UV-Based Advanced Oxidation Processes of Remazol Brilliant Blue R Dye Catalyzed by Carbon Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Titanium(IV)-Doped Carbon Dots (Ti-CD)
2.3. UV Advanced Oxidation Process (AOP)
2.4. Equipment
2.5. Data Analysis
3. Results
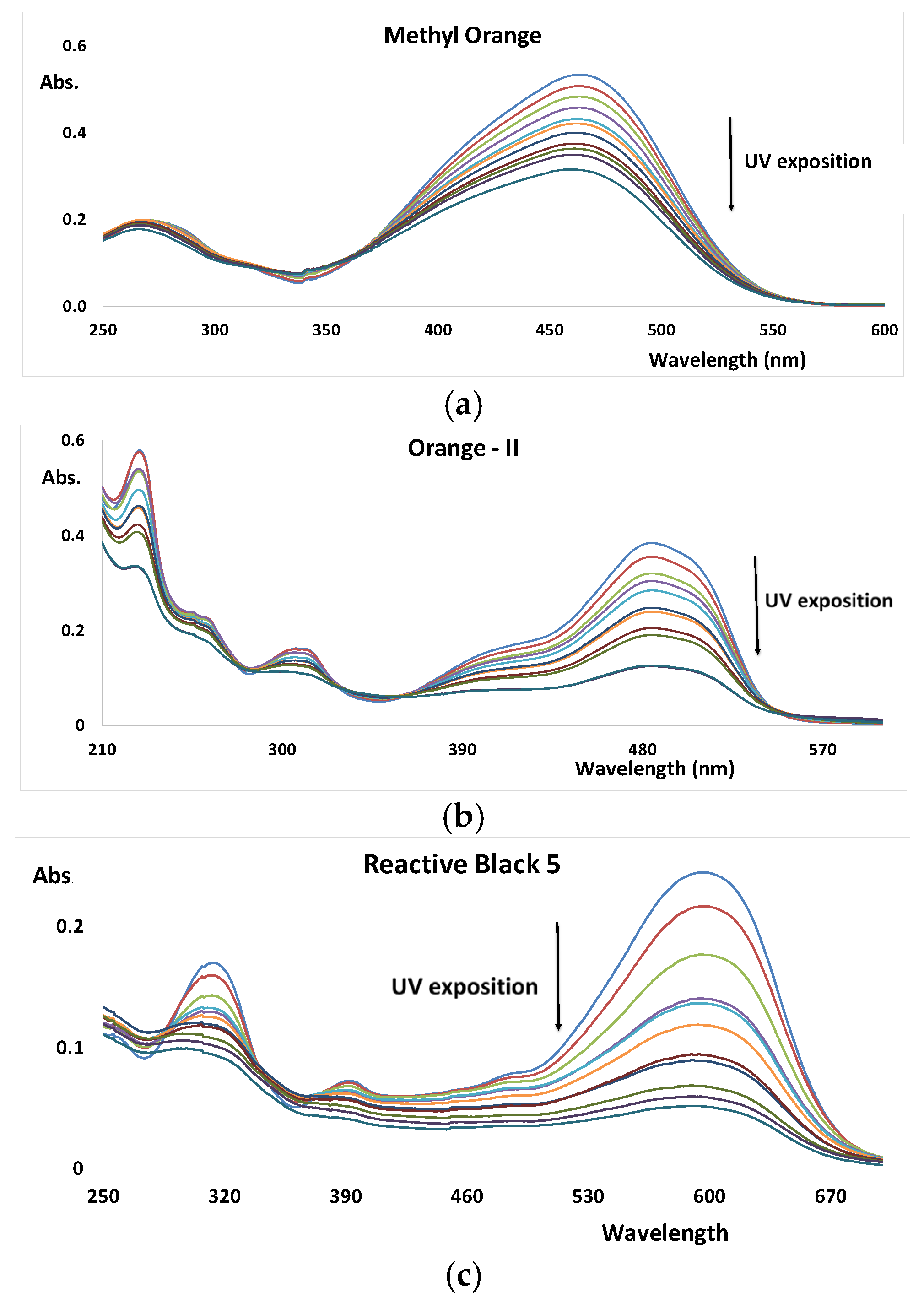
