Growth of Highly-Ordered Metal Nanoparticle Arrays in the Dimpled Pores of an Anodic Aluminum Oxide Template
Abstract
:1. Introduction
2. Materials and Methods
3. Results
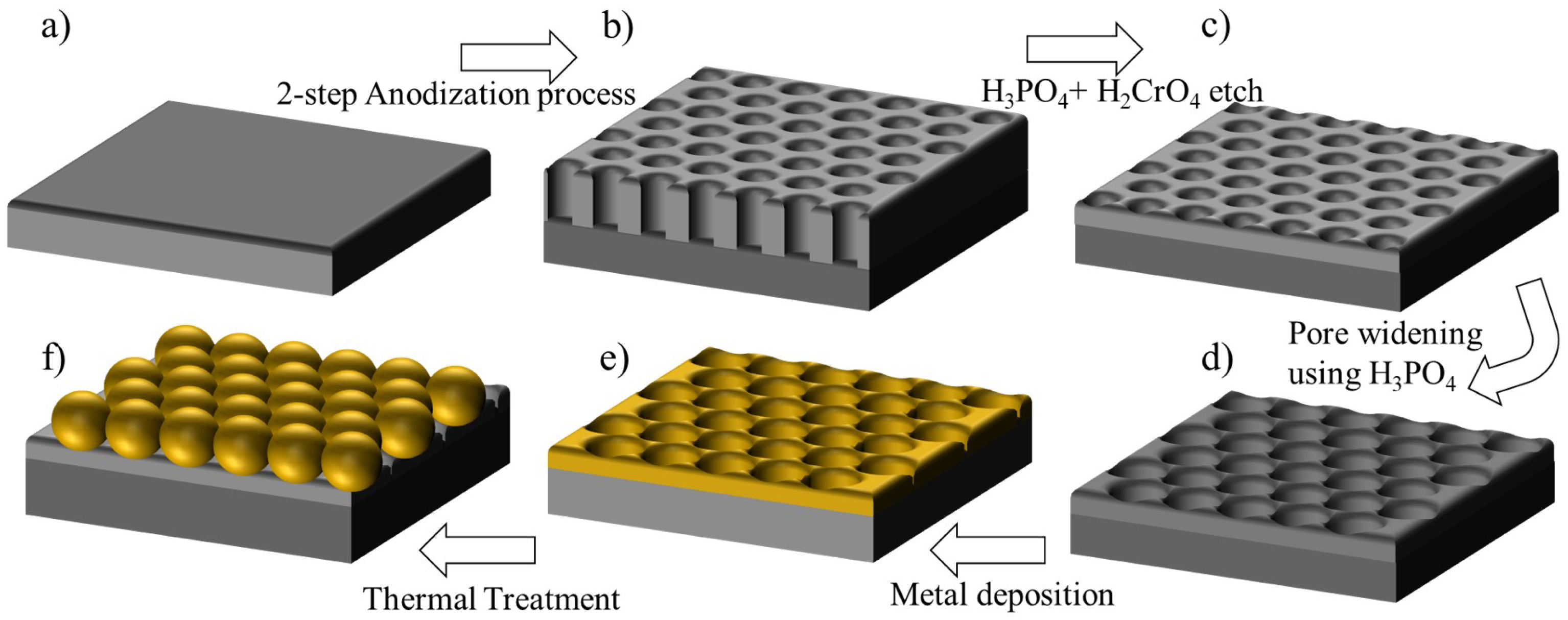
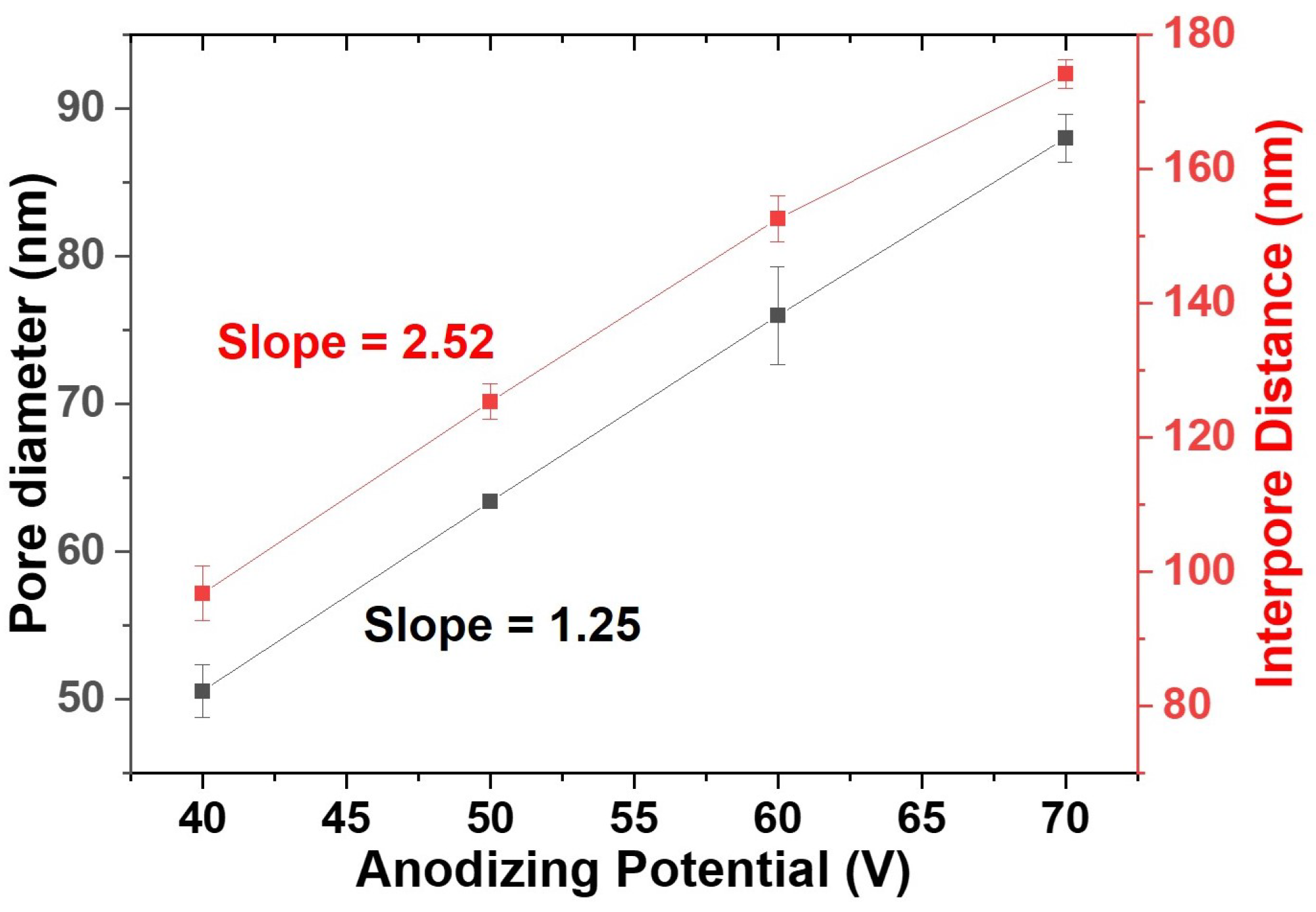
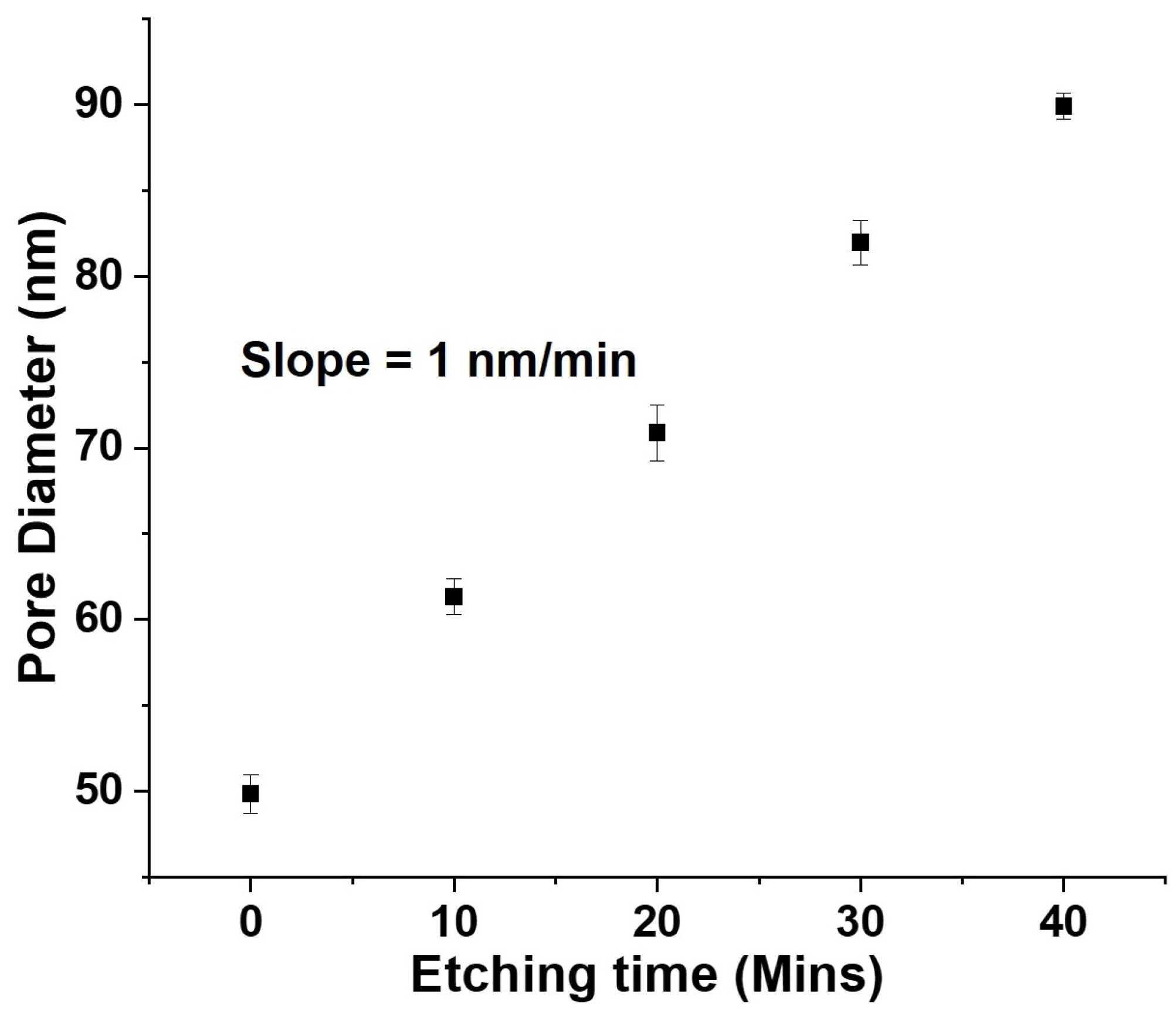
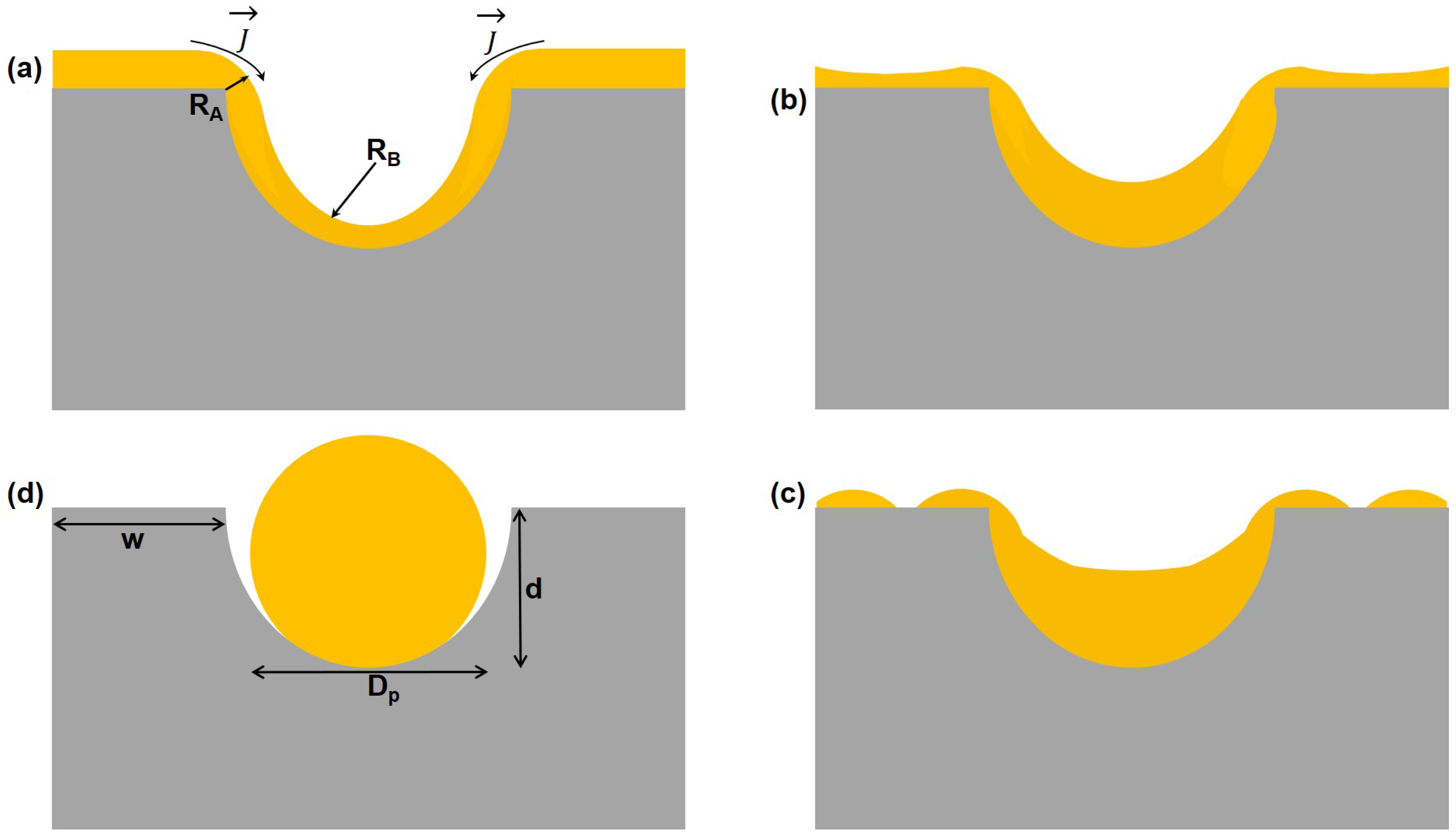
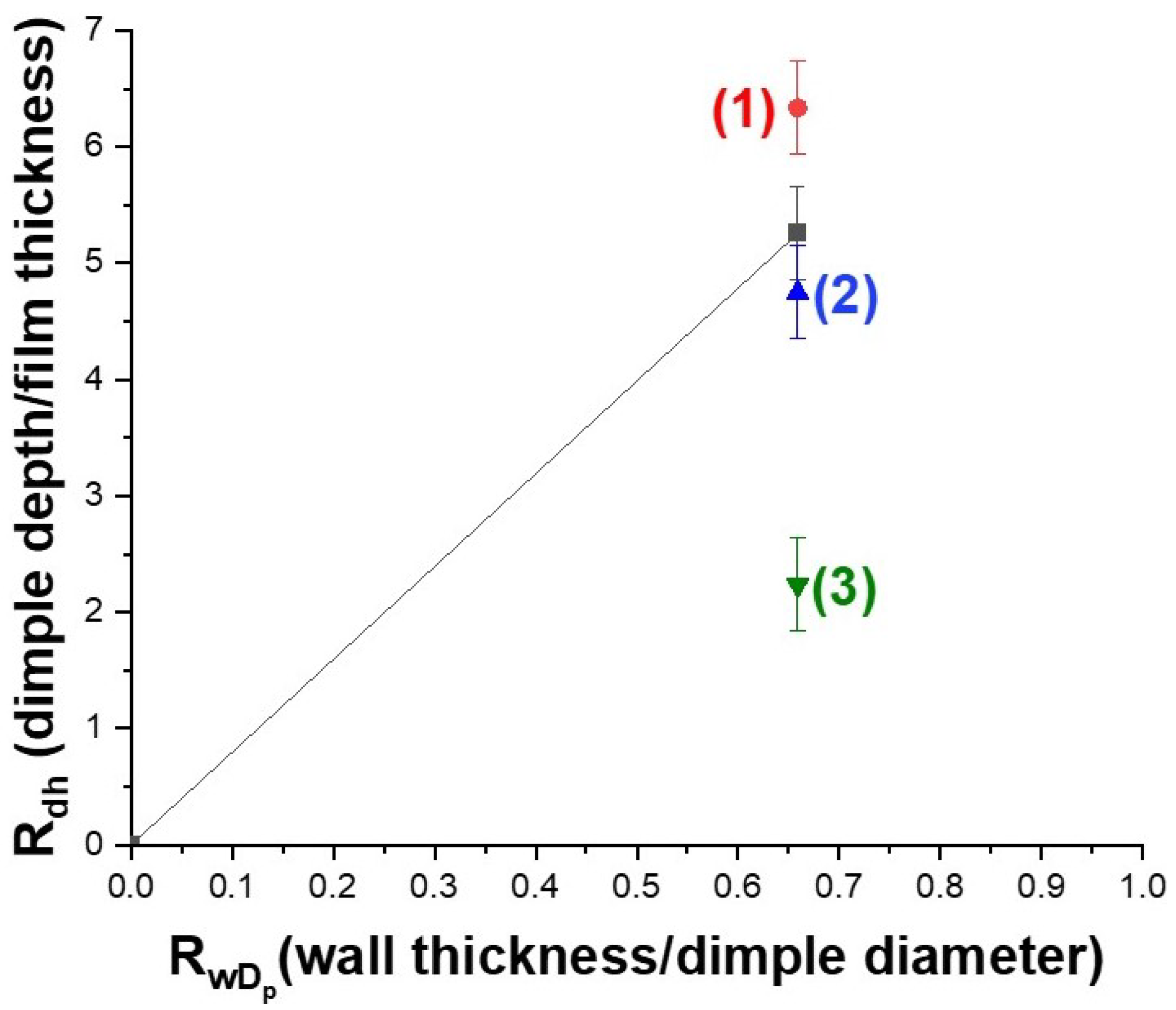
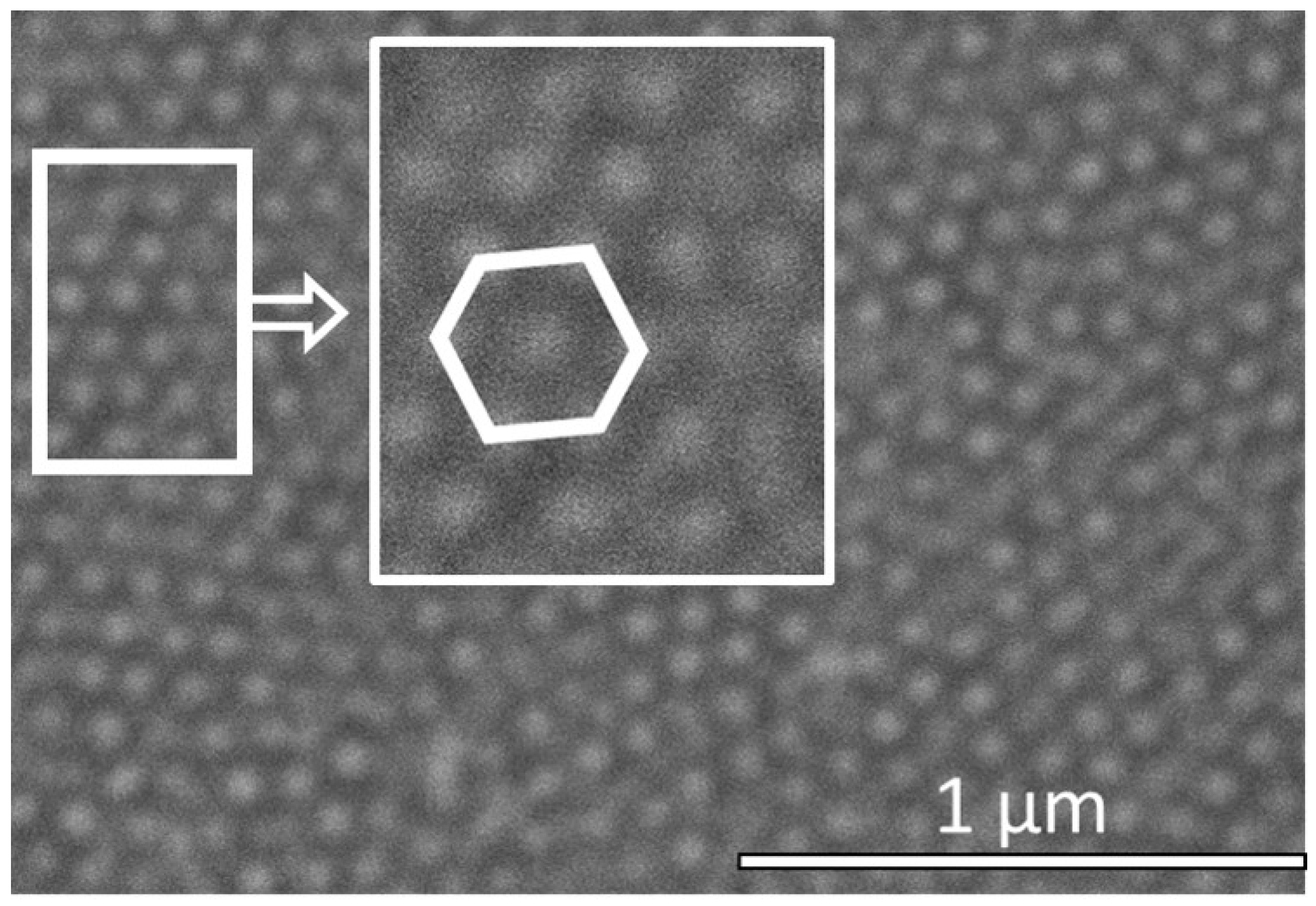
3.1. Engineering the Structural Properties of the Pores AAO Membrane
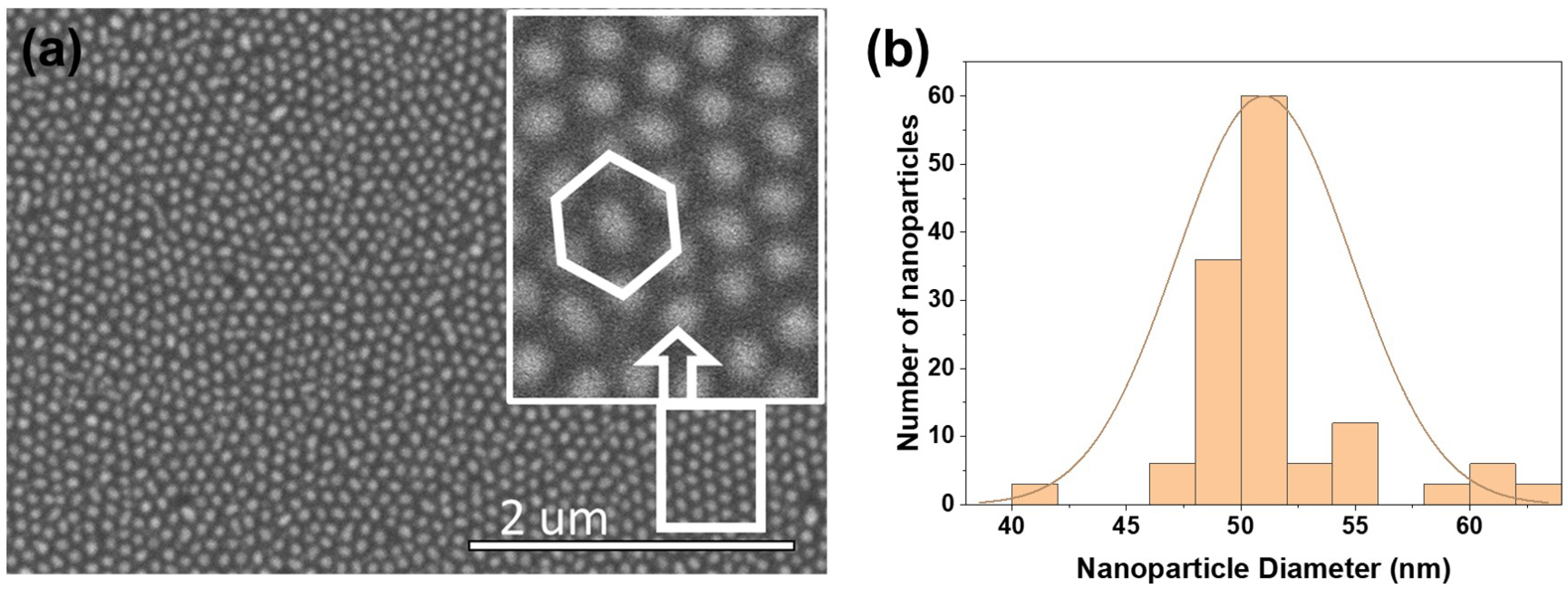
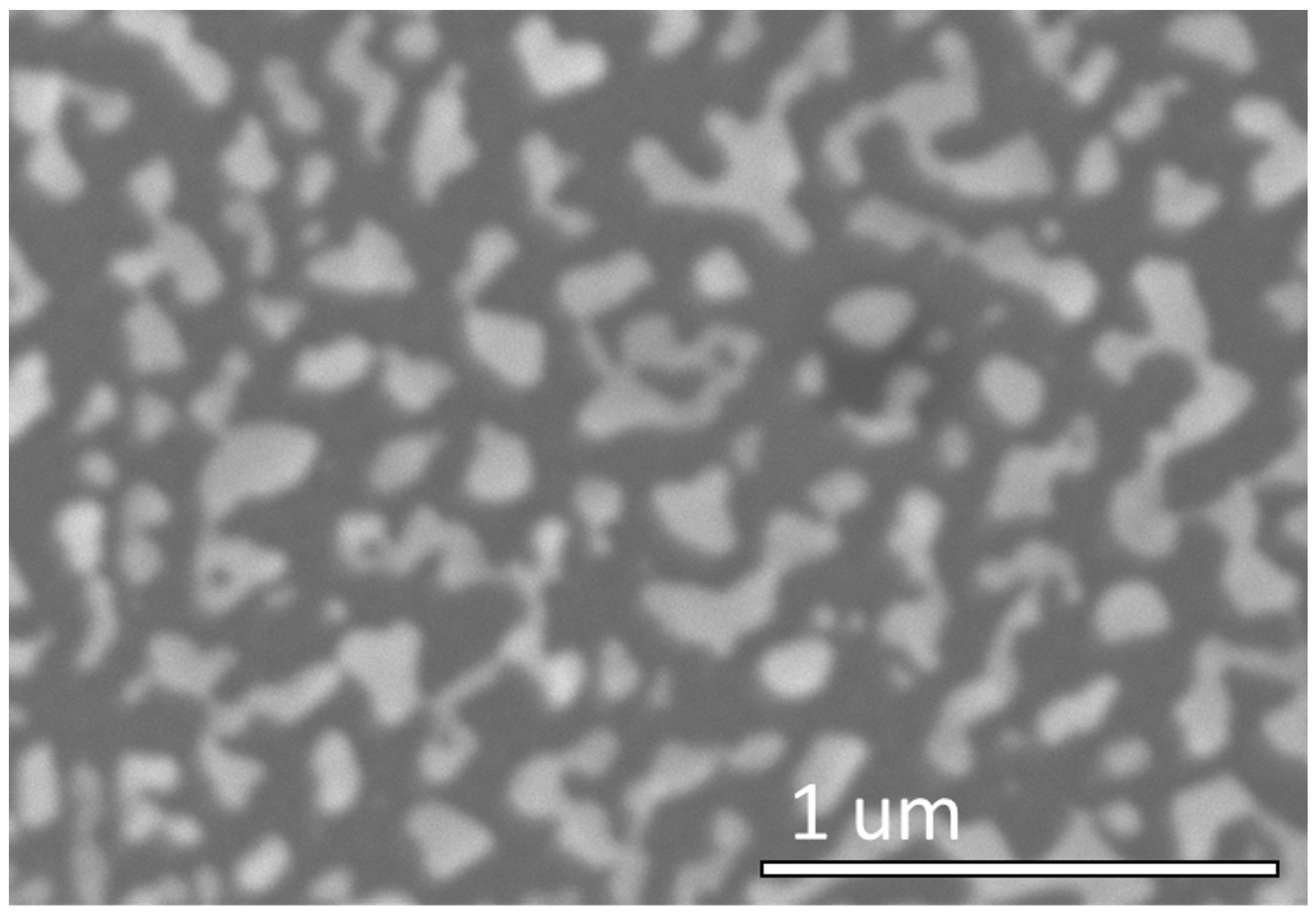
3.2. Fabrication of Ordered Array of Metal Nanoparticles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bora, T. Recent Developments on Metal Nanoparticles for SERS Applications; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Dykman, L.A.; Khlebtsov, N.G. Gold Nanoparticles in Biology and Medicine: Recent Advances and Prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef] [Green Version]
- Haynes, C.; Duyne, R. Nanosphere Lithography: A Versatile Nanofabrication Tool for Studies of Size-Dependent Nanoparticle Optics. J. Phys. Chem. B 2001, 105, 5599–5611. [Google Scholar] [CrossRef]
