Quantitative Detection of Mastitis Factor IL-6 in Dairy Cow Using the SERS Improved Immunofiltration Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
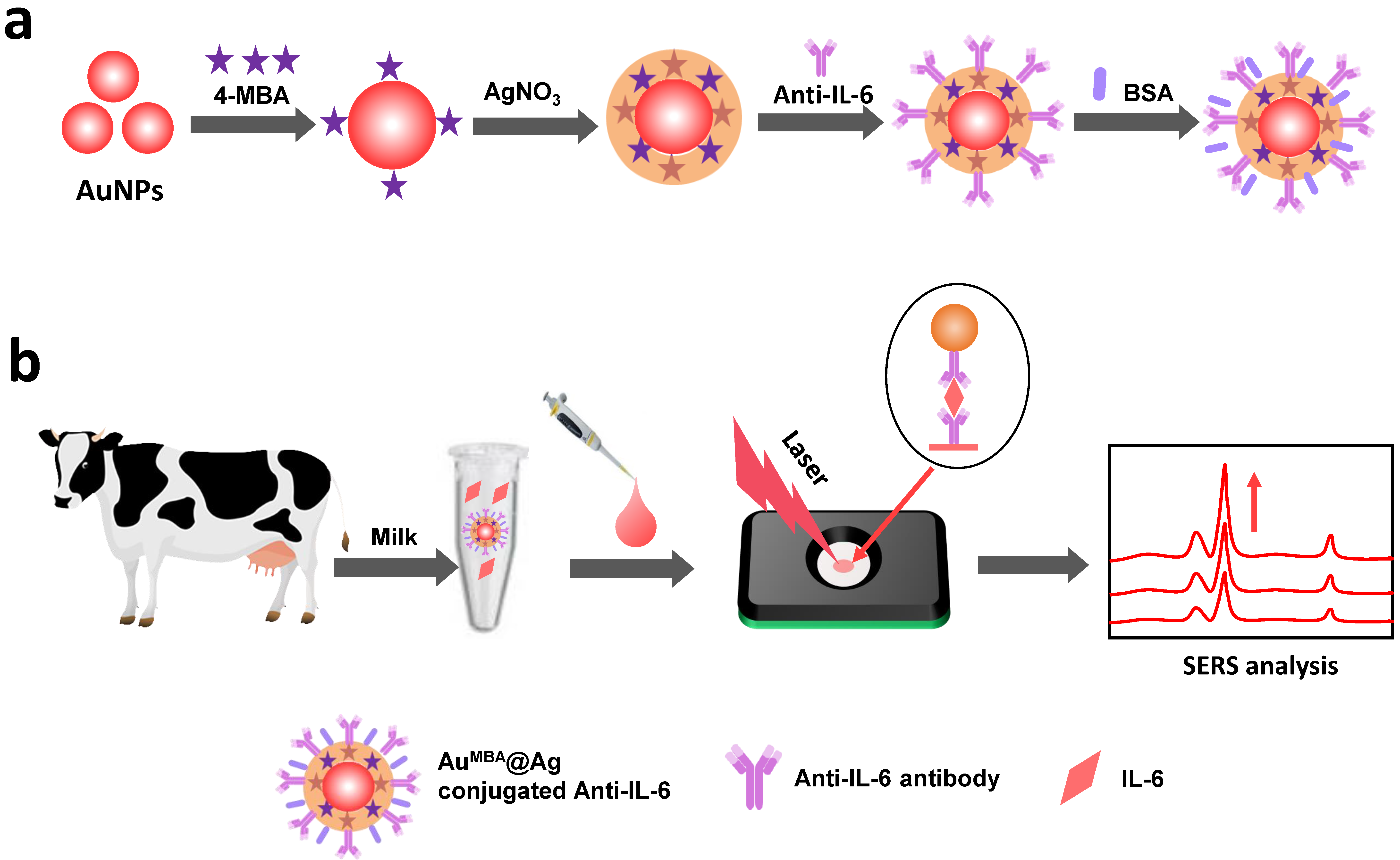
2.2. Synthesis of Au4-MBA@Ag SERS Nanotags
2.3. Conjugation of IL-6 Antibody with Au4-MBA@Ag
2.4. Preparation of IL-6 Solutions
2.5. Detection of IL-6 in Milk
2.6. Data Analysis
2.7. The Principle of the SERS IFA Method
3. Results
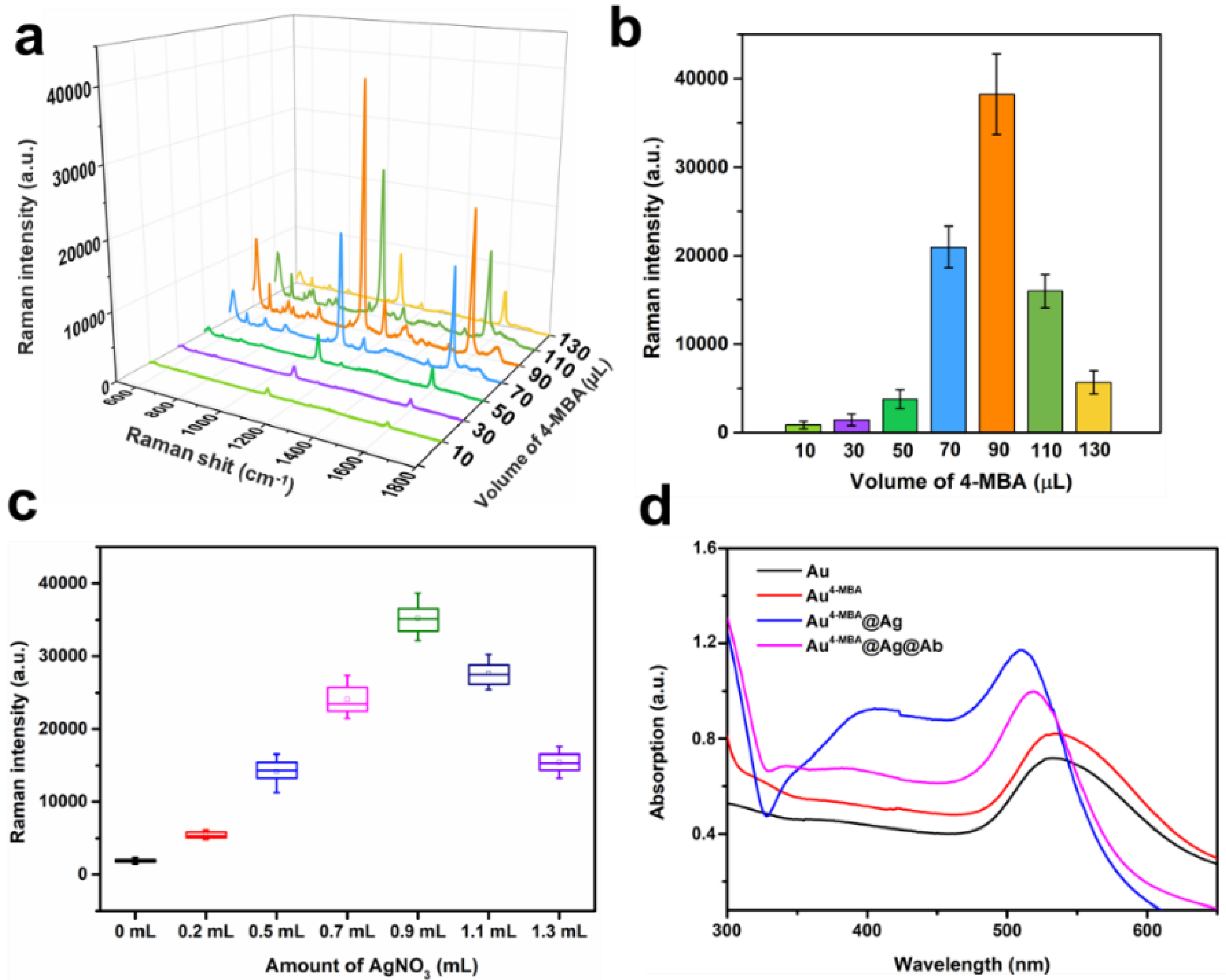
3.1. Characterization of Au4-MBA@Ag SERS Nanotag
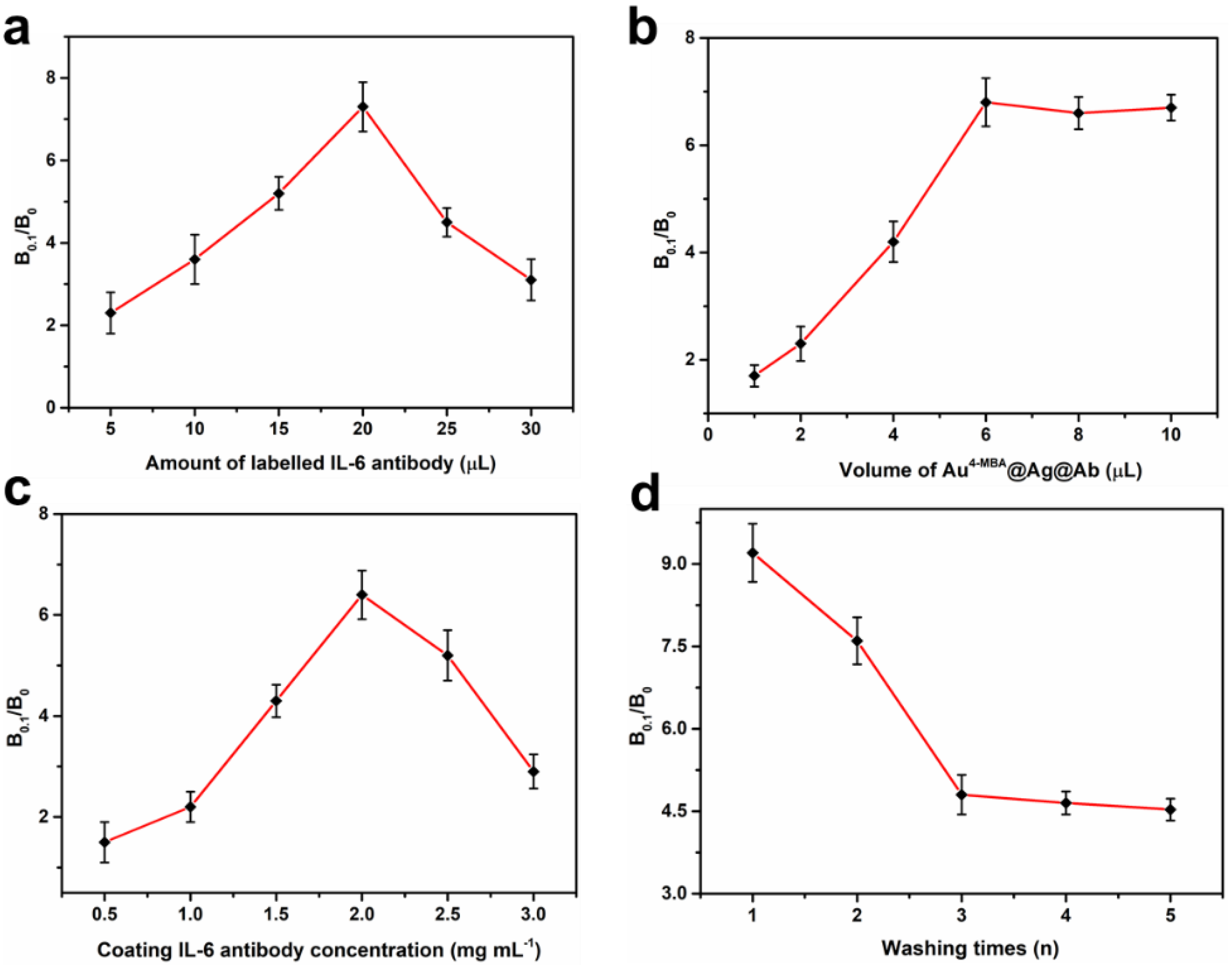
3.2. Optimization of the Assay Conditions
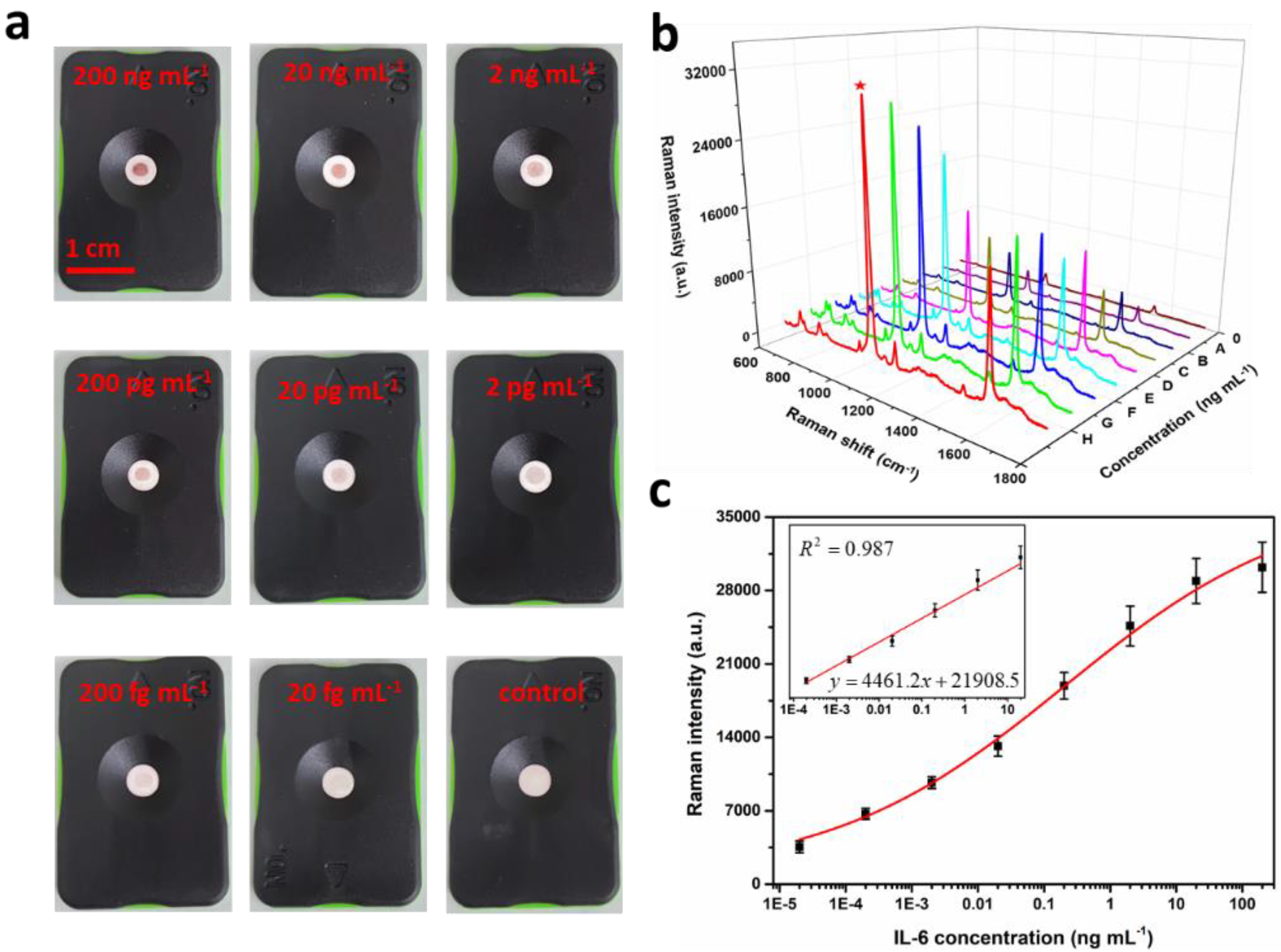
3.3. Quantitative SERS Measurements of IL-6
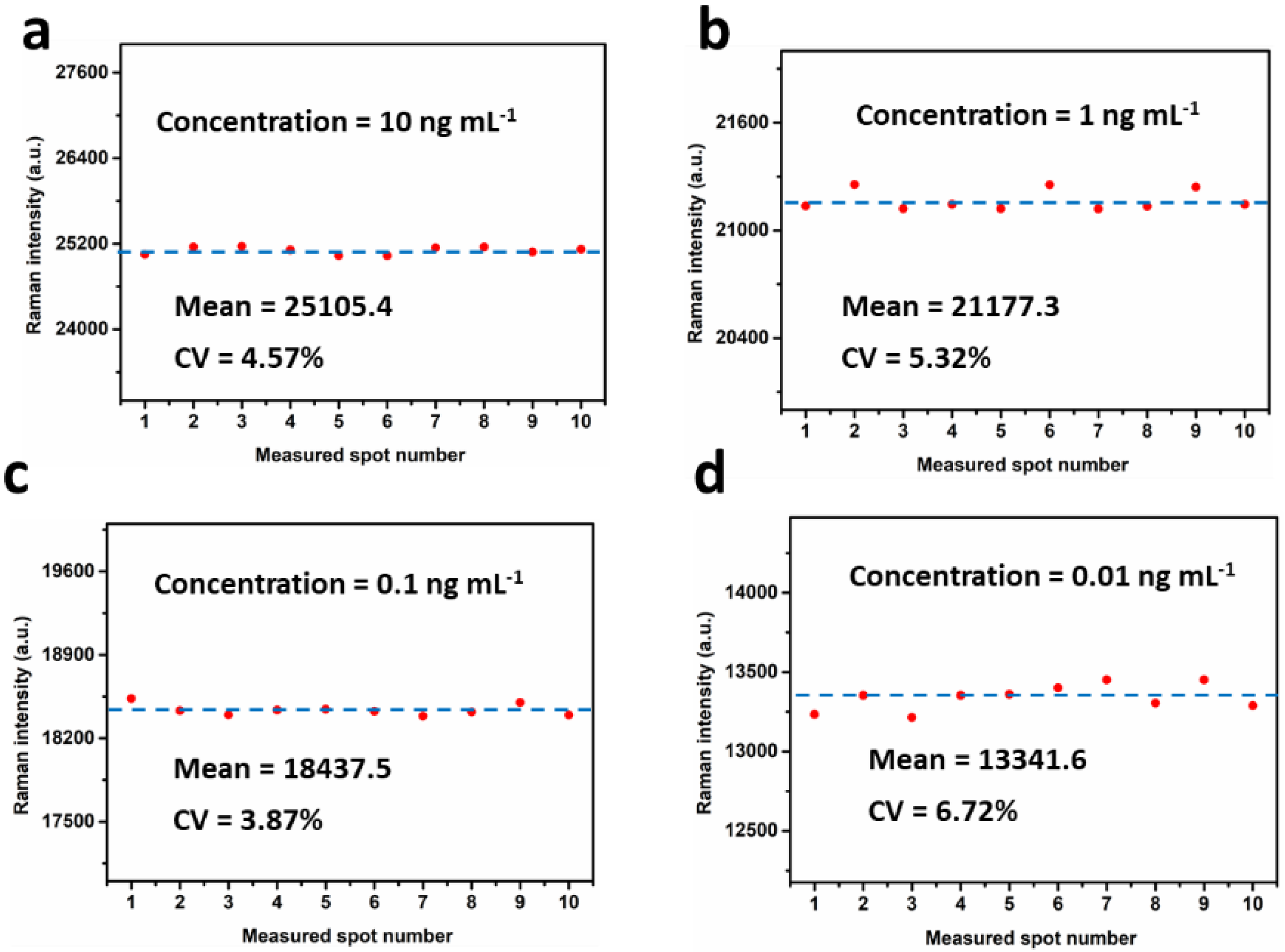
3.4. Reproducibility of the SERS IFA
3.5. Specificity of the SERS IFA
3.6. Detection of IL-6 in Spiked Milk Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morin, D.E.; Petersen, G.C.; Whitmore, H.L.; Hungerford, L.L.; Hinton, R.A. Economic analysis of a mastitis monitoring and control program in four dairy herds. J. Am. Vet. Med. Assoc. 1993, 202, 540–548. [Google Scholar] [PubMed]
- Santos, J.; Cerri, R.; Ballou, M.; Higginbotham, G.; Kirk, J. Effect of timing of first clinical mastitis occurrence on lactational and reproductive performance of Holstein dairy cows. Anim. Reprod. Sci. 2004, 80, 31–45. [Google Scholar] [CrossRef]
- Maity, S.; Das, D.; Ambatipudi, K. Quantitative alterations in bovine milk proteome from healthy, subclinical and clinical mastitis during S. aureus infection. J. Proteom. 2020, 223, 103815. [Google Scholar] [CrossRef] [PubMed]
- Viguier, C.; Arora, S.; Gilmartin, N.; Welbeck, K.; O’Kennedy, R. Mastitis detection: Current trends and future perspectives. Trends Biotechnol. 2009, 27, 486–493. [Google Scholar] [CrossRef]
