Micro and Nano Interdigitated Electrode Array (IDEA)-Based MEMS/NEMS as Electrochemical Transducers: A Review
Abstract
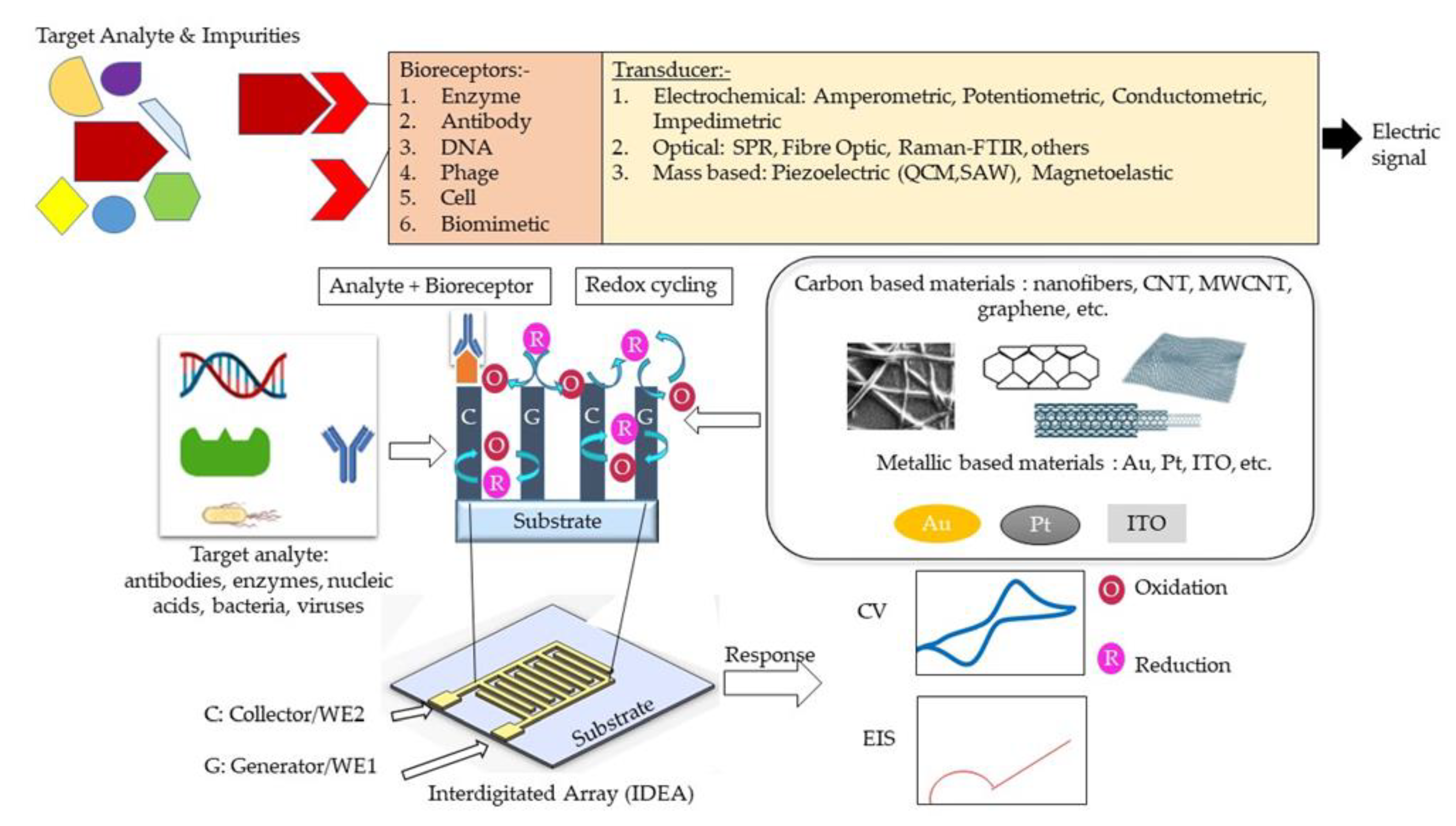
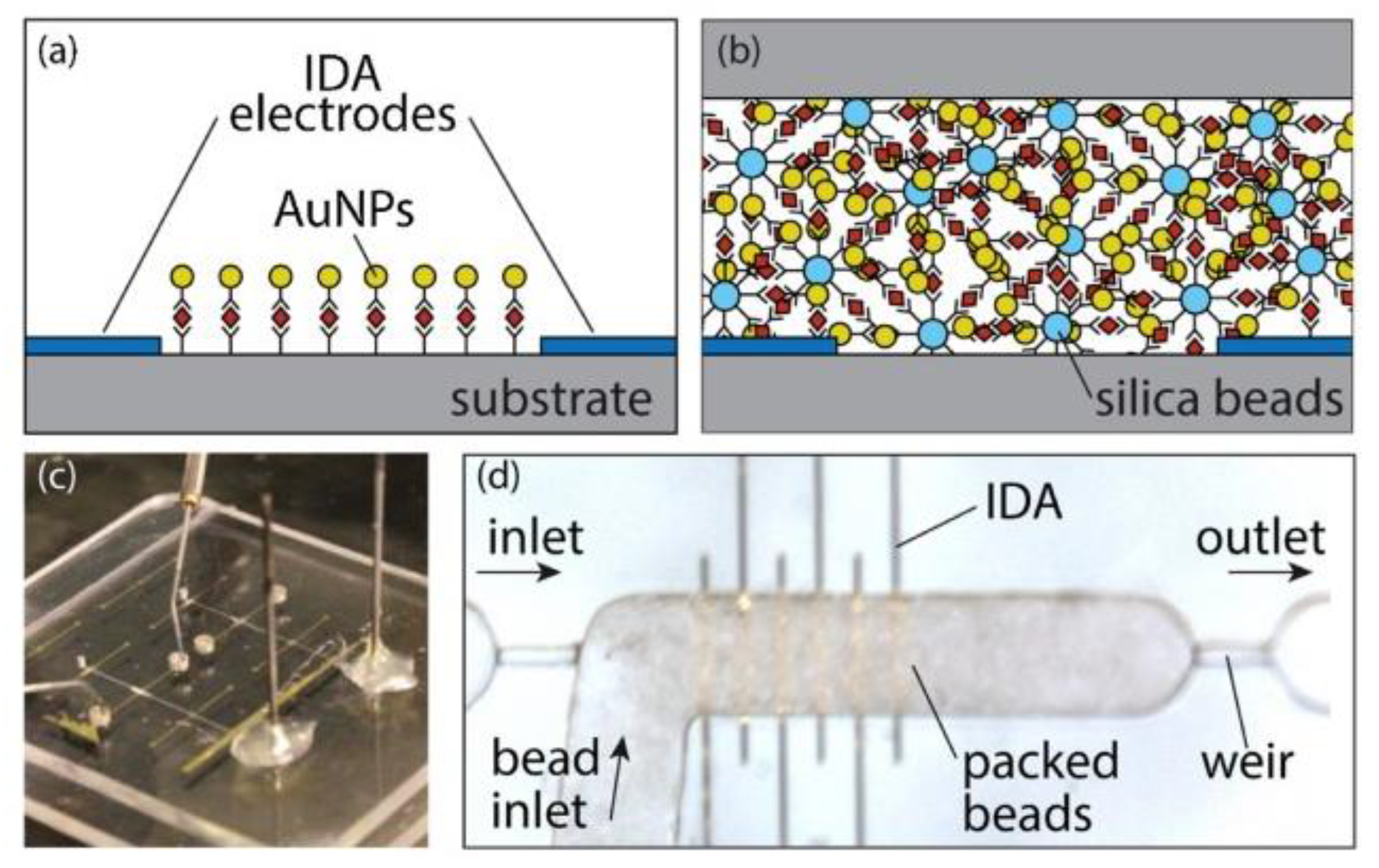
:1. Introduction
| Type of Electrochemical Biosensor | Working Principle | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Amperometric | Measures current resulting from redox cycling at a constant voltage (CA) and controlled potential (CV). | - Low fabrication cost; - High sensitivity. | - A signal reduction from fouling agents, and interferents in a sample. | [58,59] |
| Impedimetric | Measures impedance and changes in ionic concentration under no current flow between reference and ion-selective electrodes. | - Detect current changes without redox reaction; - Simple detection method. | - Slow dynamic response. - Low detection method; | [5,60] |
| Conductometric | Measures of conductance and interfacial electric arise from the biorecognition process. | - High signal-to-noise (S/N) ratio; - Directly detect the binding events; - No interference. | - Slow response; - Accuracy of detection depends on instrumental and experiment procedures. | [61] |
| Potentiometric | Measures potential difference from changes in ion concentration. | - No reference electrode; - Efficient at low amplitude alternating voltage; - Simplicity. | - Low specificity; - Low S/N ratio. | [62,63] |
2. Features in IDEA
2.1. Electrical Double Layer (EDL)

2.2. Crucial Parameters of the Sensor
3. Micro and Nano IDEA
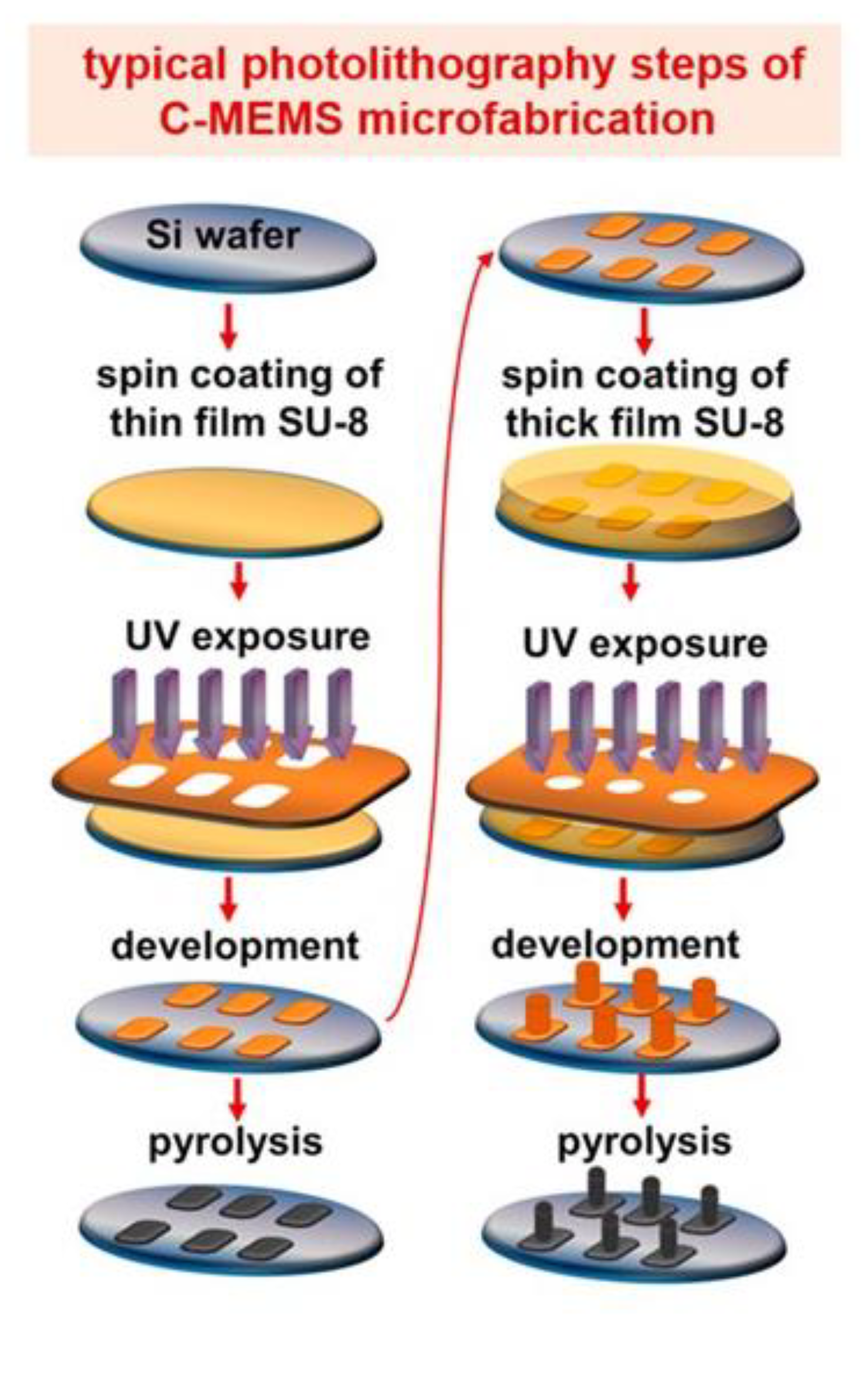
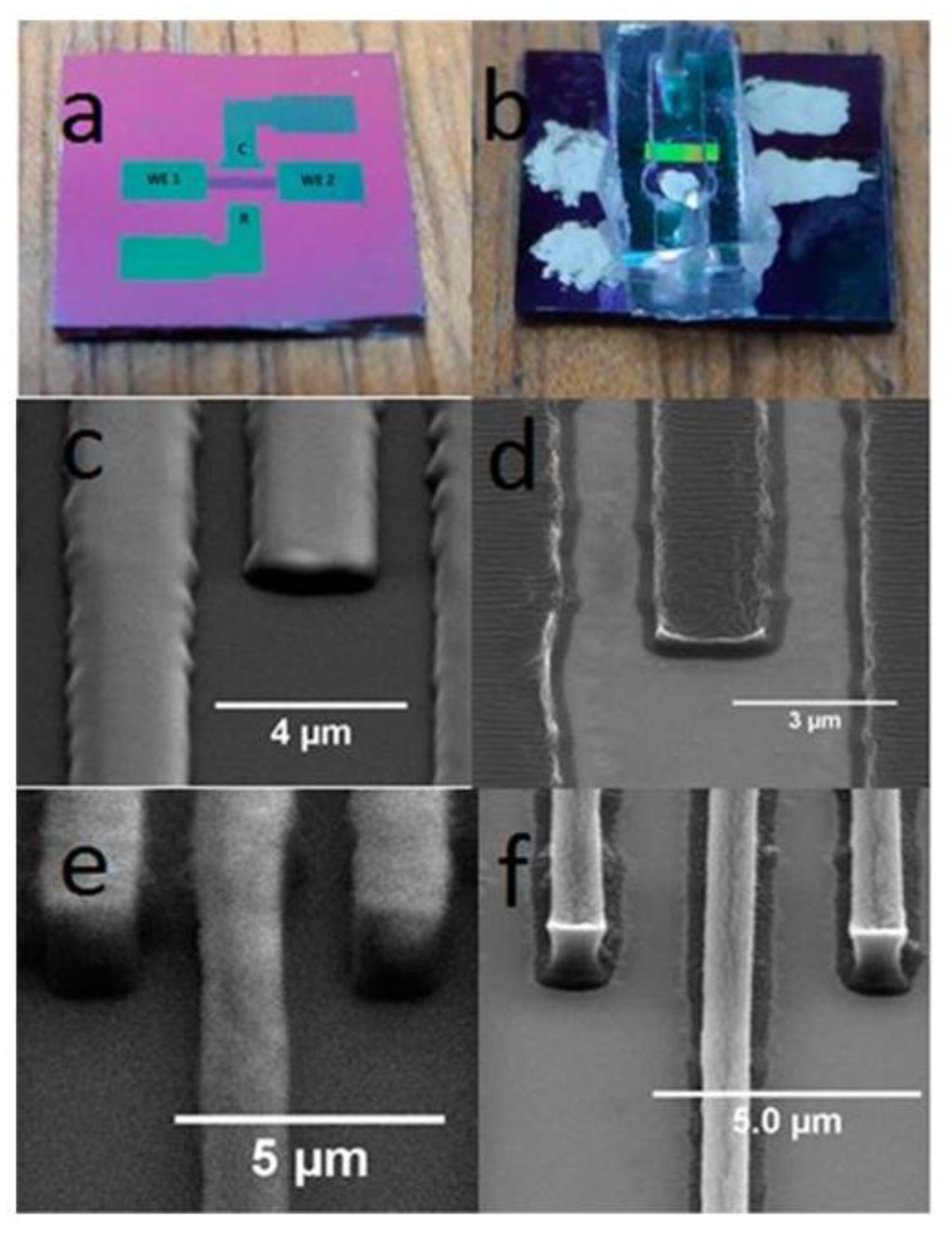
3.1. Substrate, Electrode Material, and Fabrication Techniques
3.1.1. Metal-Based IDEA
3.1.2. Carbon-Based IDEA
3.2. Method to Increase Surface Area in IDEA-Based Electrochemical Sensor
3.2.1. Microchannel Insertion
3.2.2. IDEA Geometry and Structures
3.2.3. Three-Dimensional (3D) IDEA in a Two-Electrode Configuration
4. Nanocomposites IDEA
4.1. Vertically Aligned Carbon Nanotube (VACNT)
4.2. Nanoparticles
4.3. Summary of IDEA-Based Electrochemical Sensors
5. Challenges and Future Directions
5.1. Applications of IDEA-Based Sensors
5.2. Electrode Material
5.3. Optimization of the Fabrication Process
5.4. Modification of Planar IDEAs to 3D IDEAs
5.5. Reproducibility and Commercialization of IDEAs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, D.W.G.; Dokmeci, M.; Demirci, U.; Khademhosseini, A. Clinical Applications of Micro- and Nanoscale Biosensors. In Biomedical Nanostructures; Gonsalves, K.E., Laurencin, C.L., Halberstadt, C.R., Nair, L.S., Eds.; John Wiley & Sons, Inc.: Toronto, ON, Canada, 2008. [Google Scholar]
- Kahn, K.; Plaxco, K.W. Principles of biomolecular recognition. In Recognition Receptors in Biosensors; Zourob, M., Ed.; Springer: New York, NY, 2010; pp. 3–45. [Google Scholar] [CrossRef]
- Lowe, C.R. Overview of Biosensor and Bioarray Technologies. In Handbook of Biosensors and Biochips; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An overview of foodborne pathogen detection: In the perspective of biosensors. Biotechnol. Adv. 2010, 28, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, S.; Teh, S.J.; Thong, K.L.; Thiha, A.; Dinshaw, I.J.; Lai, C.W.; Ibrahim, F.; Leo, B.F. Carbon Nanomaterial-Based Electrochemical Biosensors for Foodborne Bacterial Detection. Crit. Rev. Anal. Chem. 2019, 49, 510–533. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, A.; Malhotra, B.D. Mediated biosensors. Biosens. Biolelectron. 2002, 17, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Tothill, I.E. Biosensors and nanomaterials and their application for mycotoxin determination. World Mycotoxin J. 2011, 4, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Berrettoni, M.; Tonelli, D.; Conti, P.; Marassi, R.; Trevisani, M. Electrochemical sensor for indirect detection of bacterial population. Sens. Actuators B Chem. 2004, 102, 331–335. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, R.W. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Brosel-Oliu, S.; Ferreira, R.; Uria, N.; Abramova, N.; Gargallo, R.; Muñoz-Pascual, F.-X.; Bratov, A. Novel impedimetric aptasensor for label-free detection of Escherichia coli O157:H7. Sens. Actuators B Chem. 2018, 255, 2988–2995. [Google Scholar] [CrossRef]
- Brosel-Oliu, S.; Abramova, N.; Uria, N.; Bratov, A. Impedimetric transducers based on interdigitated electrode arrays for bacterial detection—A review. Anal. Chim. Acta 2019, 1088, 1–19. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Wang, S.; Jin, X.; Yang, L.; Liu, G.; Zhao, J. Highly selective detection of Escherichia coli O157:H7 based on micro-gapped interdigitated electrode arrays. Biotechnol. Biotechnol. Equip. 2017, 31, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Maalouf, R.; Fournier-Wirth, C.; Coste, J.; Chebib, H.; Saïkali, Y.; Vittori, O.; Errachid, A.; Cloarec, J.-P.; Martelet, C.; Jaffrezic-Renault, N. Label-Free Detection of Bacteria by Electrochemical Impedance Spectroscopy: Comparison to Surface Plasmon Resonance. Anal. Chem. 2007, 79, 4879–4886. [Google Scholar] [CrossRef]
- Abdalhai, M.H.; Fernandes, A.M.; Xia, X.; Musa, A.; Ji, J.; Sun, X. Electrochemical Genosensor To Detect Pathogenic Bacteria (Escherichia coli O157:H7) As Applied in Real Food Samples (Fresh Beef) To Improve Food Safety and Quality Control. J. Agric. Food Chem. 2015, 63, 5017–5025. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Voeroes, J.; Reimhult, E. Electrochemical biosensors—Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Matylitskaya, V.; Kasemann, S.; Urban, G.; Dincer, C.; Partel, S. Electrochemical Characterization of Nanogap Interdigitated Electrode Arrays for Lab-on-a-Chip Applications. J. Electrochem. Soc. 2018, 165, B127–B134. [Google Scholar] [CrossRef]
- Alayo, N.; Fernández-Sánchez, C.; Baldi, A.; Esquivel, J.P.; Borrisé, X.; Pérez-Murano, F. Gold interdigitated nanoelectrodes as a sensitive analytical tool for selective detection of electroactive species via redox cycling. Microchim. Acta 2016, 183, 1633–1639. [Google Scholar] [CrossRef]
- Ben Ali, M.; Korpan, Y.; Gonchar, M.; El’Skaya, A.; Maaref, M.; Jaffrezic-Renault, N.; Martelet, C. Formaldehyde assay by capacitance versus voltage and impedance measurements using bi-layer bio-recognition membrane. Biosens. Bioelectron. 2006, 22, 575–581. [Google Scholar] [CrossRef]
- Afsarimanesh, N.; Nag, A.; Alahi, M.E.E.; Han, T.; Mukhopadhyay, S. Interdigital sensors: Biomedical, environmental and industrial applications. Sens. Actuators A Phys. 2020, 305, 111923. [Google Scholar] [CrossRef]