- Li, C.; Xu, C.; Cahen, D.; Jin, Y. Unprecedented efficient electron transport across Au nanoparticles with up to 25-nm insulating SiO2-shells. Sci. Rep. 2019, 9, 18336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Cahen, D.; Wang, P.; Li, H.; Zhang, J.; Jin, Y. Plasmonics Yields Efficient Electron Transport via Assembly of Shell-Insulated Au Nanoparticles. iScience 2018, 8, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y. Nanofabrication by electron beam lithography and its applications: A review. Microelectron. Eng. 2015, 135, 57–72. [Google Scholar] [CrossRef]
- Yu, C.-C.; Chen, H.-L. Nanoimprint Technology for Patterning Functional Materials and Its Applications. Microelectron. Eng. 2015, 132, 98–119. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, W. Plasmonic properties of two-dimensional metallic nanoholes fabricated by focused ion beam lithography. J. Nanoparticle Res. 2012, 14, 652. [Google Scholar] [CrossRef]
- Lednický, T.; Bonyár, A. Large Scale Fabrication of Ordered Gold Nanoparticle—Epoxy Surface Nanocomposites and Their Application as Label-Free Plasmonic DNA Biosensors. ACS Appl. Mater. Interfaces 2020, 12, 4804–4814. [Google Scholar] [CrossRef] [Green Version]
