Chlorophyll Detection by Localized Surface Plasmon Resonance Using Functionalized Carbon Quantum Dots Triangle Ag Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.3. Synthesis of NCQD
2.4. Preparation of AgNPs-NCQD Composite on LSPR Substrate
2.5. Detection of Chlorophyll
3. Results and Discussions
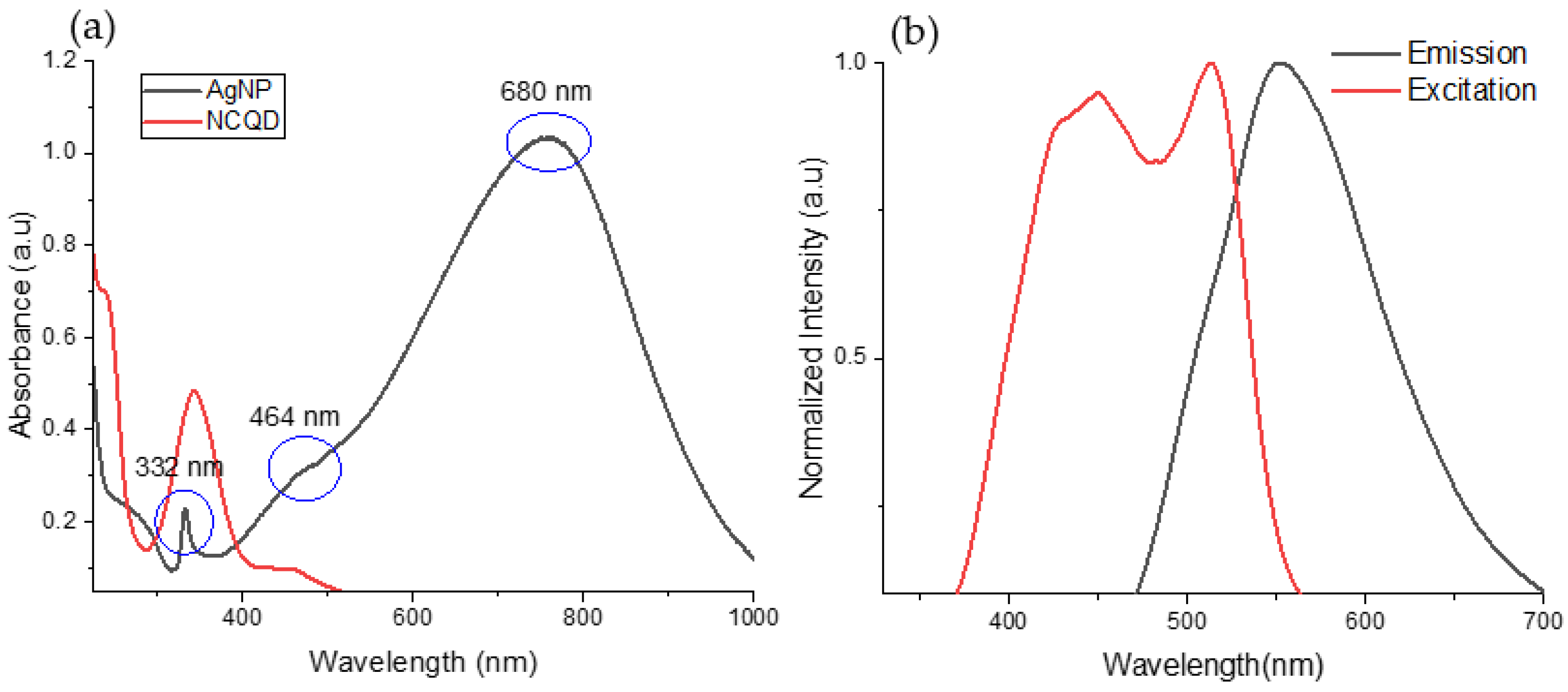
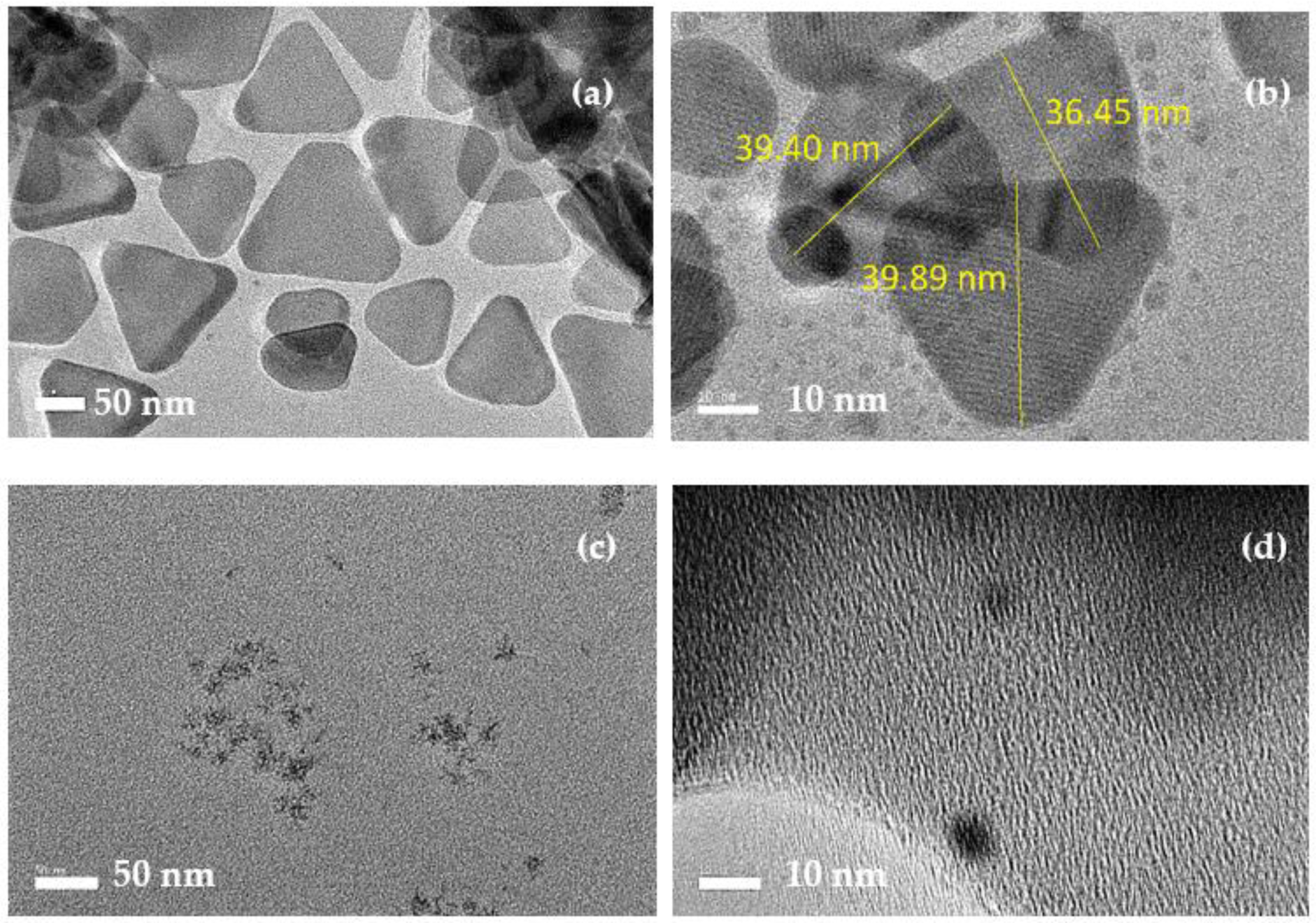
3.1. Characterization of as-Synthesized CQD and AgNPs
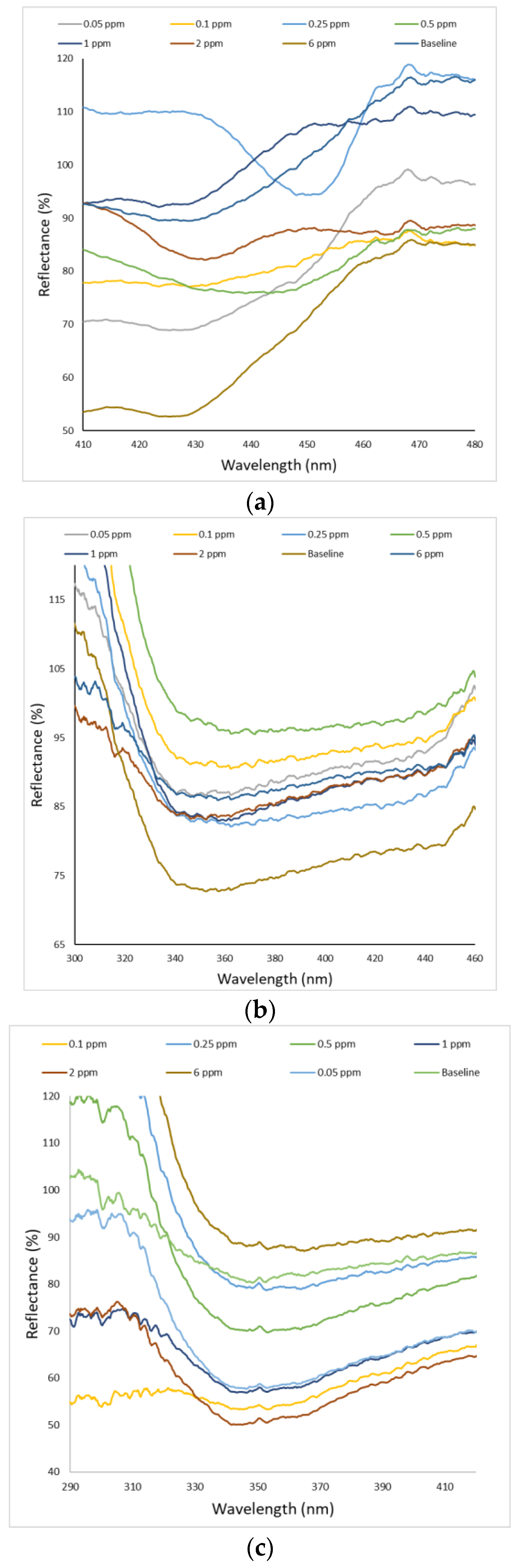
3.2. Sensing Performance
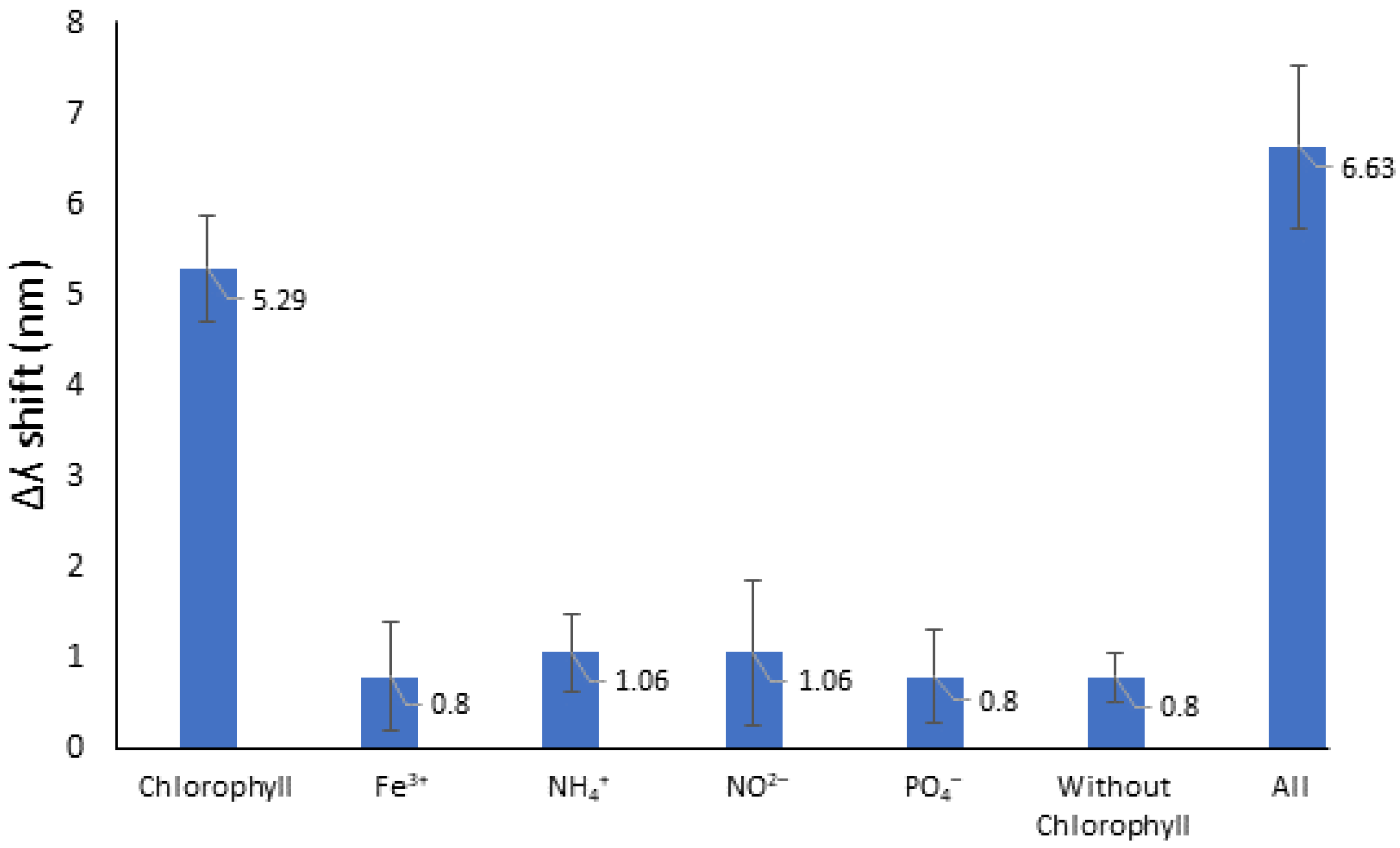
3.3. Selectivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitcher, G.C.; Figueiras, F.G.; Hickey, B.M.; Moita, M.T. The physical oceanography of upwelling systems and the development of harmful algal blooms. Prog. Oceanogr. 2010, 85, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Litchman, E. Resource Competition and the Ecological Success of Phytoplankton. In Evolution of Primary Producers in the Sea; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar] [CrossRef]
- Khataee, A.; Gholami, P.; Vahid, B.; Joo, S.W. Heterogeneous sono-Fenton process using pyrite nanorods prepared by non-Thermal plasma for degradation of an anthraquinone dye. Ultrason. Sonochemistry 2016, 32, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Orooji, Y. Sonocatalytic activity of biochar-Supported ZnO nanorods in degradation of gemifloxacin: Synergy study, effect of