- Sophocleous, M.; Atkinson, J.K. A review of screen-printed silver/silver chloride (Ag/AgCl) reference electrodes potentially suitable for environmental potentiometric sensors. Sens. Actuators A Phys. 2017, 267, 106–120. [Google Scholar] [CrossRef] [Green Version]
- Michalska, A.; Kisiel, A.; Maksymiuk, K. Screen-Printed Disposable Reference Electrodes. In Handbook of Reference Electrodes; Springer: Berlin, Heidelberg, Germany, 2013; pp. 325–330. [Google Scholar] [CrossRef]
- Khan, M.; Rahaman, R.; Khalilian, A.; Kang, S.-W. Fast, highly-sensitive, and wide-dynamic-range interdigitated capacitor glucose biosensor using solvatochromic dye-containing sensing membrane. Sensors 2016, 16, 265. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.H.; Pyun, J.-C.; Cho, S. Electrical impedance characterization of cell growth on interdigitated microelectrode array. J. Nanosci. Nanotechnol. 2014, 14, 8342–8346. [Google Scholar] [CrossRef] [PubMed]
- Banga, I.; Paul, A.; France, K.; Micklich, B.; Cardwell, B.; Micklich, C.; Prasad, S.E.C. Tech-electrochemical handheld breathalyzer COVID sensing technology. Sci. Rep. 2022, 12, 4370. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, N.F.; Tucker, R.C.; Wu, C. Electrical conductivity measurements using microfabricated interdigitated electrodes. Anal. Chem. 1993, 65, 1199–1202. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Morita, M. Electrochemical measurements with interdigitated array microelectrodes. Curr. Sep. 1995, 14, 2–8. [Google Scholar]
- Dizon, A.; Orazem, M.E. On the impedance response of interdigitated electrodes. Electrochim. Acta 2019, 327, 135000. [Google Scholar] [CrossRef]
- Barnes, E.O.; Lewis, G.E.M.; Dale, S.; Marken, F.; Compton, R.G. Generator-collector double electrode systems: A review. Analyst 2012, 137, 1068–1081. [Google Scholar] [CrossRef]
- Tomčík, P.; Bustin, D. Voltammetric determination of iodide by use of an interdigitated microelectrode array. Fresenius J. Anal. Chem. 2001, 371, 562–564. [Google Scholar] [CrossRef]
- Bustin, D.; Jursa, S.; Tomčík, P. Titrations with electrogenerated halogens in the diffusion layer of an interdigitated microelectrode array. Analyst 1996, 121, 1795–1799. [Google Scholar] [CrossRef]
- McAdams, E.; Lackermeier, A.; McLaughlin, J.; Macken, D.; Jossinet, J. The linear and non-linear electrical properties of the electrode-electrolyte interface. Biosens. Bioelectron. 1995, 10, 67–74. [Google Scholar] [CrossRef]
- Bandarenka, A.S. Exploring the interfaces between metal electrodes and aqueous electrolytes with electrochemical impedance spectroscopy. Analyst 2013, 138, 5540–5554. [Google Scholar] [CrossRef]
- Yun, J.; Kang, G.; Park, Y.; Kim, H.W.; Cha, J.-J.; Lee, J.-H. Electrochemical impedance spectroscopy with interdigitated electrodes at the end of hypodermic needle for depth profiling of biotissues. Sens. Actuators B Chem. 2016, 237, 984–991. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Lee, C.-K.; Lin, C.-T. Improving sensitivity of a miniaturized label-free electrochemical biosensor using zigzag electrodes. Biosens. Bioelectron. 2018, 103, 130–137. [Google Scholar] [CrossRef]
- Nadzirah, S.; Hashim, U. Interdigitated microelectrode geometry for simple electrical Escherichia coli O157:H7 DNA detection. Microelectron. Int. 2017, 34, 99–107. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Moura, P.A.R.; Zhenglong, L.; Feng, L.; Arokiam, S.; Yang, J.; Hariharan, M.; Basuray, S. Effect of electrode configuration on the sensitivity of nucleic acid detection in a non-planar, flow-through, porous interdigitated electrode. Biomicrofluidics 2019, 13, 064118. [Google Scholar] [CrossRef]
- Muaz, A.; Hashim, U.; Liu, W.-W.; Ibrahim, F.; Thong, K.; Mohktar, M.S. Fabrication of interdigitated electrodes (IDE’s) by conventional photolithography technique for pH measurement using micro-gap structure. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 8–10 December 2014; pp. 146–150. [Google Scholar] [CrossRef]
- Gondosiswanto, R.; Hibbert, D.B.; Fang, Y.; Zhao, C. Redox Recycling Amplification Using an Interdigitated Microelectrode Array for Ionic Liquid-Based Oxygen Sensors. Anal. Chem. 2018, 90, 3950–3957. [Google Scholar] [CrossRef]
- Bratov, A.; Ramón-Azcón, J.; Abramova, N.; Merlos, A.; Adrian, J.; Sánchez-Baeza, F.; Marco, M.-P.; Dominguez, C. Three-dimensional interdigitated electrode array as a transducer for label-free biosensors. Biosens. Bioelectron. 2008, 24, 729–735. [Google Scholar] [CrossRef]
- Lequin, R.M. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef]
- Niwa, O.; Morita, M.; Tabei, H. Fabrication and characteristics of vertically separated interdigitated array electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1989, 267, 291–297. [Google Scholar] [CrossRef]
- Horiuchi, T.; Niwa, O.; Morita, M.; Tabei, H. Quantitative analysis of the steady-state currents of reversible redox species at a microdisk array electrode embedded in a surface electrode. J. Electroanal. Chem. Interfacial Electrochem. 1990, 295, 25–40. [Google Scholar] [CrossRef]
- Morita, M.; Niwa, O.; Horiuchi, T. Interdigitated array microelectrodes as electrochemical sensors. Electrochim. Acta 1997, 42, 3177–3183. [Google Scholar] [CrossRef]
- Zafarani, H.R.; Mathwig, K.; Sudhölter, E.J.; Rassaei, L. Electrochemical redox cycling in a new nanogap sensor: Design and simulation. J. Electroanal. Chem. 2016, 760, 42–47. [Google Scholar] [CrossRef]
- Aoki, K.; Morita, M.; Niwa, O.; Tabei, H. Quantitative analysis of reversible diffusion-controlled currents of redox soluble species at interdigitated array electrodes under steady-state conditions. J. Electroanal. Chem. Interfacial Electrochem. 1988, 256, 269–282. [Google Scholar] [CrossRef]
- Bard, A.J.; Crayston, J.A.; Kittlesen, G.P.; Varco Shea, T.; Wrighton, M.S. Digital simulation of the measured electrochemical response of reversible redox couples at microelectrode arrays: Consequences arising from closely spaced ultramicroelectrodes. Anal. Chem. 1986, 58, 2321–2331. [Google Scholar] [CrossRef]
- Heo, J.-I.; Lim, Y.; Shin, H. The effect of channel height and electrode aspect ratio on redox cycling at carbon interdigitated array nanoelectrodes confined in a microchannel. Analyst 2013, 138, 6404–6411. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.I.; Shim, D.S.; Teixidor, G.T.; Oh, S.; Madou, M.J.; Shin, H. Carbon Interdigitated Array Nanoelectrodes for Electrochemical Applications. J. Electrochem. Soc. 2011, 158, J76–J80. [Google Scholar] [CrossRef]