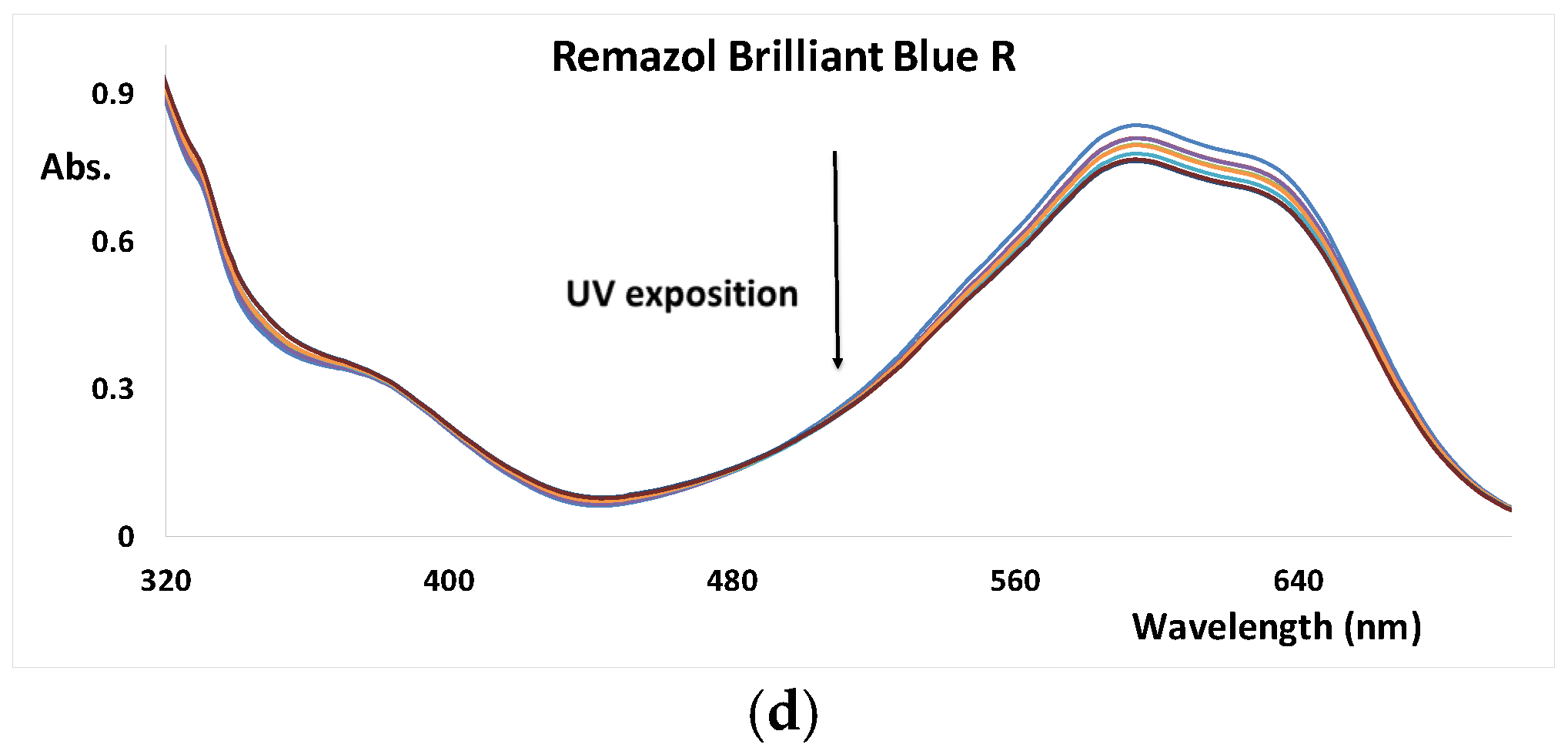
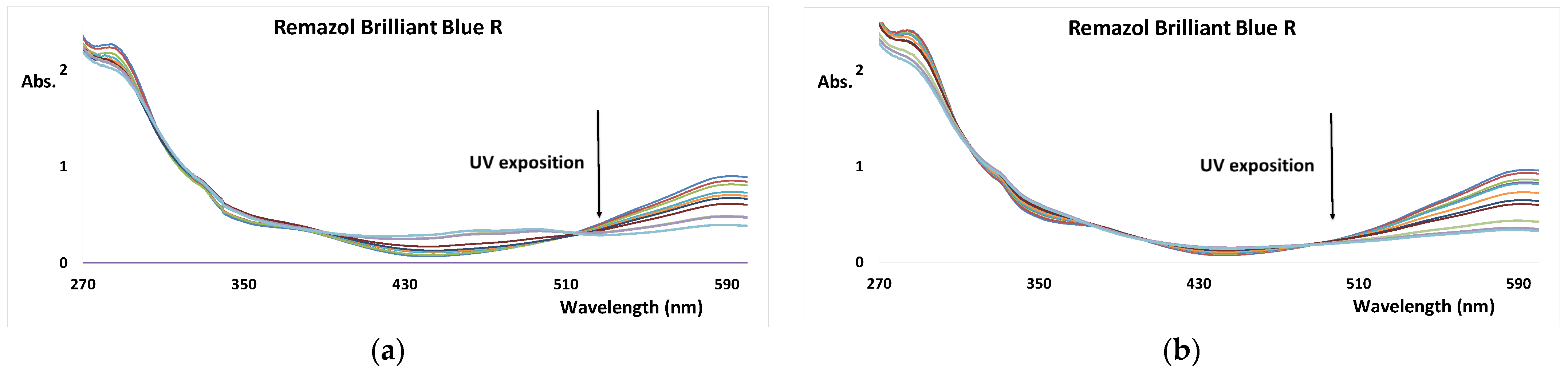
3.1. UV AOP of Dyes
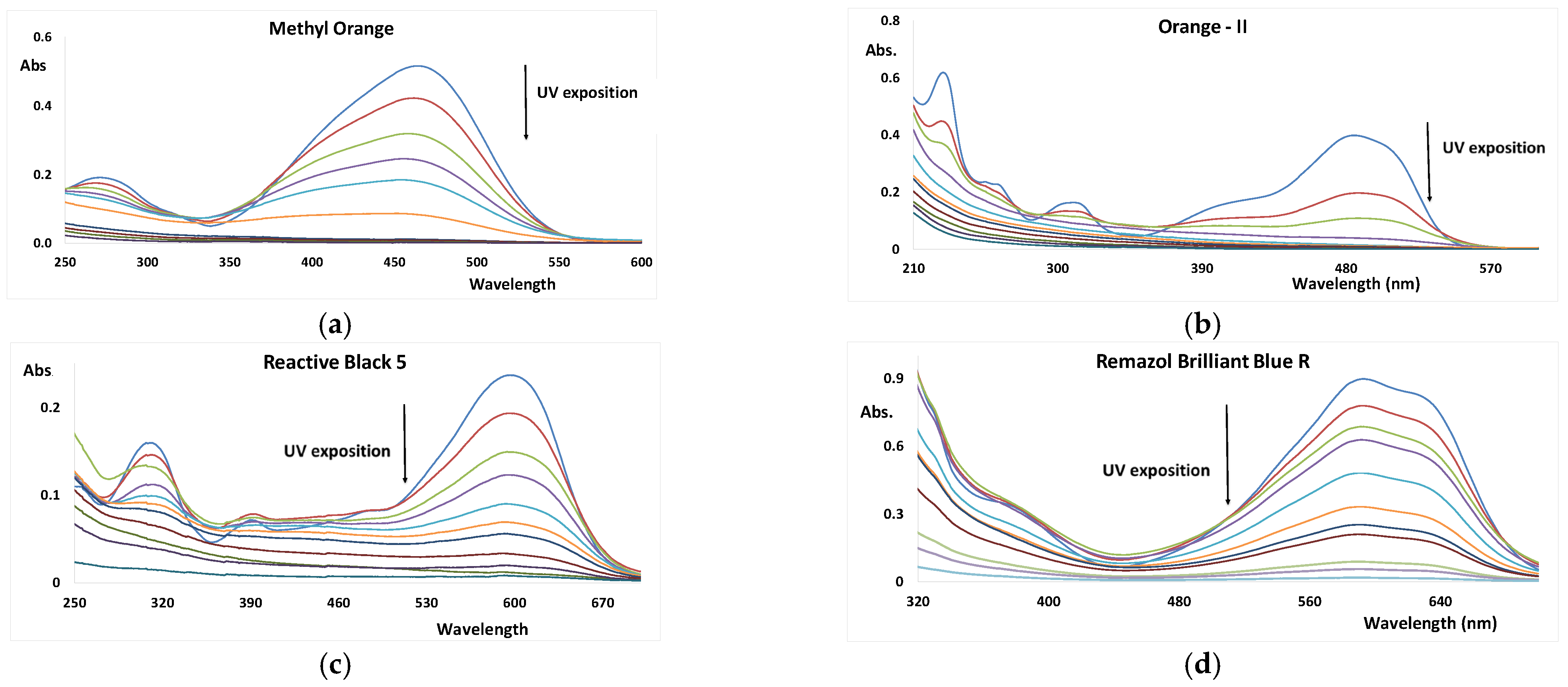
3.2. UV/H2O2 AOP of Dyes
3.3. UV/S2O82− AOP of Dyes
3.4. UV/TiO2 AOP of Dyes
3.5. Preliminary Analysis of the Catalytic Performance of the Synthesized CD
3.6. Characterization of the Nanomaterials
3.7. UV-Based AOP of RBB-R in the Presence of TiP-CD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The World Bank. How Much Do Our Wardrobes Cost to the Environment? 2019. Available online: https://www.worldbank.org/en/news/feature/2019/09/23/costo-moda-medio-ambiente (accessed on 18 April 2022).