parameters and phytotoxicity evaluation. Ultrason. Sonochemistry 2019, 55, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, J.; Chen, X.; Shi, L.; Zhang, L. Nondestructive detection of rape leaf chlorophyll level based on Vis-NIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117202. [Google Scholar] [CrossRef] [PubMed]
- Sonobe, R.; Hirono, Y.; Oi, A. Non-Destructive detection of tea leaf chlorophyll content using hyperspectral reflectance and machine learning algorithms. Plants 2020, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Bramich, J.; Bolch, C.J.S.; Fischer, A. Improved red-Edge chlorophyll-A detection for Sentinel 2. Ecol. Indic. 2020, 120, 106876. [Google Scholar] [CrossRef]
- Liu, R.; Ling, Q.; Zhang, Q.; Zhou, Y.; Le, C.; Chen, Y.; Liu, Q.; Chen, W.; Tang, J.; Liu, D. Detection of chlorophyll a and cdom absorption coefficient with a dual-Wavelength oceanic lidar: Wavelength optimization method. Remote Sens. 2020, 12, 3021. [Google Scholar] [CrossRef]
- Bakar, M.H.A.; Azeman, N.H.; Mobarak, N.N.; Nazri, N.A.A.; Abdul Aziz, T.H.T.; Zain, A.R.M.; Arsad, N.; Bakar, A.A.A. Succinyl-κ-Carrageenan Silver Nanotriangles Composite for Ammonium Localized Surface Plasmon Resonance Sensor. Polymers 2022, 14, 329. [Google Scholar] [CrossRef]
- Abdullah, S.; Azeman, N.H.; Mobarak, N.N.; Zan, M.S.D.; Ahmad, A.A. Sensitivity enhancement of localized SPR sensor towards Pb(II) ion detection using natural bio-Polymer based carrageenan. Optik 2018, 168, 784–793. [Google Scholar] [CrossRef]