- Sugime, H.; Ushiyama, T.; Nishimura, K.; Ohno, Y.; Noda, S. An interdigitated electrode with dense carbon nanotube forests on conductive supports for electrochemical biosensors. Analyst 2018, 143, 3635–3642. [Google Scholar] [CrossRef] [Green Version]
- Zevenbergen, M.A.G.; Wolfrum, B.L.; Goluch, E.D.; Singh, P.S.; Lemay, S.G. Fast Electron-Transfer Kinetics Probed in Nanofluidic Channels. J. Am. Chem. Soc. 2009, 131, 11471–11477. [Google Scholar] [CrossRef]
- Varshney, M.; Li, Y.; Srinivasan, B.; Tung, S.J.S. A label-free, microfluidics and interdigitated array microelectrode-based impedance biosensor in combination with nanoparticles immunoseparation for detection of Escherichia coli O157: H7 in food samples. Sens. Actuators B Chem. 2007, 128, 99–107. [Google Scholar] [CrossRef]
- Kamath, R.R.; Madou, M.J. Three-Dimensional Carbon Interdigitated Electrode Arrays for Redox-Amplification. Anal. Chem. 2014, 86, 2963–2971. [Google Scholar] [CrossRef]
- Madou, M. Fundamentals of Microfabrication: The Science of Miniaturization; CRC: Boca Raton, FL, USA, 2002. [Google Scholar]
- Madou, M.; Schmidt, G.; Song, X.; Kinoshita, K.; Fendorf, M.; Zettly, A.; White, R. Carbon micromachining (c-mems). In Proceedings of the Symposium on Chemical and Biological Sensors and Analytical Electrochemical Methods, Paris, France, 1 January 1997; The Electrochemical Society, Inc.: Pennington, NJ, USA; Volume 97, pp. 61–69. [Google Scholar]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical Immunosensors for Detection of Cancer Protein Biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef] [Green Version]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.; Fiore, V. Analytical problems in exposing amperometric enzyme biosensors to biological fluids. Sensors 2016, 16, 780. [Google Scholar]
- Sharma, N.K.; Nain, A.; Singh, K.; Rani, N.; Singal, A. Impedimetric Sensors: Principles, Applications and Recent Trends. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 2278–3075. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2014, 44, 248–262. [Google Scholar] [CrossRef]
- Jaffrezic-Renault, N.; Dzyadevych, S.V. Conductometric Microbiosensors for Environmental Monitoring. Sensors 2008, 8, 2569–2588. [Google Scholar] [CrossRef] [Green Version]
- Stradiotto, N.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Willander, M.; Tahira, A.; Ibupoto, Z. Potentiometric Biosensors Based on Metal Oxide Nanostructures. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Grahame, D.C. The Electrical Double Layer and the Theory of Electrocapillarity. Chem. Rev. 1947, 41, 441–501. [Google Scholar] [CrossRef]
- Smith, C.P.; White, H.S. Theory of the voltammetric response of electrodes of submicron dimensions. Violation of electroneutrality in the presence of excess supporting electrolyte. Anal. Chem. 1993, 65, 3343–3353. [Google Scholar] [CrossRef]
- He, R.; Chen, S.; Yang, A.F.; Wu, B. Dynamic Diffuse Double-Layer Model for the Electrochemistry of Nanometer-Sized Electrodes. J. Phys. Chem. B 2006, 110, 3262–3270. [Google Scholar] [CrossRef]
- Oldham, K.; Bond, A. How valid is the electroneutrality approximation in the theory of steady-state voltammetry? J. Electroanal. Chem. 2001, 508, 28–40. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G. Simulating the structure and effect of the electrical double layer at nanometre electrodes. Nanotechnology 2007, 18, 335201. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G. The effect of an electrical double layer on the voltammetric performance of nanoscale interdigitated electrodes: A simulation study. Nanotechnology 2008, 19, 465504. [Google Scholar] [CrossRef] [PubMed]
- Bearden, S.L. Manipulation of the Electrical Double Layer for Control and Sensing in a Solid State Nanopore; Clemson University: Clemson, SC, USA, 2015. [Google Scholar]
- Ngo, T.-T.; Shirzadfar, H.; Bourjilat, A.; Kourtiche, D.; Nadi, M. A method to determine the parameters of the double layer of a planar interdigital sensor. Int. J. Smart Sens. Intell. Syst. 2014, 7, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Fleischmann, M.; Pons, S.; Rolison, D.R. Ultramicroelectrodes; Datatech Systems: Birkenhead, UK, 1987. [Google Scholar]
- Wightman, R.M. Microvoltammetric electrodes. Anal. Chem. 1981, 53, 1125A–1134A. [Google Scholar] [CrossRef]
- Aoki, K.; Akimoto, K.; Tokuda, K.; Matsuda, H.; Osteryoung, J. Linear sweep voltammetry at very small stationary disk electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1984, 171, 219–230. [Google Scholar] [CrossRef]
- Wang, H.; Pilon, L. Accurate Simulations of Electric Double Layer Capacitance of Ultramicroelectrodes. J. Phys. Chem. C 2011, 115, 16711–16719. [Google Scholar] [CrossRef]
- Yasuga, H.; Shoji, K.; Koiwai, K.; Kawano, R. New Sensing Technologies: Microtas/NEMS/MEMS. In Encyclopedia of Sensors and Biosensors; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Li, D.; Batchelor-McAuley, C.; Chen, L.; Compton, R.G. Band Electrodes in Sensing Applications: Response Characteristics and Band Fabrication Methods. ACS Sens. 2019, 4, 2250–2266. [Google Scholar] [CrossRef]
- Niwa, O.; Morita, M.; Tabei, H. Electrochemical behavior of reversible redox species at interdigitated array electrodes with different geometries: Consideration of redox cycling and collection efficiency. Anal. Chem. 1990, 62, 447–452. [Google Scholar] [CrossRef]
- Huang, C.-W.; Lu, M.S.-C. Electrochemical Detection of the Neurotransmitter Dopamine by Nanoimprinted Interdigitated Electrodes and a CMOS Circuit With Enhanced Collection Efficiency. IEEE Sens. J. 2011, 11, 1826–1831. [Google Scholar] [CrossRef]
- Rassaei, L.; Mathwig, K.; Kang, S.; Heering, H.A.; Lemay, S.G. Integrated Biodetection in a Nanofluidic Device. ACS Nano 2014, 8, 8278–8284. [Google Scholar] [CrossRef]
- Bobade, S.; Kalorey, D.; Warke, S. Biosensor Devices: A review on their biological applications. Biosci. Biotechnol. Res. Commun. 2016, 9, 132–137. [Google Scholar] [CrossRef]
- Piñón, M.V.; Benítez, B.C.; Pramanick, B.; Perez-Gonzalez, V.H.; Madou, M.J.; Martinez-Chapa, S.O.; Hwang, H. Direct current-induced breakdown to enhance reproducibility and performance of carbon-based interdigitated electrode arrays for AC electroosmotic micropumps. Sens. Actuators A Phys. 2017, 262, 10–17. [Google Scholar] [CrossRef]