- Ruemmele, J.A.; Hall, W.P.; Ruvuna, L.K.; Van Duyne, R.P. A Localized Surface Plasmon Resonance Imaging Instrument for Multiplexed Biosensing. Anal. Chem. 2013, 85, 4560–4566. [Google Scholar] [CrossRef]
- Dickreuter, S.; Gleixner, J.; Kolloch, A.; Boneberg, J.; Scheer, E.; Leiderer, P. Mapping of plasmonic resonances in nanotriangles. Beilstein J. Nanotechnol. 2013, 4, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.T.; Kim, D.-J.; Kim, K.-S. Controlled Synthesis and Biomolecular Probe Application of Gold Nanoparticles. Micron 2011, 42, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Farzinpour, P.; Sundar, A.; Gilroy, K.D.; Eskin, Z.E.; Hughes, R.A.; Neretina, S. Altering the dewetting characteristics of ultrathin gold and silver films using a sacrificial antimony layer. Nanotechnology 2012, 23, 495604. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.V. Solid-State Dewetting of Thin Films. Annu. Rev. Mater. Res. 2012, 42, 399–434. [Google Scholar] [CrossRef]
- Ye, J.; Thompson, C.V. Templated solid-state dewetting to controllably produce complex patterns. Adv. Mater. 2011, 23, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Han, D.-H.; Choe, H.J.; Lee, J.-J. Synthesis of copper nanoparticles by solid-state plasma-induced dewetting. Nanotechnology 2011, 22, 245608. [Google Scholar] [CrossRef] [PubMed]