- European Parliament News. The Impact of Textile Production and Waste on the Environment (Infographic). 2020. Available online: https://www.europarl.europa.eu/news/en/headlines/society/20201208STO93327/the-impact-of-textile-production-and-waste-on-the-environment-infographic (accessed on 18 April 2022).
- ECOS. Ecodesign Requirements for Textiles are a Crucial Step Towards Stopping Fast Fashion. 2021. Available online: https://ecostandard.org/news_events/ecos-report-durable-repairable-and-mainstream/ (accessed on 18 April 2022).
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; Cardoso, R.M.F.; da Silva, L.P.; Esteves da Silva, J.C.G. Copper(II) doped carbon dots as catalyzer of ozone degradation of textile dyes. Nanomaterials 2022, 12, 1211. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, I.M.F.; Cardoso, R.M.F.; Esteves da Silva, J.C.G. Advanced oxidation processes coupled with nanomaterials for water treatment. Nanomaterials 2021, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Handbook on Advanced Photochemical Oxidation Processes; EPAl625/R-981004; United States Environmental Protection Agency: Washington, DC, USA, 1998.
- Mierzwa, J.C.; Rodrigues, R.; Teixeira, A. UV-Hydrogen Peroxide Processes. In Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Ameta, S.C., Ameta, R., Eds.; Academic Press: London, UK, 2018. [Google Scholar]
- Silva, C.G.; Faria, J.L. Photochemical and photocatalytic degradation of an azo dye in aqueous solution by UV irradiation. J. Photochem. Photobiol. A Chem. 2003, 155, 133–143. [Google Scholar] [CrossRef]
- Basturk, E.; Karatas, M. Decolorization of antraquinone dye Reactive Blue 181 solution by UV/H2O2 process. J. Photochem. Photobiol. A Chem. 2015, 299, 67–72. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Zhou, S.; Xiao, Y.; Zhuang, Z. Kinetic study of acetaminophen degradation by UV-based advanced oxidation processes. Chem. Eng. J. 2014, 253, 229–236. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, Y. Absorption of H2S from Gas Streams by the Wet Ultraviolet/Persulfate Oxidation Process: Mechanism and Kinetics. Energy Fuels 2020, 34, 8037–8045. [Google Scholar] [CrossRef]
- Brienza, M.; Katsoyiannis, I.A. Sulfate Radical Technologies as Tertiary Treatment for the Removal of Emerging Contaminants from Wastewater. Sustainability 2017, 9, 1604. [Google Scholar] [CrossRef] [Green Version]
- Baran, W.; Makowski, A.; Wardas, W. The effect of UV radiation absorption of cationic and anionic dye solutions on their photocatalytic degradation in the presence TiO2. Dyes Pigment. 2008, 76, 226–230. [Google Scholar] [CrossRef]
- Kasinathan, K.; Kennedy, J.; Elayaperumal, M.; Henini, M.; Malik, M. Photodegradation of organic pollutants RhB dye using UV simulated sunlight on ceria based TiO2 nanomaterials for antibacterial applications. Sci. Rep. 2016, 6, 38064. [Google Scholar] [CrossRef] [Green Version]
- Reza, K.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Tlatelpa, T.; Trull, J.; Romeral, L. In situ Decolorization Monitoring of Textile Dyes for an Optimized UV-LED/TiO2 Reactor. Catalysts 2019, 9, 669. [Google Scholar] [CrossRef] [Green Version]
- El Mragui, A.; Zegaoui, O.; Esteves da Silva, J.C.G. Elucidation of the photocatalytic degradation mechanism of an azo dye under visible light in the presence of cobalt doped TiO2 nanomaterials. Chemosphere 2021, 266, 128931. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Esteves da Silva, J.C.G.; Gonçalves, H.M.R. Analytical and bioanalytical applications of carbon dots. Trends Anal. Chem. 2011, 30, 1327–1336. [Google Scholar] [CrossRef]