- Day, J.; Legrand, E. Synergy of local, regional, and systemic non-specific stressors for host defense against pathogens. J. Theor. Biol. 2015, 367, 39–48. [Google Scholar] [CrossRef]
- Cravero, D.; Martignani, E.; Miretti, S.; Macchi, E.; Accornero, P.; Baratta, M. Bovine mammary epithelial cells retain stem-like phenotype in long-term cultures. Res. Vet. Sci. 2014, 97, 367–375. [Google Scholar]
- Shakeeb, N.; Varkey, P.; Ajit, A. Human Saliva as a Diagnostic Specimen for Early Detection of Inflammatory Biomarkers by Real-Time RT-PCR. Inflammation 2021, 44, 1713–1723. [Google Scholar] [CrossRef]
- Sadek, K.; Saleh, E.; Ayoub, M. Selective, reliable blood and milk bio-markers for diagnosing clinical and subclinical bovine mastitis. Trop. Anim. Health Prod. 2016, 49, 431–437. [Google Scholar] [CrossRef]
- Shuster, D.E.; Kehrli, M.; Stevens, M.G. Cytokine production during endotoxin-induced mastitis in lactating dairy cows. Am. J. Vet. Res. 1993, 54, 80–85. [Google Scholar]
- Mukherjee, J.; Varshney, N.; Chaudhury, M.; Mohanty, A.; Dang, A. Immune response of the mammary gland during different stages of lactation cycle in high versus low yielding Karan Fries crossbred cows. Livest. Sci. 2013, 154, 215–223. [Google Scholar] [CrossRef]
- Bochniarz, M.; Zdzisińska, B.; Wawron, W.; Szczubiał, M.; Dabrowski, R. Milk and serum IL-4, IL-6, IL-10, and amyloid A concentrations in cows with subclinical mastitis caused by coagulase-negative staphylococci. J. Dairy Sci. 2017, 100, 9674–9680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Fossum, C.; Berg, M.; Magnusson, U. Morphometric analysis of proinflammatory cytokines in mammary glands of sows suggests an association