properties of Rb2Ti1.95Yb0.05(PO4)3 crystals. J. Alloy. Compd. 2016, 660, 356–360. [Google Scholar] [CrossRef]
- Mendialdua, J.; Casanova, R.; Barbaux, Y. XPS studies of V2O5, V6O13, VO2 and V2O3. J. Electron Spectrosc. Relat. Phenom. 1995, 71, 249–261. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, L.; Feng, J.; Cui, X.; Pan, W. Electronic, elastic and optical properties of zircon GdVO4 investigated from experiments and LSDA+U. J. Alloy. Compd. 2012, 538, 56–60. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, L.; Pan, W. Physical properties of zircon and scheelite lutetium orthovanadate: Experiment and first-principles calculation. J. Solid State Chem. 2013, 205, 97–103. [Google Scholar] [CrossRef]
- Perego, M.; Seguini, G.; Scarel, G.; Fanciulli, M. X-ray photoelectron spectroscopy study of energy-band alignments of Lu2O3 on Ge. Surf. Interface Anal. 2006, 38, 494–497. [Google Scholar] [CrossRef]
- Lademan, W.J.; See, A.K.; Klebanoff, L.E.; van der Laan, G. Multiplet structure in high-resolution and spin-resolved X-ray photoemission from gadolinium. Phys. Rev. B 1996, 54, 17191–17198. [Google Scholar] [CrossRef] [PubMed]
- Baur, W. The geometry of polyhedral distortions. Predictive relationships for the phosphate group. Acta Cryst. 1974, B30, 1195–1215. [Google Scholar] [CrossRef]





| x | 0.1 | 0.3 | 0.5 | 0.7 | 0.9 |
|---|---|---|---|---|---|
| V (mol %) | 50.295 | 50.719 | 49.543 | 51.071 | 50.627 |
| Gd (mol %) | 44.910 | 34.566 | 25.230 | 15.590 | 4.960 |
| Lu (mol %) | 4.795 | 14.714 | 25.227 | 33.338 | 44.413 |
| Molar ratio of Lu/RE 1 | 0.10 | 0.30 | 0.50 | 0.68 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, Z.; Ji, N.; Wei, C.; Duan, X.; Jiang, H. The Local and Electronic Structure Study of LuxGd1−xVO4 (0 ≤ x ≤ 1) Solid Solution Nanocrystals. Nanomaterials 2023, 13, 323. https://doi.org/10.3390/nano13020323
Chen Y, Li Z, Ji N, Wei C, Duan X, Jiang H. The Local and Electronic Structure Study of LuxGd1−xVO4 (0 ≤ x ≤ 1) Solid Solution Nanocrystals. Nanomaterials. 2023; 13(2):323. https://doi.org/10.3390/nano13020323
Chicago/Turabian StyleChen, Yang, Ziqing Li, Nianjing Ji, Chenxi Wei, Xiulan Duan, and Huaidong Jiang. 2023. "The Local and Electronic Structure Study of LuxGd1−xVO4 (0 ≤ x ≤ 1) Solid Solution Nanocrystals" Nanomaterials 13, no. 2: 323. https://doi.org/10.3390/nano13020323