- Zannotti, M.; Vicomandi, V.; Rossi, A.; Minicucci, M.; Ferraro, S.; Petetta, L.; Giovannetti, R. Tuning of hydrogen peroxide etching during the synthesis of silver nanoparticles. An application of triangular nanoplates as plasmon sensors for Hg2+ in aqueous solution. J. Mol. Liq. 2020, 309, 113238. [Google Scholar] [CrossRef]
- Azeman, N.H.; Arsad, N.; Bakar, A.A.A. Polysaccharides as the sensing material for metal ion detection-Based optical sensor applications. Sensors 2020, 20, 3924. [Google Scholar] [CrossRef]
- Rahman, W.B.W.A.; Azeman, N.H.; Kamaruddin, N.H.; Menon, P.S.; Shabaneh, A.A.; Mahdi, M.A.; Mokhtar, M.H.H.; Arsad, N.; Bakar, A.A.A. Label-Free detection of dissolved carbon dioxide utilizing multimode tapered optical fiber coated zinc oxide nanorice. IEEE Access 2018, 7, 4538–4545. [Google Scholar] [CrossRef]
- Abu Bakar, M.H.; Azeman, N.H.; Mobarak, N.N.; Mokhtar, M.H.H.; Bakar, A.A.A. Effect of active site modification towards performance enhancement in biopolymer κ-Carrageenan derivatives. Polymers 2020, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- Afifah, N.; Nazri, A.; Azeman, N.H.; Hafiz, M.; Bakar, A.; Mobarak, N.N.; Luo, Y.; Arsad, N.; Hasnan, T.; Abd, T.; et al. Localized Surface Plasmon Resonance Decorated with Carbon Quantum Dots and Triangular Ag Nanoparticles for Chlorophyll Detection. Nanomater. 2021, 12, 35. [Google Scholar] [CrossRef]