between clinical mastitis and local production of IL-1beta, IL-6 and TNF-alpha. Vet. Res. 2007, 38, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezamand, P.; McGuire, M. Short communication: Effects of trans fatty acids on markers of inflammation in bovine mammary epithelial cells. J. Dairy Sci. 2011, 94, 316–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dernfalk, J.; Persson Waller, K.; Johannisson, A. The xMAP technique can be used for detection of the inflammatory cytokines IL-1beta, IL-6 and TNF-alpha in bovine samples. Vet. Immunol. Immunopathol. 2007, 118, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, F.F.; Xu, Y.; Wang, J.J.; Samadli, S.; Wu, Y.F.; Liu, H.H.; Chen, W.X.; Luo, H.H.; Zhang, D.D.; et al. Interleukin-6 is prone to be a candidate biomarker for predicting incomplete and IVIG nonresponsive Kawasaki disease rather than coronary artery aneurysm. Clin. Exper. Med. 2019, 19, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Schoenhoff, F.S.; Savage, W.J.; Zhang, P.; Van Eyk, J.E. Multiplex assays for biomarker research and clinical applica-tion: Translational science coming of age. Proteom. Clin. Appl. 2010, 4, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, Z.; Hou, L.; Wang, J.; Tian, W. The study of a chemiluminescence immunoassay using the peroxyoxalate chemiluminescent reaction and its application. Talanta 2007, 72, 1293–1297. [Google Scholar] [CrossRef]
- Cho, A.-R.; Lee, J.-H.; Lee, H.S.; Lee, Y.-J. Leukocyte count, C-reactive protein level and incidence risk of type 2 diabetes among non-smoking adults: A longitudinal finding over 12 years from the Korean Genome and Epidemiology Study. Prim. Care Diabetes 2021, 15, 385–390. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Oh, Y.K.; Joung, H.-A.; Kim, S.; Kim, M.-G. Vertical flow immunoassay (VFA) biosensor for a rapid one-step immunoassay. Lab Chip 2013, 13, 768–772. [Google Scholar] [CrossRef]