- Simões, E.; Leitão, J.; Esteves da Silva, J.C.G. Carbon dots from tryptophan doped glucose for peroxynitrite sensing. Anal. Chim. Acta 2014, 852, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Algarra, M.; Martin, M.P.; Rueda, M.C.; Jiménez-Jiménez, J.; Esteves da Silva, J.C.G.; Bandosz, T.; Castellón, E.R.; Casado, J.; López-Navarrete, J.T. Carbon Dots obtained using hydrothermal treatment of formaldehyde. Cell imaging in-vitro. Nanoscale 2014, 6, 9071–9077. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, S.-H. Carbon dots: Large-scale synthesis, sensing and bioimaging. Mater. Today 2016, 19, 382–393. [Google Scholar] [CrossRef]
- Algarra, M.; Contreras-Caceres, R.; Bandosz, T.J.; Castellón, E.R.; Esteves da Silva, J.C.G.; Campos, B.B.; Jimenez-Jimenez, J. Carbon Dots as Fluorescent Sensor for Detection of Explosive Nitrocompounds. Carbon 2016, 106, 171–178. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.L.; Li, C.M.; Huang, C.Z. Fluorescent carbon dots functionalization. Adv. Colloid Interface Sci. 2019, 270, 165–190. [Google Scholar] [CrossRef]
- Sendão, R.M.S.; Crista, D.M.A.; Afonso, A.C.; de Yuso, M.M.; Algarra, M.; Esteves da Silva, J.C.G.; da Silva, L.P. Insight into the Hybrid Luminescence Showed by Carbon Dots and Molecular Fluorophores in Solution. Phys. Chem. Chem. Phys. 2019, 21, 20919–20926. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon dots: Synthesis, properties and biomedical applications. J. Mater. Chem. B 2021, 9, 6553. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon Dots: Synthesis, Properties and Applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of Mono-disperse carbon Quantum Dots from fennel Seeds: Photoluminescence analysis using Machine Learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef]
- Huang, G.; Lin, Y.; Zhang, L.; Yan, Z.; Wang, Y.; Liu, Y. Synthesis of Sulfur-Selenium Doped Carbon Quantum Dots for Biological Imaging and Scavenging Reactive Oxygen Species. Sci. Rep. 2019, 9, 19651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.; Li, R.; Wang, H.; Fu, J. Carbon Dot–Doped Titanium Dioxide Sheets for the Efficient Photocatalytic Performance of Refractory Pollutants. Front. Chem. 2021, 9, 706343. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.; Perumal, S.; Vinodh, R.; Lee, Y. In-situ green synthesis of nitrogen-doped carbon dots for bioimaging and TiO2 nanoparticles@nitrogen-doped carbon composite for photocatalytic degradation of organic pollutants. J. Alloys Compd. 2018, 766, 12–24. [Google Scholar] [CrossRef]
- Ramachandran, P.; Lee, C.; Doong, R.; Oon, C.; Thanh, N.; Lee, H. A titanium dioxide/nitrogen-doped graphene quantum dot nanocomposite to mitigate cytotoxicity: Synthesis, characterisation, and cell viability evaluation. RSC Adv. 2020, 10, 21795. [Google Scholar] [CrossRef]
- Jaiswal, A.; Ghosh, S.S.; Chattopadhyay, A. One step synthesis of C-dots by microwave mediated caramelization of poly(ethylene glycol). Chem. Commun. 2012, 48, 407–409. [Google Scholar] [CrossRef]
- Peng, Z.; Ji, C.; Zhou, Y.; Zhao, T.; Leblanc, R.M. Polyethylene glycol (PEG) derived carbon dots: Preparation and applications. Appl. Mat. Today 2020, 20, 100677. [Google Scholar] [CrossRef]