- Sundar, A.; Decker, C.J.; Hughes, R.A.; Neretina, S. The templated assembly of highly faceted three-dimensional gold microstructures into periodic arrays. Mater. Lett. 2012, 76, 155–158. [Google Scholar] [CrossRef]
- Jiran, E.; Thompson, C.V. Capillary instabilities in thin, continuous films. Thin Solid Film. 1992, 208, 23–28. [Google Scholar] [CrossRef]
- Giermann, A.L.; Thompson, C.V. Solid-state dewetting for ordered arrays of crystallographically oriented metal particles. Appl. Phys. Lett. 2005, 86, 121903. [Google Scholar] [CrossRef]
- Giermann, A.L.; Thompson, C.V. Requirements for graphoepitaxial alignment through solid-state dewetting of Au films. J. Appl. Phys. 2011, 109, 083520. [Google Scholar] [CrossRef]
- Yang, S.; Cao, B.; Kong, L.; Wang, Z. Template-directed dewetting of a gold membrane to fabricate highly SERS-active substrates. J. Mater. Chem. 2011, 21, 14031–14035. [Google Scholar] [CrossRef]
- Mahmud, A.; Habiballah, A.; Jani, A.M.M. The Effect of Applied Voltage and Anodisation Time on Anodized Aluminum Oxide Nanostructures. Mater. Sci. Forum 2015, 819, 103–108. [Google Scholar] [CrossRef]