- Chinnasamy, T.; Segerink, L.I.; Nystrand, M.; Gantelius, J.; Andersson Svahn, H. Point-of-care vertical flow allergen microar-ray assay: Proof of concept. Clin. Chem. 2014, 60, 1209–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas, L.; Reuterswärd, P.; Rasti, R.; Herrmann, B.; Mårtensson, A.; Alfvén, T.; Gantelius, J.; Andersson-Svahn, H. A vertical flow paper-microarray assay with isothermal DNA amplification for detection of Neisseria meningitidis. Talanta 2018, 183, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, J.; Kneipp, H.; Kneipp, K. SERS—A single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 2008, 37, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Park, J.K. Functional packaging of lateral flow strip allows simple delivery of multiple reagents for multistep assays. Anal. Chem. 2016, 88, 10374–10378. [Google Scholar] [CrossRef]
- Cheng, Z.; Choi, N.; Wang, R.; Lee, S.; Moon, K.C.; Yoon, S.-Y.; Chen, L.; Choo, J. Simultaneous Detection of Dual Prostate Specific Antigens Using Surface-Enhanced Raman Scattering-Based Immunoassay for Accurate Diagnosis of Prostate Cancer. ACS Nano 2017, 11, 4926–4933. [Google Scholar] [CrossRef]
- Kamińska, A.; Winkler, K.; Kowalska, A.; Witkowska, E.; Szymborski, T.; Janeczek, A.; Waluk, J. SERS-based Immunoassay in a Microfluidic System for the Multiplexed Recognition of Interleukins from Blood Plasma: Towards Picogram Detection. Sci. Rep. 2017, 7, 10656. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Jiang, X.; Sun, B.; Zhu, Y.; Wang, H.; Su, Y.; He, Y. Simultaneous Capture, Detection, and Inactivation of Bacteria as Enabled by a Surface-Enhanced Raman Scattering Multifunctional Chip. Angew. Chem. Int. Ed. 2015, 54, 5132–5136. [Google Scholar] [CrossRef]