- Chen, S.; Jia, Q.; Zheng, X.; Wen, Y.; Liu, W.; Zhang, H.; Ge, J.; Wang, P. PEGylated carbon dot/MnO2 nanohybrid: A new pH/H2O2-d-riven, turn-on cancer nanotheranostics. Sci. China Mater. 2018, 61, 1325–1338. [Google Scholar] [CrossRef] [Green Version]
- Arsalania, N.; Nezhad-Mokhtaria, P.; Jabbari, E. Microwave-assisted and one-step synthesis of PEG passivated fluorescent carbon dots from gelatin as an efficient nanocarrier for methotrexate delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.-H.; Wu, C.-H.; Ho, T.-H.; Hong, P.K.A. Decolorization of C.I. Reactive Black 5 in UV/TiO2, UV/oxidant and UV/TiO2/oxidant systems: A comparative study. Chem. Eng. J. 2010, 158, 578–583. [Google Scholar] [CrossRef]
- Haji, S.; Benstaali, B.; Al-Bastaki, N. Degradation of methyl orange by UV/H2O2 advanced oxidation process. Chem. Eng. J. 2011, 168, 134–139. [Google Scholar] [CrossRef]
- Feng, J.; Hu, X.; Po, L.Y. Discoloration and Mineralization of Orange II Using Different Heterogeneous Catalysts Containing Fe: A Comparative Study. Environ. Sci. Technol. 2004, 38, 5773–5778. [Google Scholar] [CrossRef] [PubMed]
- El-Dein, A.; Libra, J.A.; Wiesmann, U. Mechanism and kinetic model for the decolorization of the azo dye Reactive Black 5 by hydrogen peroxide and UV radiation. Chemosphere 2003, 52, 1069–1077. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A. Degradation of Reactive Black 5 by Fenton/UV-C and ferrioxalate/H2O2/solar light processes. Dyes Pigment. 2007, 74, 622–629. [Google Scholar] [CrossRef]
- Hoang, N.T.; Nguyen, V.T.; Tuan, N.D.; Manh, T.D.; Le, P.-C.; Tac, D.; Mwazighe, F.M. Degradation of dyes by UV/Persulfate and comparison with other UV-based advanced oxidation processes: Kinetics and role of radicals. Chemosphere 2022, 298, 134197. [Google Scholar] [CrossRef]
- Yu, X.; Sun, J.; Li, G.; Huang, Y.; Li, Y.; Xia, D.; Jiang, F. Integration of SO4−-based AOP mediated by reusable iron particles and a sulfidogenic process to degrade and detoxify Orange II. Water Res. 2020, 174, 115622. [Google Scholar] [CrossRef]
- Fadaei, S.; Noorisepehr, M.; Pourzamani, H.; Salari, M.; Moradnia, M.; Darvishmotevalli, M.; Mengelizadeh, N. Heterogeneous activation of peroxymonosulfate with Fe3O4 magnetic nanoparticles for degradation of Reactive Black 5: Batch and column study. J. Environ. Chem. Eng. 2021, 9, 105414. [Google Scholar] [CrossRef]
- Feng, S.; Xiao, B.; Wu, M.; Wang, Y.; Chen, R.; Liu, H. Copper phosphide: A dual-catalysis-center catalyst for the efficient activation of peroxydisulfate and degradation of Orange II. Sep. Purif. Technol. 2020, 248, 117004. [Google Scholar] [CrossRef]
- Su, S.; Liu, Y.; He, W.; Tang, X.; Jin, W.; Zhao, Y. A novel graphene oxide-carbon nanotubes anchored α-FeOOH hybrid activated persulfate system for enhanced degradation of Orange II. J. Environ. Sci. 2019, 83, 73–84. [Google Scholar] [CrossRef]
- Al-Mamun, M.; Hossain, K.T.; Mondal, S.; Khatun, M.; Islam, M.; Khan, D.M. Synthesis, characterization, and photocatalytic performance of methyl orange in aqueous TiO2 suspension under UV and solar light irradiation. S. Afr. J. Chem. Eng. 2022, 40, 113–125. [Google Scholar] [CrossRef]