- Pramanick, B.; Vazquez-Pinon, M.; Torres-Castro, A.; Martinez-Chapaa, S.O.; Madou, M. Effect of pyrolysis process parameters on electrical, physical, chemical and electro-chemical properties of SU-8-derived carbon structures fabricated using the C-MEMS process. Mater. Today Proc. 2018, 5, 9669–9682. [Google Scholar] [CrossRef]
- Martinez-Duarte, R. SU-8 Photolithography as a Toolbox for Carbon MEMS. Micromachines 2014, 5, 766–782. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Peng, T.; Meng, Q.; Zhu, X.; Guo, L.; Yao, K.; Wang, Z.; Zheng, P.; Ren, Z.; He, Z.; et al. Rapid and ultrasensitive detection of Salmonella typhimurium using a novel impedance biosensor based on SiO2@MnO2 nanocomposites and interdigitated array microelectrodes. Sens. Actuators B Chem. 2020, 324, 128654. [Google Scholar] [CrossRef]
- Aliabadi, E.S. Carbon Interdigitated Electrode Arrays for Biosensing. Ph.D. Thesis, UC Irvine, Irvine, CA, USA, 2021. [Google Scholar]
- Zou, Z.; Kai, J.; Rust, M.J.; Han, J.; Ahn, C.H. Functionalized nano interdigitated electrodes arrays on polymer with integrated microfluidics for direct bio-affinity sensing using impedimetric measurement. Sens. Actuators A Phys. 2007, 136, 518–526. [Google Scholar] [CrossRef]
- Bhura, D.K. 3D Interdigitated Electrode Array (IDEA) Biosensor For Detection of Serum Biomarker. Master’s Thesis, Portland State University, Portland, OR, USA, 2011. [Google Scholar] [CrossRef]
- Webster, M.S.; Timoshkin, I.V.; Macgregor, S.J.; Mattey, M. Computer aided modelling of an interdigitated microelectrode array impedance biosensor for the detection of bacteria. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1356–1363. [Google Scholar] [CrossRef]
- Yuan, Q.; Yang, K.; Wu, J. Optimization of planar interdigitated microelectrode array for biofluid transport by AC electrothermal effect. Microfluid. Nanofluid. 2013, 16, 167–178. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Patella, B.; Daly, R.; Seymour, I.; Robinson, C.; Lovera, P.; Rohan, J.; Inguanta, R.; O’Riordan, A. A simulation and experimental study of electrochemical pH control at gold interdigitated electrode arrays. Electrochim. Acta 2021, 395, 139113. [Google Scholar] [CrossRef]
- MacKay, S.; Hermansen, P.; Wishart, D.; Hiebert, W.; Chen, J. Simulating electrical properties of interdigitated electrode designs for impedance-based biosensing applications. In Proceedings of the 2015 IEEE 28th Canadian Conference on Electrical and Computer Engineering (CCECE), Halifax, NS, Canada, 3–6 May 2015; pp. 370–375. [Google Scholar] [CrossRef]
- Lavacchi, A.; Bardi, U.; Borri, C.; Caporali, S.; Fossati, A.; Perissi, I. Cyclic voltammetry simulation at microelectrode arrays with COMSOL Multiphysics®. J. Appl. Electrochem. 2009, 39, 2159–2163. [Google Scholar] [CrossRef]
- Jun, L.Q.; bin Djaswadi, G.W.; bin Hawari, H.F.; Zakariya, M.A.B. Simulation of interdigitated electrodes (IDEs) geometry using COMSOL Multiphysics. In Proceedings of the 2018 International Conference on Intelligent and Advanced System (ICIAS), Kuala Lumpur, Malaysia, 13–14 August 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Deshpande, S.; Bhand, S.; Bacher, G. Investigation of the effect of metallization ratio and side shift on the interdigitated electrodes performance for biochemical sensing. J. Appl. Electrochem. 2021, 51, 893–904. [Google Scholar] [CrossRef]
- Lvovich, V.F.; Liu, C.; Smiechowski, M.F. Optimization and fabrication of planar interdigitated impedance sensors for highly resistive non-aqueous industrial fluids. Sens. Actuators B Chem. 2006, 119, 490–496. [Google Scholar] [CrossRef]
- Madou, M.J.; Perez-Gonzalez, V.H.; Pramanick, B. Carbon: The Next Silicon?: Book 2—Applications; Momentum Press: New York, NY, USA, 2016. [Google Scholar]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Upadhyayula, V.K. Functionalized gold nanoparticle supported sensory mechanisms applied in detection of chemical and biological threat agents: A review. Anal. Chim. Acta 2012, 715, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [Green Version]
- Rishi, M.; Amreen, K.; Gohel, K.; Javed, A.; Dubey, S.K.; Goel, S. Three Different Rapidly Prototyped Polymeric Substrates with Interdigitated Electrodes for Escherichia coli Sensing: A Comparative Study. IEEE Trans. NanoBiosci. 2022. [Google Scholar] [CrossRef]
- Daly, R.; Narayan, T.; Shao, H.; O’Riordan, A.; Lovera, P. Platinum-Based Interdigitated Micro-Electrode Arrays for Reagent-Free Detection of Copper. Sensors 2021, 21, 3544. [Google Scholar] [CrossRef]
- Hwang, C.; Park, N.; Kim, E.S.; Kim, M.; Kim, S.D.; Park, S.; Kim, N.Y.; Kim, J.H. Ultra-fast and recyclable DNA biosensor for point-of-care detection of SARS-CoV-2 (COVID-19). Biosens. Bioelectron. 2021, 185, 113177. [Google Scholar] [CrossRef]
- Ferreira, P.A.; Araujo, M.C.; Prado, C.M.; de Lima, R.A.; Rodríguez, B.A.; Dutra, R.F. An ultrasensitive Cystatin C renal failure immunosensor based on a PPy/CNT electrochemical capacitor grafted on interdigitated electrode. Colloids Surf. B Biointerfaces 2020, 189, 110834. [Google Scholar] [CrossRef]
- Oh, C.; Yang, L.; Park, B.; Bohn, P. Interdigitated Electrode Arrays for Electrochemical Immunosensing of Interleukin-6 in Cerebrospinal Fluid (CSF) and Serum. ECS Meet. Abstr. 2021, MA2021-01, 1380. [Google Scholar] [CrossRef]
- Wang, C.; Zaouk, R.; Park, B.Y.; Madou, M.J. Carbon as a MEMS material: Micro and nanofabrication of pyrolysed photoresist carbon. Int. J. Manuf. Technol. Manag. 2008, 13, 360. [Google Scholar] [CrossRef]
- Shaw, J.M.; Gelorme, J.D.; Labianca, N.C.; Conley, W.E.; Holmes, S.J. Negative photoresists for optical lithography. IBM J. Res. Dev. 1997, 41, 81–94. [Google Scholar] [CrossRef]
- Sanders, D.P. Advances in Patterning Materials for 193 nm Immersion Lithography. Chem. Rev. 2010, 110, 321–360. [Google Scholar] [CrossRef]
- Ueno, K.; Hayashida, M.; Ye, J.-Y.; Misawa, H. Fabrication and electrochemical characterization of interdigitated nanoelectrode arrays. Electrochem. Commun. 2005, 7, 161–165. [Google Scholar] [CrossRef]
- Moreau, W.M. Semiconductor Lithography: Principles, Practices, and Materials; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Elliott, D.J. Integrated Circuit Fabrication Technology; McGraw-Hill: New York, NY, USA, 1982. [Google Scholar]
- Wang, C.; Jia, G.; Taherabadi, L.; Madou, M. A novel method for the fabrication of high-aspect ratio C-MEMS structures. J. Microelectromech. Syst. 2005, 14, 348–358. [Google Scholar] [CrossRef]