- Zhou, W.; Tian, Y.F.; Yin, B.C.; Ye, B.C. Simultaneous surface-enhanced Raman spectroscopy detection of multiplexed Mi-croRNA biomarkers. Anal. Chem. 2017, 89, 6120–6128. [Google Scholar] [CrossRef]
- Choi, H.-K.; Park, W.-H.; Park, C.-G.; Shin, H.-H.; Lee, K.S.; Kim, Z.H. Metal-Catalyzed Chemical Reaction of Single Molecules Directly Probed by Vibrational Spectroscopy. J. Am. Chem. Soc. 2016, 138, 4673–4684. [Google Scholar] [CrossRef]
- Xi, W.; Haes, A.J. Elucidation of HEPES Affinity to and Structure on Gold Nanostars. J. Am. Chem. Soc. 2019, 141, 4034–4042. [Google Scholar] [CrossRef]
- Pallaoro, A.; Mirsafavi, R.Y.; Culp, W.T.; Braun, G.B.; Meinhart, C.D.; Moskovits, M. Screening for canine transitional cell carcinoma (TCC) by SERS-based quantitative urine cytology. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Xu, M.; Xu, L.-J.; Wei, T.; Ma, X.; Zheng, X.-S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.P.; Du, X.; Cui, Y.J.; Zhang, X.Y.; Ge, Q.Y.; Dong, J.; Zhao, X.W. Vertical flow assay for inflammatory biomarkers based on nanofluidic channel array and SERS nanotags. Small 2020, 16, 2002801. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, B.; Ni, H.; Chang, N.; Luan, C.; Ge, Q.; Dong, J.; Zhao, X. Vertical flow assays based on core–shell SERS nanotags for multiplex prostate cancer biomarker detection. Analyst 2019, 144, 4051–4059. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Moshaii, A.; Nikkhah, M. Controlled synthesis of colloidal monodisperse gold nanoparticles in a wide range of sizes; investigating the effect of reducing agent. Mater. Res. Express 2019, 6, 1150f2. [Google Scholar] [CrossRef]