- Yoo, D.; Park, Y.; Cheon, B.; Park, M.H. Carbon Dots as an Effective Fluorescent Sensing Platform for Metal Ion Detection. Nanoscale Res. Lett. 2019, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhu, Z.; Chen, M.; Zhou, X.; Chen, W. Microwave-Assisted synthesis of polyamine-functionalized carbon dots from xylan and their use for the detection of tannic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 301–308. [Google Scholar] [CrossRef]
- Qian, K.; Guo, H.; Chen, G.; Ma, C.; Xing, B. Distribution of different surface modified carbon dots in pumpkin seedlings. Sci. Rep. 2018, 8, 7991. [Google Scholar] [CrossRef]
- Nazri, N.A.A.; Azeman, N.H.; Luo, Y.; Bakar, A.A.A. Carbon quantum dots for optical sensor applications: A review. Opt. Laser Technol. 2021, 139, 106928. [Google Scholar] [CrossRef]
- Lee, K.S.; El-Sayed, M.A. Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef]
- Caro, C.; Castillo, P.M.; Klippstein, R.; Pozo, D.; Zaderenko, A.P. Silver Nanoparticles: Sensing and Imaging Applications. Silver Nanopart. 2010, 95, 201–224. Available online: https://www.intechopen.com/chapters/9730 (accessed on 21 July 2022).