- Masuda, H.; Tanaka, H.; Baba, N. Preparation of Porous Material by Replacing Microstructure of Anodic Alumina Film with Metal. Chem. Lett. 1990, 19, 621–622. [Google Scholar] [CrossRef]
- Belwalkar, A.; Grasing, E.; Geertruyden, W.V.; Huang, Z.; Misiolek, W.Z. Effect of Processing Parameters on Pore Structure and Thickness of Anodic Aluminum Oxide (AAO) Tubular Membranes. J. Membr. Sci. 2008, 319, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Sulka, G.D.; Stępniowski, W.J. Structural features of self-organized nanopore arrays formed by anodization of aluminum in oxalic acid at relatively high temperatures. Electrochim. Acta 2009, 54, 3683–3691. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef]
- Kumeria, T.; Santos, A.; Losic, D. Nanoporous anodic alumina platforms: Engineered surface chemistry and structure for optical sensing applications. Sensors 2014, 14, 11878–11918. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zheng, M.; Ma, L. Fabrication of highly ordered nanoporous alumina films by stable high-field anodization. Nanotechnology 2006, 17, 5101–5105. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Caballero-Calero, O.; Martín-González, M. Revisiting anodic alumina templates: From fabrication to applications. Nanoscale 2021, 13, 2227–2265. [Google Scholar] [CrossRef]
- Ono, S.; Saito, M.; Ishiguro, M.; Asoh, H. Controlling Factor of Self-Ordering of Anodic Porous Alumina. J. Electrochem. Soc. 2004, 151, B473. [Google Scholar] [CrossRef]
- Nielsch, K.; Choi, J.; Schwirn, K.; Wehrspohn, R.B.; Gösele, U. Self-ordering Regimes of Porous Alumina: The 10 Porosity Rule. Nano Lett. 2002, 2, 677–680. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.-J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhang, H.; Di, W.; Zhao, T. Study on Wet Etching of AAO Template. Appl. Phys. Res. 2009, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Crouse, D.; Lo, Y.-H.; Miller, A.E.; Crouse, M. Self-ordered pore structure of anodized aluminum on silicon and pattern transfer. Appl. Phys. Lett. 2000, 76, 49. [Google Scholar] [CrossRef]
- Chen, J.; Wei, T.; Chang, C.; Ko, H.; Chu, C.; Chi, M.; Tsai, C. Fabrication of Polymer Nanopeapods in the Nanopores of Anodic Aluminum Oxide Templates Using a Double-Solution Wetting Method. Macromolecules 2014, 47, 5227–5235. [Google Scholar] [CrossRef]