- Liu, H.; Chen, C.; Zhang, C.; Du, X.; Li, P.; Wang, S. Functionalized Au MBA @Ag Nanoparticles as an Optical and SERS Dual Probe in a Lateral Flow Strip for the Quantitative Detection of Escherichia coli O157:H7. J. Food Sci. 2019, 84, 2916–2924. [Google Scholar] [CrossRef]
- Lin, L.-K.; Stanciu, L.A. Bisphenol A detection using gold nanostars in a SERS improved lateral flow immunochromatograph-ic assay. Sens. Actuator B Chem. 2018, 276, 222–229. [Google Scholar] [CrossRef]
- Xiong, Z.; Chen, X.; Liou, P.; Lin, M. Development of nanofibrillated cellulose coated with gold nanoparticles for measurement of melamine by SERS. Cellulose 2017, 24, 2801–2811. [Google Scholar] [CrossRef]
- Zhou, J.; Nie, W.; Chen, Y.; Yang, C.; Gong, L.; Zhang, C.; Chen, Q.; He, L.; Feng, X. Quadruplex gold immunochromatogaraphic assay for four families of antibiotic residues in milk. Food Chem. 2018, 256, 304–310. [Google Scholar] [CrossRef]
- Han, M.; Gong, L.; Wang, J.; Zhang, X.; Jin, Y.; Zhao, R.; Yang, C.; He, L.; Feng, X.; Chen, Y. An octuplex lateral flow immu-noassay for rapid detection of antibiotic residues, aflatoxin M1 and melamine in milk. Sens. Actuator B Chem. 2019, 292, 94–104. [Google Scholar] [CrossRef]
- Doyen, M.; Bartik, K.; Bruylants, G. UV-Vis and NMR study of the formation of gold nanoparticles by citrate reduction: Ob-servation of gold-citrate aggregates. J. Colloid Interface Sci. 2013, 399, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ren, Y.-J.; Zhao, S.-M.; Zhao, J.-W. The effect of inserted gold nanosphere on the local field enhancement of gold nanoshell. Mater. Chem. Phys. 2012, 133, 1060–1065. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Wang, F.; Jia, Z.; Zhou, J.; Jiang, T.; Petti, L.; Chen, Y.; Xiong, Q.; Wang, X. Classification analyses for prostate cancer, benign prostate hyperplasia and healthy subjects by SERS-based immunoassay of multiple tumour markers. Talanta 2018, 188, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Han, G.; Zhang, Z.; Liu, R.; Jiang, C.; Wang, S.; Han, M.-Y. Shell Thickness-Dependent Raman Enhancement for Rapid Identification and Detection of Pesticide Residues at Fruit Peels. Anal. Chem. 2012, 84, 255–261. [Google Scholar] [CrossRef]
- Shi, Q.; Huang, J.; Sun, Y.; Yin, M.; Hu, M.; Hu, X.; Zhang, Z.; Zhang, G. Utilization of a lateral flow colloidal gold immuno-assay strip based on surface-enhanced Raman spectroscopy for ultrasensitive detection of antibiotics in milk. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 197, 107–113. [Google Scholar] [CrossRef]
- Deng, D.; Yang, H.; Liu, C.; Zhao, K.; Li, J.; Deng, A. Ultrasensitive detection of diclofenac in water samples by a novel sur-face-enhanced Raman scattering (SERS)-based immunochromatographic assay using AgMBA@SiO2-Ab as immunoprobe. Sens. Actuator B Chem. 2019, 283, 563–570. [Google Scholar] [CrossRef]
- Huang, W.; Guo, E.; Li, J.; Deng, A. Quantitative and ultrasensitive detection of brombuterol by a surface-enhanced Raman scattering (SERS)-based lateral flow immunochromatographic assay (FLIA) using AgMBA@Au–Ab as an immunoprobe. Analyst 2021, 146, 296–304. [Google Scholar] [CrossRef]
- Rathnayake, N.; Akerman, S.; Klinge, B.; Lundegren, N.; Jansson, H.; Sorsa, Y.T.; Gustafsson, A. Salivary biomarkers for detection of systemic diseases. PLoS ONE 2013, 8, e61356–e61361. [Google Scholar] [CrossRef] [Green Version]