- Forouzanfar, S.; Alam, F.; Pala, N.; Wang, C. Highly sensitive label-free electrochemical aptasensors based on photoresist derived carbon for cancer biomarker detection. Biosens. Bioelectron. 2020, 170, 112598. [Google Scholar] [CrossRef]
- Vomero, M.; Zucchini, E.; Delfino, E.; Gueli, C.; Mondragon, N.C.; Carli, S.; Fadiga, L.; Stieglitz, T. Glassy Carbon Electrocorticography Electrodes on Ultra-Thin and Finger-Like Polyimide Substrate: Performance Evaluation Based on Different Electrode Diameters. Materials 2018, 11, 2486. [Google Scholar] [CrossRef] [Green Version]
- Quang, L.N.; Larsen, P.E.; Boisen, A.; Keller, S.S. Tailoring stress in pyrolytic carbon for fabrication of nanomechanical string resonators. Carbon 2018, 133, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Song, X.; Kinoshita, K.; Madou, M.; White, R. Electrochemical Studies of Carbon Films from Pyrolyzed Photoresist. J. Electrochem. Soc. 1998, 145, 2314–2319. [Google Scholar] [CrossRef]
- Forouzanfar, S.; Pala, N.; Madou, M.; Wang, C. Perspectives on C-MEMS and C-NEMS biotech applications. Biosens. Bioelectron. 2021, 180, 113119. [Google Scholar] [CrossRef]
- Liu, F.; Kolesov, G.; Parkinson, B.A. Preparation, Applications, and Digital Simulation of Carbon Interdigitated Array Electrodes. Anal. Chem. 2014, 86, 7391–7398. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Divan, R.; Parkinson, B.A. Fabrication of Carbon-Platinum Interdigitated Array Electrodes and Their Application for Investigating Homogeneous Hydrogen Evolution Catalysis. J. Electrochem. Soc. 2015, 162, H645–H650. [Google Scholar] [CrossRef] [Green Version]
- Ueno, K.; Kim, H.-B.; Kitamura, N. Characteristic Electrochemical Responses of Polymer Microchannel−Microelectrode Chips. Anal. Chem. 2003, 75, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Maruyama, K.; Sobue, K.; Ohya, S.; Niwa, O.; Suzuki, K. Electrochemical Behavior of Parallel Opposed Dual Electrode in a Microchannel. Electroanalysis 2004, 16, 2035–2041. [Google Scholar] [CrossRef]
- Mathew, D.G.; Beekman, P.; Lemay, S.G.; Zuilhof, H.; Le Gac, S.; Van Der Wiel, W.G. Electrochemical Detection of Tumor-Derived Extracellular Vesicles on Nanointerdigitated Electrodes. Nano Lett. 2020, 20, 820–828. [Google Scholar] [CrossRef]
- Kamath, R.; Madou, M.J. Selective Detection of Dopamine against Ascorbic Acid Interference Using 3D Carbon Interdigitated Electrode Arrays. ECS Trans. 2014, 61, 65. [Google Scholar] [CrossRef]
- Rahimi, M.; Mikkelsen, S.R. Cyclic Biamperometry at Micro-Interdigitated Electrodes. Anal. Chem. 2011, 83, 7555–7559. [Google Scholar] [CrossRef]
- Varshney, M.; Li, Y. Interdigitated array microelectrodes based impedance biosensors for detection of bacterial cells. Biosens. Bioelectron. 2009, 24, 2951–2960. [Google Scholar] [CrossRef]
- Min, J.; Baeumner, A.J. Characterization and Optimization of Interdigitated Ultramicroelectrode Arrays as Electrochemical Biosensor Transducers. Electroanalysis 2004, 16, 724–729. [Google Scholar] [CrossRef]
- Amato, L.; Keller, S.S.; Heiskanen, A.; Dimaki, M.; Emnéus, J.; Boisen, A.; Tenje, M. Fabrication of high-aspect ratio SU-8 micropillar arrays. Microelectron. Eng. 2012, 98, 483–487. [Google Scholar] [CrossRef]
- Amato, L.; Heiskanen, A.; Hansen, R.; Gammelgaard, L.; Rindzevičius, T.; Tenje, M.; Emnéus, J.; Keller, S.S. Dense high-aspect ratio 3D carbon pillars on interdigitated microelectrode arrays. Carbon 2015, 94, 792–803. [Google Scholar] [CrossRef]
- Han, M.; Lee, W.; Lee, S.-K.; Lee, S.S. Microfabrication of 3D oblique structures by inclined UV lithography. In Micro Total Analysis Systems 2002; Springer: Dordrecht, The Netherlands, 2002; pp. 106–108. [Google Scholar] [CrossRef]
- Han, M.; Lee, W.; Lee, S.-K.; Lee, S.S. 3D microfabrication with inclined/rotated UV lithography. Sens. Actuators A Phys. 2004, 111, 14–20. [Google Scholar] [CrossRef]
- Lee, J.B.; Choi, K.-H.; Yoo, K. Innovative SU-8 Lithography Techniques and Their Applications. Micromachines 2014, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Del Campo, A.; Greiner, C. SU-8: A photoresist for high-aspect-ratio and 3D submicron lithography. J. Micromech. Microeng. 2007, 17, R81–R95. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, H.; Despont, M.; Fahrni, N.; Brugger, J.; Vettiger, P.; Renaud, P. High-aspect-ratio, ultrathick, negative-tone near-UV photoresist and its applications for MEMS. Sens. Actuators A Phys. 1998, 64, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, E.N. Single walled and multi walled carbon nanotube structure, synthesis and applications. Int. J. Innov. Technol. Explor. Eng. 2013, 2, 311–320. [Google Scholar]
- Brownlee, B.J.; Claussen, J.C.; Iverson, B.D. 3D Interdigitated Vertically Aligned Carbon Nanotube Electrodes for Electrochemical Impedimetric Biosensing. ACS Appl. Nano Mater. 2020, 3, 10166–10175. [Google Scholar] [CrossRef]
- Lewis, P.M.; Sheridan, L.B.; Gawley, R.E.; Fritsch, I. Signal Amplification in a Microchannel from Redox Cycling with Varied Electroactive Configurations of an Individually Addressable Microband Electrode Array. Anal. Chem. 2010, 82, 1659–1668. [Google Scholar] [CrossRef] [Green Version]
- Hong, F.; Cao, J.; Cheng, P. A parametric study of AC electrothermal flow in microchannels with asymmetrical interdigitated electrodes. Int. Commun. Heat Mass Transf. 2011, 38, 275–279. [Google Scholar] [CrossRef]
- Sharma, D.; Lee, J.; Shin, H. An electrochemical immunosensor based on a 3D carbon system consisting of a suspended mesh and substrate-bound interdigitated array nanoelectrodes for sensitive cardiac biomarker detection. Biosens. Bioelectron. 2018, 107, 10–16. [Google Scholar] [CrossRef]
- Bratov, A.; Abramova, N.; Marco, M.P.; Sanchez-Baeza, F. Three-Dimensional Interdigitated Electrode Array as a Tool for Surface Reactions Registration. Electroanalysis 2012, 24, 69–75. [Google Scholar] [CrossRef]
- Han, D.; Kim, Y.-R.; Kang, C.M.; Chung, T.D. Electrochemical Signal Amplification for Immunosensor Based on 3D Interdigitated Array Electrodes. Anal. Chem. 2014, 86, 5991–5998. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.M.; Jenkins, A.; Kawamura, K. Polymeric Carbons: Carbon Fibre, Glass and Char; Cambridge University Press: Cambridge, UK, 1976. [Google Scholar]
- Bose, A.; Sengupta, S. Fabrication and characterization of pillar interdigitated electrode for blood glucose sensing. Sens. Rev. 2021, 41, 200–207. [Google Scholar] [CrossRef]