- Bakar, N.A.; Shapter, J.G.; Salleh, M.M.; Umar, A.A. Self-Assembly of high density of triangular silver nanoplate films promoted by 3-Aminopropyltrimethoxysilan. Appl. Sci. 2015, 5, 209–221. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Li, Y.; Wei, H.P.; Li, X.S.; Zhang, X.H.; Chen, S.M.; Chen, X.Q. Synthesis of polyethyleneimine capped carbon dots for preconcentration and slurry sampling analysis of trace chromium in environmental water samples. Talanta 2015, 134, 16–23. [Google Scholar] [CrossRef]
- Pudza, M.Y.; Abidin, Z.Z.; Rashid, S.A.; Yasin, F.M.; Noor, A.S.M.; Issa, M.A. Eco-Friendly sustainable fluorescent carbon dots for the adsorption of heavy metal ions in aqueous environment. Nanomaterials 2020, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liyanage, P.Y.; Geleroff, D.L.; Peng, Z.; Mintz, K.J.; Hettiarachchi, S.D.; Pandey, R.R.; Chusuei, C.C.; Blackwelder, P.L.; Leblanc, R.M. Photoluminescent Carbon Dots: A Mixture of Heterogeneous Fractions. ChemPhysChem 2018, 19, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Azeman, N.H.; Bakar, M.H.A.; Nazri, N.A.A.; Mobarak, N.N.; Khushaini, M.A.A.; Aziz, T.H.T.A.; Zain, A.R.M.; Bakar, A.A.A. Carboxymethyl chitosan/graphene oxide/silver nanotriangles nanohybrid as the sensing materials for the enhancement of ammonia localized surface plasmon resonance sensor. Opt. Laser Technol. 2021, 148, 107789. [Google Scholar] [CrossRef]
- Hamdhani, H.; Eppehimer, D.E.; Walker, D.; Bogan, M.T. Performance of a handheld chlorophyll-A fluorometer: Potential use for rapid algae monitoring. Water 2021, 13, 1409. [Google Scholar] [CrossRef]