- Tertis, M.; Leva, P.I.; Bogdan, D.; Suciu, M.; Graur, F.; Cristea, C. Impedimetric aptasensor for the label-free and selective detection of interleukin-6 for colorectal cancer screening. Biosens. Bioelectron. 2019, 137, 1293–1297. [Google Scholar] [CrossRef]
- Barman, S.C.; Sharifuzzaman, M.; Zahed, M.A.; Park, C.; Yoon, S.H.; Zhang, S.P.; Kim, H.; Yoon, H.; Park, J.Y. A highly selective and stable cationic polyelectrolyte encapsulated black phosphorene based impedimetric immunosensor for interleukin-6 detection. Biosens. Bioelectron. 2021, 186, 1873–4235. [Google Scholar]
- Wang, X.M.; Ma, L.; Hu, C.M.; Liu, T.W.; Sun, S.J.; Liu, X.H.; Guan, M. Simultaneous quantitative detection of IL-6 and PCT using SERS magnetic immunoassay with sandwich structure. Nanotechnology 2021, 32, 255702. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Wang, H.; Zhao, Y.; Nan, X.; Wei, W.; Du, C.; Zhang, F.; Luo, Q.; Yang, L.; Xiong, B. Quantitative Detection of Mastitis Factor IL-6 in Dairy Cow Using the SERS Improved Immunofiltration Assay. Nanomaterials 2022, 12, 1091. https://doi.org/10.3390/nano12071091
Chen R, Wang H, Zhao Y, Nan X, Wei W, Du C, Zhang F, Luo Q, Yang L, Xiong B. Quantitative Detection of Mastitis Factor IL-6 in Dairy Cow Using the SERS Improved Immunofiltration Assay. Nanomaterials. 2022; 12(7):1091. https://doi.org/10.3390/nano12071091
Chicago/Turabian StyleChen, Ruipeng, Hui Wang, Yiguang Zhao, Xuemei Nan, Wensong Wei, Chunmei Du, Fan Zhang, Qingyao Luo, Liang Yang, and Benhai Xiong. 2022. "Quantitative Detection of Mastitis Factor IL-6 in Dairy Cow Using the SERS Improved Immunofiltration Assay" Nanomaterials 12, no. 7: 1091. https://doi.org/10.3390/nano12071091