- Thivina, V.; Hashim, U.; Arshad, M.M.; Ruslinda, A.; Ayoib, A.; Nordin, N. Design and fabrication of Interdigitated Electrode (IDE) for detection of Ganoderma boninense. In Proceedings of the 2016 IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 17–19 August 2016; pp. 50–53. [Google Scholar]
- Mantis, I.; Hemanth, S.; Caviglia, C.; Heiskanen, A.; Keller, S.S. Suspended highly 3D interdigitated carbon microelectrodes. Carbon 2021, 179, 579–589. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.; Rho, J.; Jang, W.; Han, S.H.; Chung, T.D. 3D interdigitated electrode array in the microchannel free of reference and counter electrodes. Biosens. Bioelectron. 2018, 101, 317–321. [Google Scholar] [CrossRef]
- Brownlee, B.J.; Marr, K.M.; Claussen, J.C.; Iverson, B.D. Improving sensitivity of electrochemical sensors with convective transport in free-standing, carbon nanotube structures. Sens. Actuators B Chem. 2017, 246, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Marr, K.M.; Chen, B.; Mootz, E.J.; Geder, J.; Pruessner, M.; Melde, B.J.; Vanfleet, R.R.; Medintz, I.L.; Iverson, B.D.; Claussen, J.C. High aspect ratio carbon nanotube membranes decorated with Pt nanoparticle urchins for micro underwater vehicle propulsion via H2O2 decomposition. ACS Nano 2015, 9, 7791–7803. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-J.; Anandan, V.; Zhang, G. Electrochemical fabrication and evaluation of highly sensitive nanorod-modified electrodes for a biotin/avidin system. Biosens. Bioelectron. 2008, 23, 1117–1124. [Google Scholar] [CrossRef]
- Buzid, A.; Hayes, P.E.; Glennon, J.D.; Luong, J.H. Captavidin as a regenerable biorecognition element on boron-doped diamond for biotin sensing. Anal. Chim. Acta 2019, 1059, 42–48. [Google Scholar] [CrossRef]
- Ding, S.; Das, S.R.; Brownlee, B.J.; Parate, K.; Davis, T.M.; Stromberg, L.R.; Chan, E.K.; Katz, J.; Iverson, B.D.; Claussen, J.C. CIP2A immunosensor comprised of vertically-aligned carbon nanotube interdigitated electrodes towards point-of-care oral cancer screening. Biosens. Bioelectron. 2018, 117, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Ohno, R.; Ohnuki, H.; Wang, H.; Yokoyama, T.; Endo, H.; Tsuya, D.; Izumi, M. Electrochemical impedance spectroscopy biosensor with interdigitated electrode for detection of human immunoglobulin A. Biosens. Bioelectron. 2013, 40, 422–426. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Huerta, F.; Montilla, F.; Morallón, E. Study on electroactive and electrocatalytic surfaces of single walled carbon nanotube-modified electrodes. Electrochim. Acta 2011, 56, 2464–2470. [Google Scholar] [CrossRef]
- Laureyn, W.; Van Gerwen, P.; Suls, J.; Jacobs, P.; Maes, G. Characterization of Nanoscaled Interdigitated Palladium Electrodes of Various Dimensions in KCl Solutions. Electroanalysis 2001, 13, 204–211. [Google Scholar] [CrossRef]
- Banks, C.E.; Moore, R.R.; Davies, T.J.; Compton, R.G. Investigation of modified basal plane pyrolytic graphite electrodes: Definitive evidence for the electrocatalytic properties of the ends of carbon nanotubes. Chem. Commun. 2004, 1804–1805. [Google Scholar] [CrossRef]
- Banks, C.E.; Davies, T.J.; Wildgoose, G.G.; Compton, R.G. Electrocatalysis at Graphite and Carbon Nanotube Modified Electrodes: Edge-Plane Sites and Tube Ends Are the Reactive Sites. ChemInform 2005, 36, 241–829. [Google Scholar] [CrossRef]
- 157. Dahlan, N.A.; Thiha, A.; Ibrahim, F.; Milić, L.; Muniandy, S.; Jamaluddin, N.F.; Petrović, B.; Kojić, S.; Stojanović, G. Role of Nanomaterials in the Fabrication of bioNEMS/MEMS for Biomedical Applications and towards Pioneering Food Waste Utilisation. Nanomaterials 2022, 12, 4025. [Google Scholar] [CrossRef]
- Hasan, S. A review on nanoparticles: Their synthesis and types. Res. J. Recent Sci. 2015, 2277, 2502. [Google Scholar]
- Tiwari, D.K.; Behari, J.; Sen, P. Application of nanoparticles in waste water treatment 1. World Appl. Sci. J. 2008, 3, 417–433. [Google Scholar]
- Ealia, S.A.M.; Saravanakumar, M. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Mir, N. Synthesis and characterization of metallic copper nanoparticles via thermal decomposition. Polyhedron 2008, 27, 3514–3518. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Aval, S.F.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.-V.; Ngo, N.M.; Medhi, R.; Srinoi, P.; Liu, T.; Rittikulsittichai, S.; Lee, T.R. Multifunctional Iron Oxide Magnetic Nanoparticles for Biomedical Applications: A Review. Materials 2022, 15, 503. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, B.; Kumar, R.; Chhabra, D.; Ghosh, M.; Manuja, M.; Brar, B.; Pal, Y.; Tripathi, B.; Prasad, M. Metal/metal oxide nanoparticles: Toxicity concerns associated with their physical state and remediation for biomedical applications. Toxicol. Rep. 2021, 8, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Waris, A.; Din, M.; Ali, A.; Afridi, S.; Baset, A.; Khan, A.U.; Ali, M. Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review. Open Life Sci. 2021, 16, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.R.; Nayak, V.; Singh, J.; Singh, A.K.; Singh, R.P. Potentialities of bioinspired metal and metal oxide nanoparticles in biomedical sciences. RSC Adv. 2021, 11, 24722–24746. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef]
- Sharma, D.; Lee, J.; Seo, J.; Shin, H. Development of a Sensitive Electrochemical Enzymatic Reaction-Based Cholesterol Biosensor Using Nano-Sized Carbon Interdigitated Electrodes Decorated with Gold Nanoparticles. Sensors 2017, 17, 2128. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Aldeen, T.S.; Mohamed, H.E.A.; Maaza, M. ZnO nanoparticles prepared via a green synthesis approach: Physical properties, photocatalytic and antibacterial activity. J. Phys. Chem. Solids 2022, 160, 110313. [Google Scholar] [CrossRef]
- Lingaraju, K.; Basavaraj, R.B.; Jayanna, K.; Bhavana, S.; Devaraja, S.; Swamy, H.M.K.; Nagaraju, G.; Nagabhushana, H.; Naika, H.R. Biocompatible fabrication of TiO2 nanoparticles: Antimicrobial, anticoagulant, antiplatelet, direct hemolytic and cytotoxicity properties. Inorg. Chem. Commun. 2021, 127, 108505. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Gao, J. Biocompatible Iron Oxide Nanoparticles for Targeted Cancer Gene Therapy: A Review. Nanomaterials 2022, 12, 3323. [Google Scholar] [CrossRef]