- Yap, S.H.K.; Chan, K.K.; Zhang, G.; Tjin, S.C.; Yong, K.T. Carbon Dot-Functionalized Interferometric Optical Fiber Sensor for Detection of Ferric Ions in Biological Samples. ACS Appl. Mater. Interfaces 2019, 11, 28546–28553. [Google Scholar] [CrossRef]
- Shin, Y.H.; Barnett, J.Z.; Song, E.; Gutierrez-Wing, M.T.; Rusch, K.A.; Choi, J.W. A portable fluorescent sensor for on-Site detection of microalgae. Microelectron. Eng. 2015, 144, 6–11. [Google Scholar] [CrossRef]
- Gosset, A.; Durrieu, C.; Renaud, L.; Deman, A.L.; Barbe, P.; Bayard, R.; Chateaux, J.F. Xurography-Based microfluidic algal biosensor and dedicated portable measurement station for online monitoring of urban polluted samples. Biosens. Bioelectron. 2018, 117, 669–677. [Google Scholar] [CrossRef]
- Attivissimo, F.; Carducci, C.G.C.; Lanzolla, A.M.L.; Massaro, A.; Vadrucci, M.R. A portable optical sensor for sea quality monitoring. IEEE Sensors J. 2014, 15, 146–153. [Google Scholar] [CrossRef]




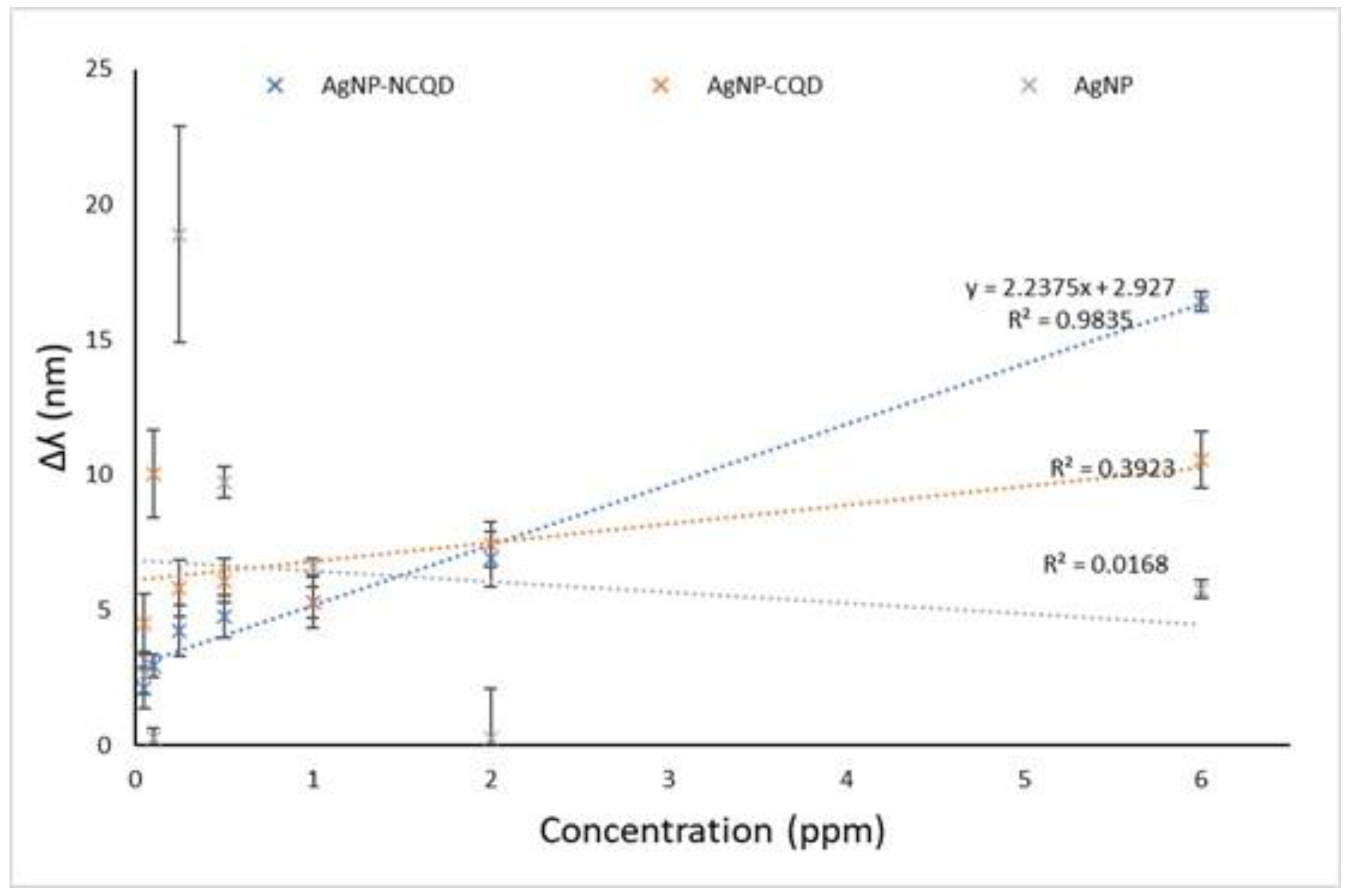

| Compound Film | R2 | Sensitivity (nm ppm−1) | Range (ppm) | LOD (ppm) | LOQ (ppm) |
|---|---|---|---|---|---|
| AgNPs | 0.0168 | 0.40 | 0.05-6 | 79.21 | 264.05 |
| AgNPs-CQD | 0.3923 | 0.70 | 0.05-6 | 12.31 | 41.04 |
| AgNPs-NCQD | 0.9835 | 2.23 | 0.05-6 | 1.02 | 3.40 |
| Methods | Analytes | Sensitivity | Range (ppm) | LOD (ppm) | References |
|---|---|---|---|---|---|