- Altomare, M.; Nguyen, N.T.; Schmuki, P. Templated dewetting: Designing entirely self-organized platforms for photocatalysis. Chem. Sci. 2016, 7, 6865–6886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonvicini, S.N.; Fu, B.; Fulton, A.J.; Jia, Z.; Shi, Y. Formation of Au, Pt, and bimetallic Au–Pt nanostructures from thermal dewetting of single-layer or bilayer thin films. Nanotechnology 2022, 33, 235604. [Google Scholar] [CrossRef]
- Masoud, N.; Partsch, T.; de Jong, K.P.; de Jongh, P.E. Thermal stability of oxide-supported gold nanoparticles. Gold Bull. 2019, 52, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Lu, P.; Cao, Z.; Campbell, C.T.; Xia, Y. The physical chemistry and materials science behind sinter-resistant catalysts. Chem. Soc. Rev. 2018, 47, 4314–4331. [Google Scholar] [CrossRef]
- Prieto, G.; Tüysüz, H.; Duyckaerts, N.; Knossalla, J.; Wang, G.-H.; Schüth, F. Hollow Nano- and Microstructures as Catalysts. Chem. Rev. 2016, 116, 14056–14119. [Google Scholar] [CrossRef]
- Fan, X.; Hao, Q.; Jin, R.; Huang, H.; Luo, Z.; Yang, X.; Chen, Y.; Han, X.; Sun, M.; Jing, Q.; et al. Assembly of gold nanoparticles into aluminum nanobowl array. Sci. Rep. 2017, 7, 2322. [Google Scholar] [CrossRef]