- Stanicki, D.; Vangijzegem, T.; Ternad, I.; Laurent, S. An update on the applications and characteristics of magnetic iron oxide nanoparticles for drug delivery. Expert Opin. Drug Deliv. 2022, 19, 321–335. [Google Scholar] [CrossRef]
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.; Lee, Y.-W.; Nagaraj, H.; Rotello, V.M. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020, 156, 188–213. [Google Scholar] [CrossRef]
- Tong, S.; Zhu, H.; Bao, G. Magnetic iron oxide nanoparticles for disease detection and therapy. Mater. Today 2019, 31, 86–99. [Google Scholar] [CrossRef]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechniol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef]
- Baudler, A.; Schmidt, I.; Langner, M.; Greiner, A.; Schröder, U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef] [Green Version]
- Saxena, U.; Das, A.B. Nanomaterials towards fabrication of cholesterol biosensors: Key roles and design approaches. Biosens. Bioelectron. 2016, 75, 196–205. [Google Scholar] [CrossRef]
- Wiederoder, M.S.; Misri, I.; DeVoe, D.L. Impedimetric immunosensing in a porous volumetric microfluidic detector. Sens. Actuators B Chem. 2016, 234, 493–497. [Google Scholar] [CrossRef] [Green Version]
- Taton, T.A.; Mirkin, C.A.; Letsinger, R.L. Scanometric DNA array detection with nanoparticle probes. Science 2000, 289, 1757–1760. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Feng, Z.-Y.; Li, D.; Jin, B.; Lan, Y.; Meng, L.-Y. Recent trends in carbon-based microelectrodes as electrochemical sensors for neurotransmitter detection: A review. TrAC Trends Anal. Chem. 2022, 148, 116541. [Google Scholar] [CrossRef]
- Kucherenko, I.; Kucherenko, D.Y.; Soldatkin, O.; Lagarde, F.; Dzyadevych, S.; Soldatkin, A. A novel conductometric biosensor based on hexokinase for determination of adenosine triphosphate. Talanta 2016, 150, 469–475. [Google Scholar] [CrossRef]
- Costas, A.; Florica, C.; Preda, N.; Besleaga, C.; Kuncser, A.; Enculescu, I. Self-connected CuO–ZnO radial core–shell heterojunction nanowire arrays grown on interdigitated electrodes for visible-light photodetectors. Sci. Rep. 2022, 12, 6834. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nam, K.; Muhammad, W.; Shin, D.; Seo, S.; Kim, S.-D. Influence of N2O plasma treatment on PET-based flexible bending sensors with ZnO nanorod array cross-linked with interdigitated electrode structures. Ceram. Int. 2022, 48, 25696–25704. [Google Scholar] [CrossRef]
- Liu, F.; Tao, K.; Peiqi, D.; Shi, J. Photoelectrochemical oxygen evolution with interdigitated array electrodes: The example of TiO2. Nanotechnology 2022, 33, 325701. [Google Scholar] [CrossRef] [PubMed]
- Vahidpour, F.; Alghazali, Y.; Akca, S.; Hommes, G.; Schöning, M.J. An Enzyme-Based Interdigitated Electrode-Type Biosensor for Detecting Low Concentrations of H2O2 Vapor/Aerosol. Chemosensors 2022, 10, 202. [Google Scholar] [CrossRef]
- Brosel-Oliu, S.; Chacón-Aparicio, S.; Ezenarro, J.J.; Abramova, N.; Uría, N.; Bratov, A. 3D Impedimetric Biosensor for Cyanobacteria Detection in Natural Water Sources. Chemosensors 2021, 10, 7. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.; Ismail, Z.H.; Arshad, M.M.; Poopalan, P. Aptasensing nucleocapsid protein on nanodiamond assembled gold interdigitated electrodes for impedimetric SARS-CoV-2 infectious disease assessment. Biosens. Bioelectron. 2022, 197, 113735. [Google Scholar] [CrossRef]
- Srinivasan, K.P.; Muthuramalingam, T. Fabrication and Performance Evolution of AgNP Interdigitated Electrode Touch Sensor for Automotive Infotainment. Sensors 2021, 21, 7961. [Google Scholar] [CrossRef]
- Bibi, A.; Rubio, Y.; Santiago, K.; Jia, H.-W.; Ahmed, M.; Lin, Y.-F.; Yeh, J.-M. H2S-Sensing Studies Using Interdigitated Electrode with Spin-Coated Carbon Aerogel-Polyaniline Composites. Polymers 2021, 13, 1457. [Google Scholar] [CrossRef]
- Wang, Y.; Du, H.; Xiao, D.; Zhang, Y.; Hu, F.; Sun, L. On-chip integration of bulk micromachined three-dimensional Si/C/CNT@ TiC micro-supercapacitors for alternating current line filtering. RSC Adv. 2022, 12, 2048–2056. [Google Scholar] [CrossRef]
- al Rumon, M.A.; Shahariar, H. Fabrication of interdigitated capacitor on fabric as tactile sensor. Sens. Int. 2021, 2, 100086. [Google Scholar] [CrossRef]
- Tabei, H.; Morita, M.; Niwa, O.; Horiuchi, T. Fabrication and electrochemical features of new carbon based interdigitated array microelectrodes. J. Electroanal. Chem. 1992, 334, 25–33. [Google Scholar] [CrossRef]
- Huang, X.-J.; O’Mahony, A.M.; Compton, R.G. Microelectrode Arrays for Electrochemistry: Approaches to Fabrication. Small 2009, 5, 776–788. [Google Scholar] [CrossRef]
- Fiaccabrino, G.; Tang, X.-M.; Skinner, N.; De Rooij, N.; Koudelka-Hep, M. Electrochemical characterization of thin-film carbon interdigitated electrode arrays. Anal. Chim. Acta 1996, 326, 155–161. [Google Scholar] [CrossRef]
- Niwa, O.; Horiuchi, T.; Tabei, H. Electrochemical properties of carbon based interdigitated microarray electrodes fabricated by the pyrolysis of electrochemically prepared conducting films. J. Electroanal. Chem. 1994, 367, 265–269. [Google Scholar] [CrossRef]
- Mamishev, A.; Sundara-Rajan, K.; Yang, F.; Du, Y.; Zahn, M. Interdigital sensors and transducers. Proc. IEEE 2004, 92, 808–845. [Google Scholar] [CrossRef] [Green Version]
- Rassaei, L.; Singh, P.S.; Lemay, S.G. Lithography-Based Nanoelectrochemistry. Anal. Chem. 2011, 83, 3974–3980. [Google Scholar] [CrossRef]
- Hassan, Y.; Caviglia, C.; Hemanth, S.; Mackenzie, D.; Alstrøm, T.; Petersen, D.; Keller, S. High temperature SU-8 pyrolysis for fabrication of carbon electrodes. J. Anal. Appl. Pyrolysis 2017, 125, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Hassan, Y.M.; Caviglia, C.; Hemanth, S.; Mackenzie, D.M.A.; Petersen, D.H.; Keller, S.S. Pyrolytic carbon microelectrodes for impedance based cell sensing. ECS Trans. 2016, 72, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Pu, Q.; Yun, J.; Temkin, A.H.; Liu, S. Ion-Enrichment and Ion-Depletion Effect of Nanochannel Structures. Nano Lett. 2004, 4, 1099–1103. [Google Scholar] [CrossRef]
- Stein, D.; Kruithof, M.; Dekker, C. Surface-Charge-Governed Ion Transport in Nanofluidic Channels. Phys. Rev. Lett. 2004, 93, 035901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Lim, Y.; Lee, Y.; Shin, H. Glucose sensor based on redox-cycling between selectively modified and unmodified combs of carbon interdigitated array nanoelectrodes. Anal. Chim. Acta 2015, 889, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Uda, M.N.A.; Gopinath, S.C.B.; Hashim, U.; Hakimi, A.; Anuar, A.; A A Bakar, M.; Sulaiman, M.K.; A Parmin, N. Harumanis Mango: Perspectives in Disease Management and Advancement using Interdigitated Electrodes (IDE) Nano-Biosensor. IOP Conf. Ser. Mater. Sci. Eng. 2020, 864, 012180. [Google Scholar] [CrossRef]