| Fluorescence | Green algae | - | - | 1 | [29] |
| Fluorescence | Chlorophyll-a | - | - | 0.001 | [30] |
| Fluorescence | Chlorophyll-a | - | 0.025–0.15 | - | [27] |
| Fluorescence | Chlorophyll-a | 1 mV 2.5 μg L−1 | 0–0.2 | - | [31] |
| LSPR | Chlorophyll | 0.80 nm ppm−1 | 0.2–10 | 4.71 | [19] |
| LSPR | Chlorophyll | 2.23 nm ppm−1 | 0.05–6 | 1.02 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazri, N.A.A.; Azeman, N.H.; Bakar, M.H.A.; Mobarak, N.N.; Aziz, T.H.T.A.; Zain, A.R.M.; Arsad, N.; Luo, Y.; Bakar, A.A.A. Chlorophyll Detection by Localized Surface Plasmon Resonance Using Functionalized Carbon Quantum Dots Triangle Ag Nanoparticles. Nanomaterials 2022, 12, 2999. https://doi.org/10.3390/nano12172999
Nazri NAA, Azeman NH, Bakar MHA, Mobarak NN, Aziz THTA, Zain ARM, Arsad N, Luo Y, Bakar AAA. Chlorophyll Detection by Localized Surface Plasmon Resonance Using Functionalized Carbon Quantum Dots Triangle Ag Nanoparticles. Nanomaterials. 2022; 12(17):2999. https://doi.org/10.3390/nano12172999
Chicago/Turabian StyleNazri, Nur Afifah Ahmad, Nur Hidayah Azeman, Mohd Hafiz Abu Bakar, Nadhratun Naiim Mobarak, Tg Hasnan Tg Abd Aziz, Ahmad Rifqi Md Zain, Norhana Arsad, Yunhan Luo, and Ahmad Ashrif A. Bakar. 2022. "Chlorophyll Detection by Localized Surface Plasmon Resonance Using Functionalized Carbon Quantum Dots Triangle Ag Nanoparticles" Nanomaterials 12, no. 17: 2999. https://doi.org/10.3390/nano12172999