- Repetto, L.; Batič, B.Š.; Firpo, G.; Piano, E.; Valbusa, U. Anisotropic dewetting of ion irradiated solid films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 315, 244–247. [Google Scholar] [CrossRef]
- Mullins, W.W. Mass transport at interfaces in single component systems. Metall Mater. Trans. A 1995, 26, 1917–1929. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farmer, G.; Abraham, J.; Littler, C.; Syllaios, A.J.; Philipose, U. Growth of Highly-Ordered Metal Nanoparticle Arrays in the Dimpled Pores of an Anodic Aluminum Oxide Template. Nanomaterials 2022, 12, 3929. https://doi.org/10.3390/nano12223929
Farmer G, Abraham J, Littler C, Syllaios AJ, Philipose U. Growth of Highly-Ordered Metal Nanoparticle Arrays in the Dimpled Pores of an Anodic Aluminum Oxide Template. Nanomaterials. 2022; 12(22):3929. https://doi.org/10.3390/nano12223929
Chicago/Turabian StyleFarmer, Gavin, James Abraham, Chris Littler, A. J. Syllaios, and U. Philipose. 2022. "Growth of Highly-Ordered Metal Nanoparticle Arrays in the Dimpled Pores of an Anodic Aluminum Oxide Template" Nanomaterials 12, no. 22: 3929. https://doi.org/10.3390/nano12223929