- Li, W.; Liu, G.; Jiang, D.; Wang, C.; Li, W.; Guo, T.; Zhao, J.; Xi, F.; Liu, W.; Zhang, C. Interdigitated Electrode-Based Triboelectric Sliding Sensor for Security Monitoring. Adv. Mater. Technol. 2018, 3, 1800189. [Google Scholar] [CrossRef]
- Kanade, P.P.; Oyunbaatar, N.-E.; Lee, D.-W. Polymer-Based Functional Cantilevers Integrated with Interdigitated Electrode Arrays—A Novel Platform for Cardiac Sensing. Micromachines 2020, 11, 450. [Google Scholar] [CrossRef]
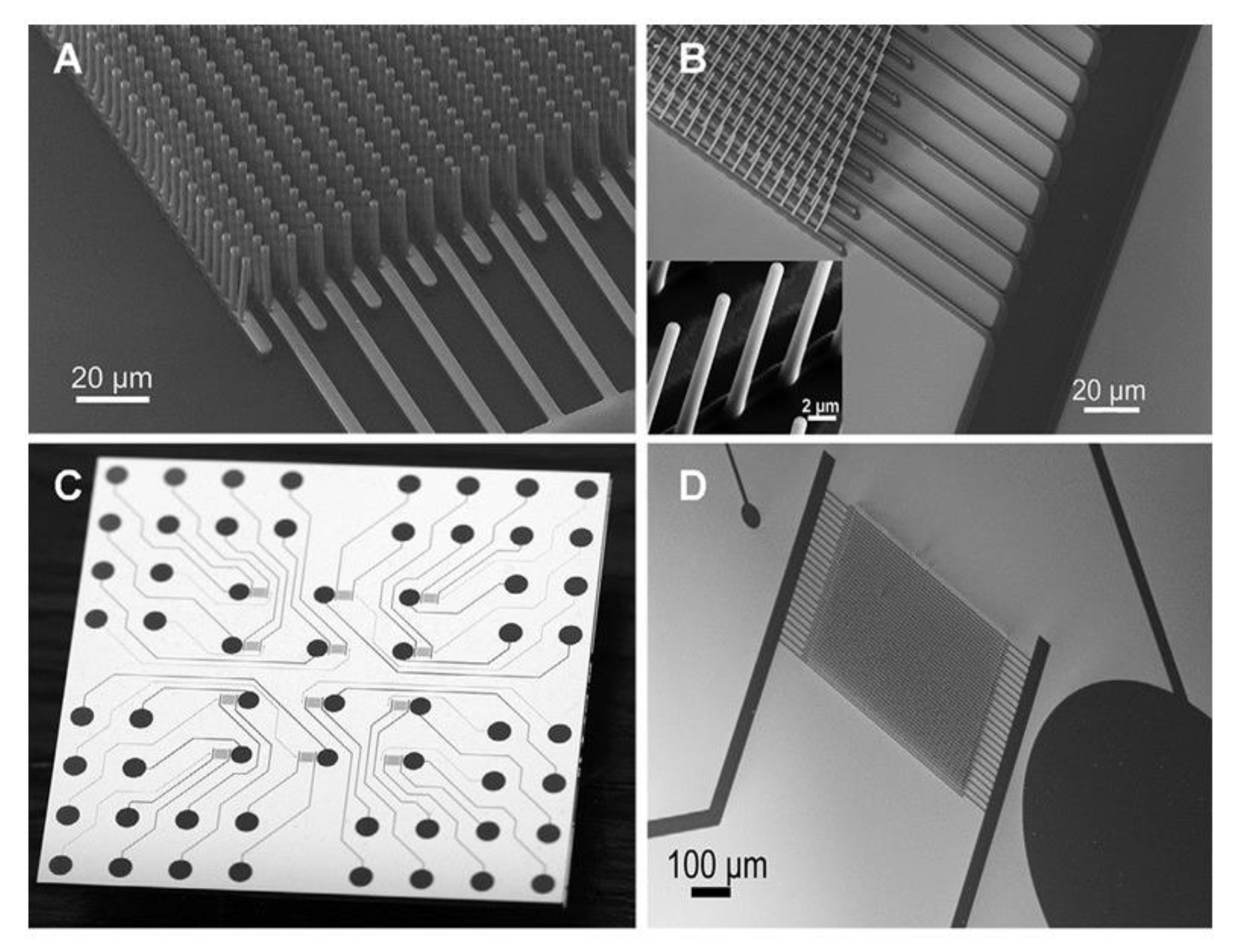
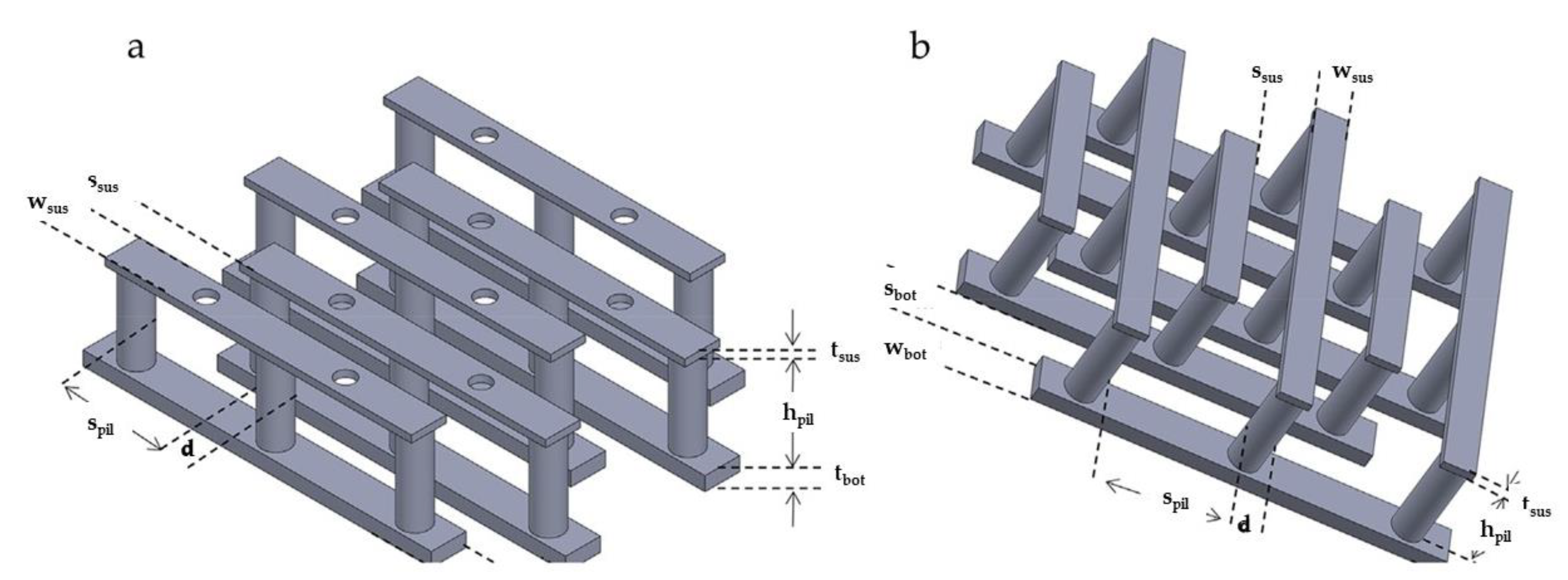
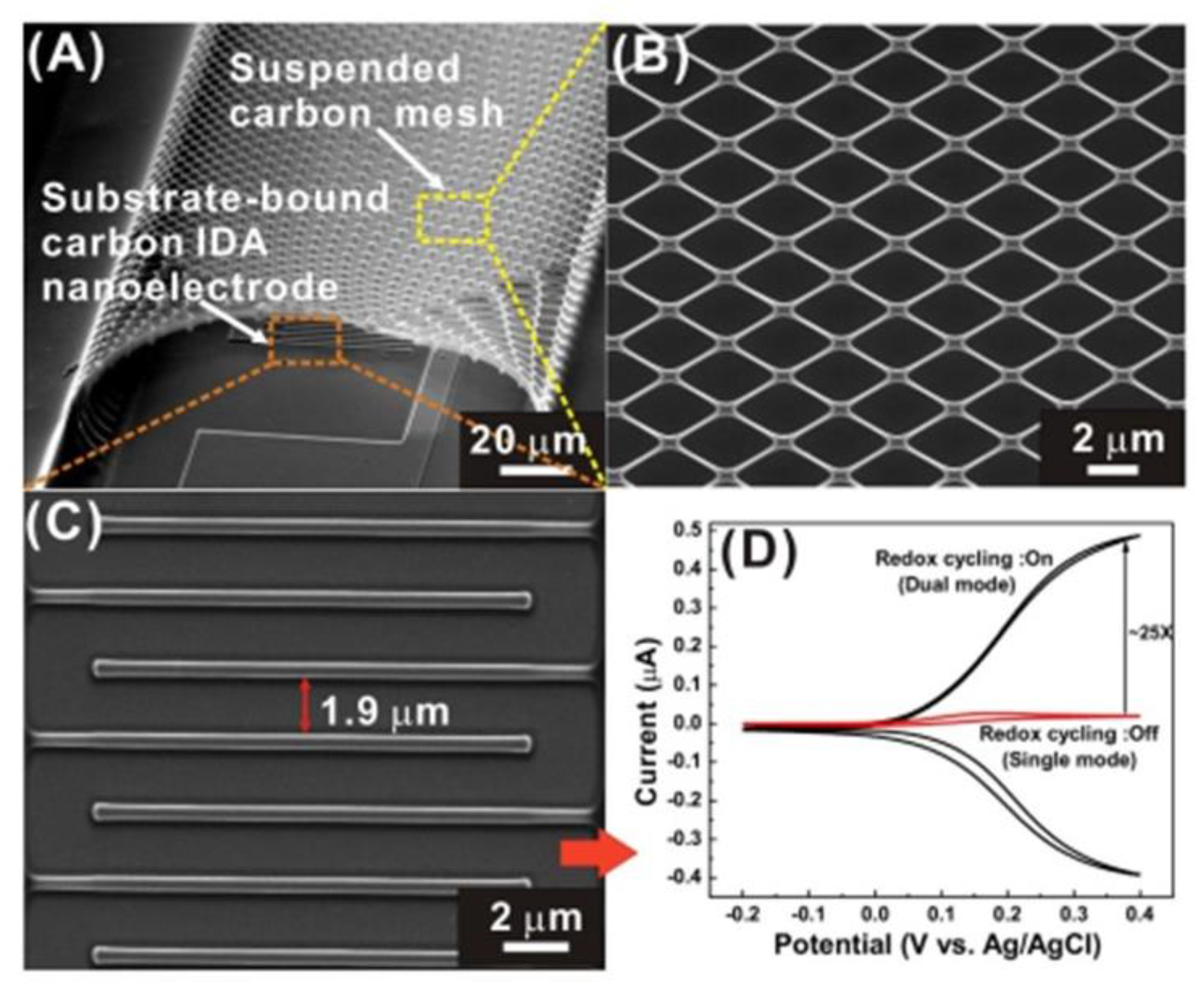
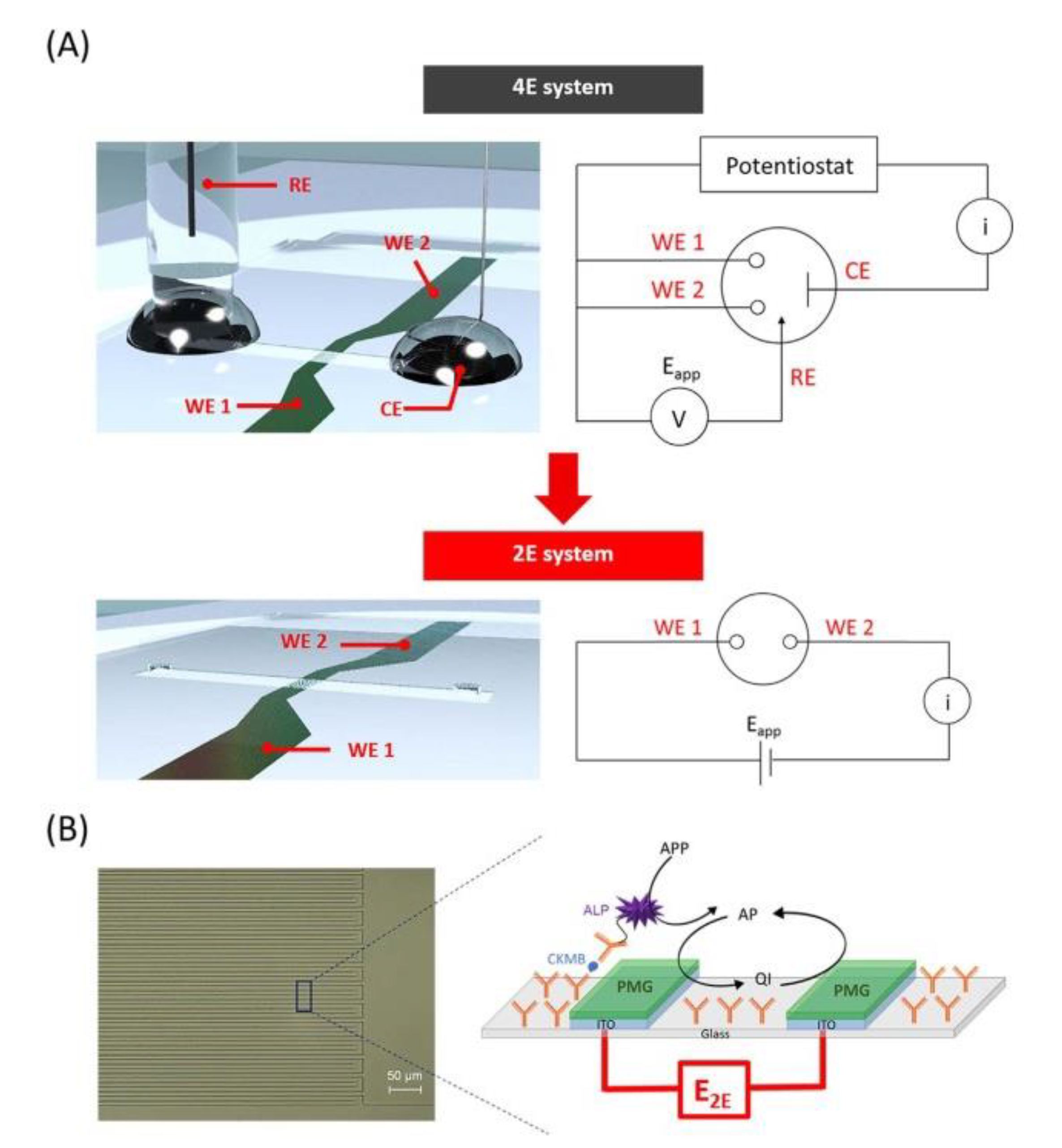
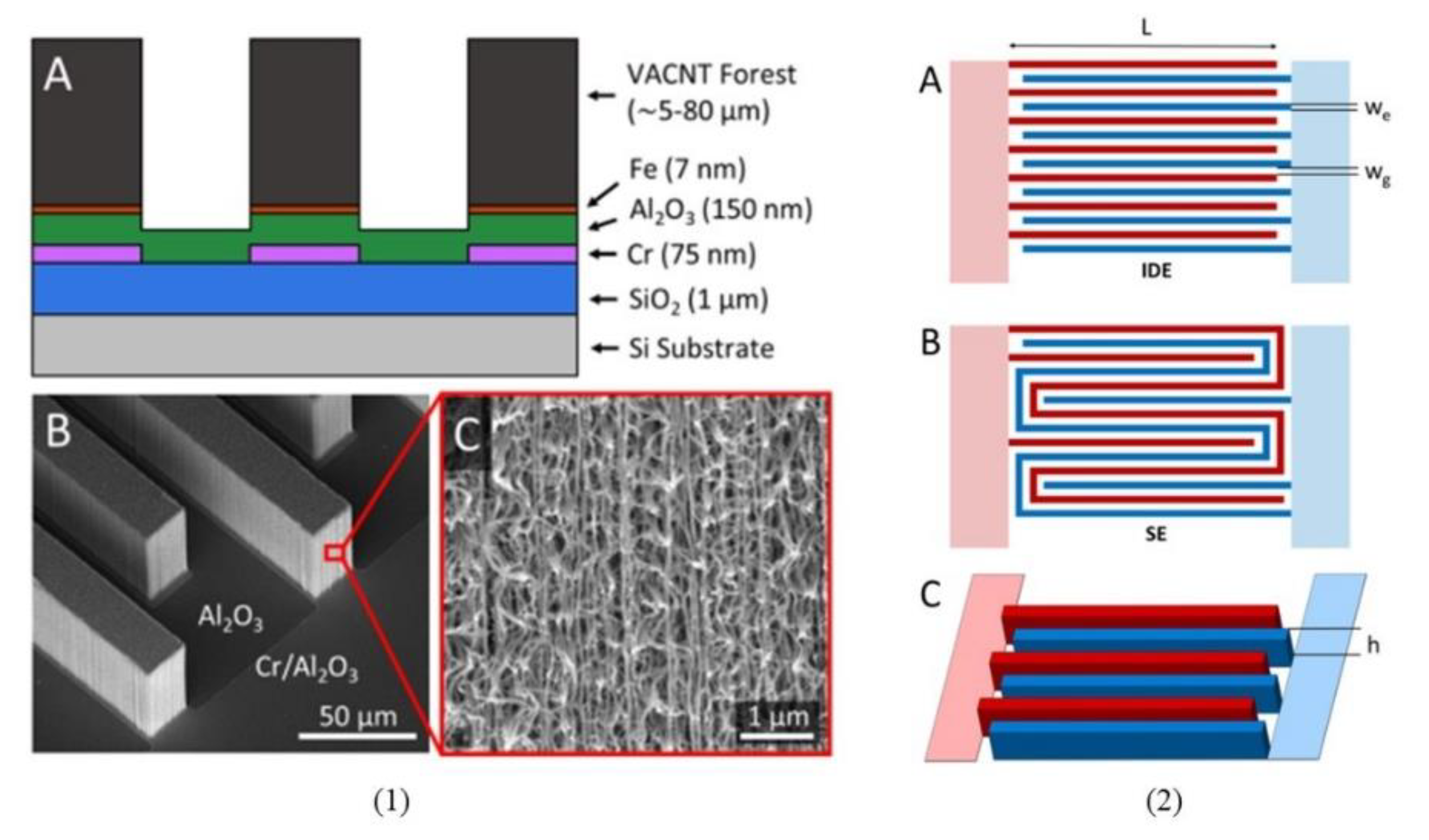
| Electrode C-IDEA Sources | Dimension | IDEA Structure | Sensor Performance | Ref. |
|---|---|---|---|---|
| SU-8 | w = 650 nm, h = 650 nm, g = 2.35 μm | 3D C-IDEA | (1) AF = 10.8, CE = 96.8% in bulk solution (2) AF = 139 in h = 6 mm channel, (3) AF = 230 in h = 10 mm channel | [49] |
| SU-8 2000.5, SU- 8 2002, SU-8 2005 | h = 1.1 μm and a w/g ratio of 1.58 (w bottom = 2.7, w top = 1.95, g bottom = 1.1 μm and g top = 1.85 μm) | 3D C-IDEA | AF = 37, CE = 98.6% | [54] |
| SU-8 | w = 1.3 μm g = 2.7 μm | 2D C-IDEA | AF = 13, CE = 98% | [119] |
| SU-8, Pt | w < 2 μm, g < 3 μm | 2D C-Pt IDEA | CE = 68%, 31% higher than C-C IDEA | [120] |
| SU-8 2005, SU-8 2075 | (1) g = 5 µm, (2) 2D C-IDEA: h = 0.4 µm, w = 3.6 µm (3) 3D C-IDEA: h = 11 µm, diameter = 1.4 µm | 3D carbon pillars on top of 2D C-IDEA | CV: 168 ± 12 mV for carbon 3D IDEA with pillars of 1.4 µm in diameter (aspect ratio of 8) | [129] |
| SU-8 2035, SU-8 2075 | 2Dp-25 and 3D#-25 bottom IDEA and pillars: wbot = 25 µm, sbot 25 µm, tbot = 17 µm, d = 20 µm, spil = 60 µm, hpil = 100 µm, 3D#-25 suspended IDEA: wsus = 10 µm, ssus = 20 µm, tsus = 17 µm | 2D C-IDEA and 3D C-IDEA with suspended | 3D#-25: Ip = 0.527 ± 0.003 mA, 2Dp – 25: Ip = 0.23 ± 0.02 mA | [145] |
| Techniques | Substrate/Electrode Materials | IDEA Dimensions | IDEA Structure | Sensor Performance | Ref. |
|---|---|---|---|---|---|
| Amperometric | Pt | w = g = h = 100 nm | Pt-nIDEA | AF = 161 for FcMeOH, CE = 99% -no biosensing test | [18] |
| Impedimetric | Polyimide sheet | w = 917 µm, g = 553 µm | 2.5 CFU/mL of E. Coli detection | [102] | |
| Impedimetric | Au | w = g = 10 μm, h = ∼60 nm | PPy/CNT film on 2 Au 2D IDEA | LOD: 28 ng/mL CysC | [105] |
| Amperometric and Impedimetric | Fe | (a) w = 20 μm, g = 15 μm, h = 80 μm, (b) w = 25 μm, g = 25 μm, h = 5 μm, (c) w = 25 μm, g = 25 μm, h = 20 μm, and (d) w = 25 μm, g = 25 μm, h = 80 μm. | 3D VACNT IDEA | LOD: 1 ng/mL F-biotin | [136] |
| Impedimetric | SU-8 2002, SU-8 2025 | Suspended carbon mesh: w ~300 nm, IDEA g ~1.9 µm | Suspended carbon mesh on top of the 2D C-IDEA | LOD: 0.43 pg/mL cMyo human serum | [139] |
| Amperometric, chronocoulometric | ITO | w = 5 μm, g = 10 μm between the bottom and ceiling and h = several tens µm | Closed 2D IDEA and 3D IDEA | LOD: 10 fg/mL (3D IDEA) and ∼100 fg/mL (Closed-2D IDEA) for mouseIgG 3D IDEA: 100 fg/mL for cTnI | [141] |
| Amperometric, Impedimetric | ITO electrode modified with PMG and PDA | w = 5 μm, g = 10 μm, h = 30 μm | 3D IDEA without reference and counter electrodes. | LOD: 0.32 pg/mL of Creatine Kinase-MB | [146] |
| Amperometric and Impedimetric | Fe | w = g = 25 µm, h = 75 µm | 3D VACNT IDEA | LOD: 0.24 pg/mL of CIP2A in salivasupernatant | [151] |
| Amperometric | SU-8 2002 | w = 620 nm, h = 650 nm and g = 1.9 µm | AuNPs on top of 3D C-IDEA | LOD: ~1.28 µM of cholesterol | [168] |
| Impedimetric | Cr/Au | w = 10 µm, g = 100 µm | 2D IDEA with porous sensor on top | LOD: 10 ng/mL for an IgG | [180] |
| Conductometric | Au | w = g = 20 μm | 2D IDEA | LOD: 15 µM of ATP | [183] |
| IDEA Source Materials | Integration in IDEA | Applications | Sensor Performance | Ref. |
|---|---|---|---|---|
| Ti/Pt IDEA | CuO–ZnO radial core–shell heterojunction nanowire arrays on metallic IDEA | photodetectors | responsivity: 26.3 A/W, detectivity: 5.8 × 1013 Jones | [184] |
| Metal IDEA on PET substrates | grown zinc oxide nanorod (NR) arrays cross-linked with IDEA | bending detection characteristics and sensing mechanism | no plasma treatment: highest gauge factor of 196 at a bending strain of 1.75% in the convex direction | [185] |
| C-Pt-IDEA | TiO2 nanoparticles | photoelectrochemical (PEC) water splitting | shining of 365 nm LED light | [186] |
| Ti/Pt IDEA onglass substrates | one IDEA activated by enzyme immobilization with HRP | capacitive detection of the H2O2 vapor/aerosol | sensitivity of 57.8 nF/c(H2O2), the response time (<60 s) | [187] |
| TaSi2 3D-IDEA | with 4 µm high insulating barriers | detection ofcyanobacteria cells | LOD: 100 cells·mL−1 | [188] |
| Au-IDEA | carbon nanodiamond | detection of SARS-CoV-2 nucleocapsid protein (NCP) | LOD: 0.389 fM | [189] |
| IDEA on polycarbonate substrates to make printed capacitive sensors | Ag nanoparticles | automotive infotainment | capacitance is increased when thickness increases | [190] |
| IDEA on ITO glass | carbon aerogel (CA)-polyaniline (PANI) composites | H2S gas sensing | PANI-CA-3 sensitivity:452% | [191] |
| 3D IDEA micro-supercapacitors (MSCs) | Si/C/CNT@TiC composite nanostructure | alternating current line filtering | capacitance: 7.42 mF cm−2 (3.71 F g−1) at 5 mV s−1 | [192] |
| IDEA capacitor on woven fabric | - | tactile sensor | capacitance change- 1.28 pF/gm. | [193] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosri, E.; Ibrahim, F.; Thiha, A.; Madou, M. Micro and Nano Interdigitated Electrode Array (IDEA)-Based MEMS/NEMS as Electrochemical Transducers: A Review. Nanomaterials 2022, 12, 4171. https://doi.org/10.3390/nano12234171
Kosri E, Ibrahim F, Thiha A, Madou M. Micro and Nano Interdigitated Electrode Array (IDEA)-Based MEMS/NEMS as Electrochemical Transducers: A Review. Nanomaterials. 2022; 12(23):4171. https://doi.org/10.3390/nano12234171
Chicago/Turabian StyleKosri, Elyana, Fatimah Ibrahim, Aung Thiha, and Marc Madou. 2022. "Micro and Nano Interdigitated Electrode Array (IDEA)-Based MEMS/NEMS as Electrochemical Transducers: A Review" Nanomaterials 12, no. 23: 4171. https://doi.org/10.3390/nano12234171