- May-Lozano, M.; Lopez-Medina, R.; Escamilla, V.M.; Rivadeneyra-Romero, G.; Alonzo-Garcia, A.; Morales-Mora, M.; González-Díaz, M.O.; Martinez-Degadillo, S.A. Intensification of the Orange II and Black 5 degradation by sonophotocatalysis using Ag-graphene oxide/TiO2 systems. Chem. Eng. Process. Process Intensif. 2020, 158, 108175. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bhunia, H.; Bajpai, P.K. Photocatalytic Decolorization Kinetics and Mineralization of Reactive Black 5 Aqueous Solution by UV/TiO2 Nanoparticles. CLEAN Soil Air Water 2012, 40, 1290–1296. [Google Scholar] [CrossRef]
- Szeto, W.; Li, J.; Huang, H.; Leung, D.Y.C. VUV/TiO2 photocatalytic oxidation process of methyl orange and simultaneous utilization of the lamp-generated ozone. Chem. Eng. Sci. 2018, 177, 380–390. [Google Scholar] [CrossRef]
- Marques, S.M.; Tavares, C.J.; Oliveira, L.F.; Oliveira-Campos, A.M.F. Photocatalytic degradation of C.I. Reactive Blue 19 with nitrogen-doped TiO2 catalysts thin films under UV/visible light. J. Mol. Struct. 2010, 983, 147–152. [Google Scholar] [CrossRef]
- Mokhtari, P.N.; Arsalani, N.; Ghorbani, M.; Hamishehkar, H. Development of biocompatible fluorescent gelatin nanocarriers for cell imaging and anticancer drug targeting. J. Mater. Sci. 2018, 53, 10679–10691. [Google Scholar] [CrossRef]
- Mura, S.; Ludmerczki, R.; Stagi, L.; Garroni, S.; Carbonaro, C.M.; Ricci, P.C.; Casula, M.F.; Malfatti, L.; Innocenzi, P. Integrating sol-gel and carbon dots chemistry for the fabrication of fluorescent hybrid organic-inorganic films. Sci. Rep. 2020, 10, 4770. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Weber, F.; Weigert, F.; Wang, Y.; Choudhury, S.; Xiao, J.; Lauermann, I.; Genger, U.R.; Bande, A.; Petit, T. Influence of surface chemistry on optical, chemical and electronic properties of blue luminescent carbon dots. Nanoscale 2019, 11, 2056–2064. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.D.; Arellano, J.C.; Luna, L.A.G.; Rojas, L.L. Photo-Fenton Degradation of RB5 Dye in Aqueous Solution Using Fe Supported on Mexican Natural Zeolite. Int. J. Photoenergy 2019, 981631. [Google Scholar] [CrossRef] [Green Version]
- Chonga, M.N.; Tneua, Z.T.; Poha, P.E.; Arya, J.R. Synthesis, characterisation and application of TiO2–zeolite nanocomposites for the advanced treatment of industrial dye wastewater. J. Taiwan Inst. Chem. Eng. 2015, 50, 288–296. [Google Scholar] [CrossRef]
- Christé, S.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Evaluation of the Environmental Impact and Efficiency of N-Doping Strategies in the Synthesis of Carbon Dots. Materials 2020, 13, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crista, D.; Algarra, M.; Esteves da Silva, J.C.G.; Luque, R.; Pinto da Silva, L. Sustainable Production of Carbon Dots Nanoparticles from Spent Coffee Grounds. Sensing and Life Cycle Assessment Analysis. Nanomaterials 2020, 10, 1209. [Google Scholar] [CrossRef] [PubMed]
- Crista, D.; Pinto da Silva, L.; Esteves da Silva, J.C.G. Evaluation of Different Bottom-up Routes for the Fabrication of Carbon Dots. Nanomaterials 2020, 10, 1316. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Desipio, M.M.; Hoinkis, T.J.; Smeltz, E.J.; Thorpe, R.; Hensley, D.K.; Fischer-Drowos, S.G.; Chen, J. Influence of hydrogen peroxide in enhancing photocatalytic activity of carbon nitride under visible light: An insight into reaction intermediates. J. Environ. Chem. Eng. 2018, 6, 4927–4936. [Google Scholar] [CrossRef]
- Miller, C.M.; Valentine, R.L. Mechanistic Studies of Surface Catalysed H2O2 Decomposition and Contaminant Degradation in the Presence of Sand. Water Res. 1999, 33, 2805–2816. [Google Scholar] [CrossRef]
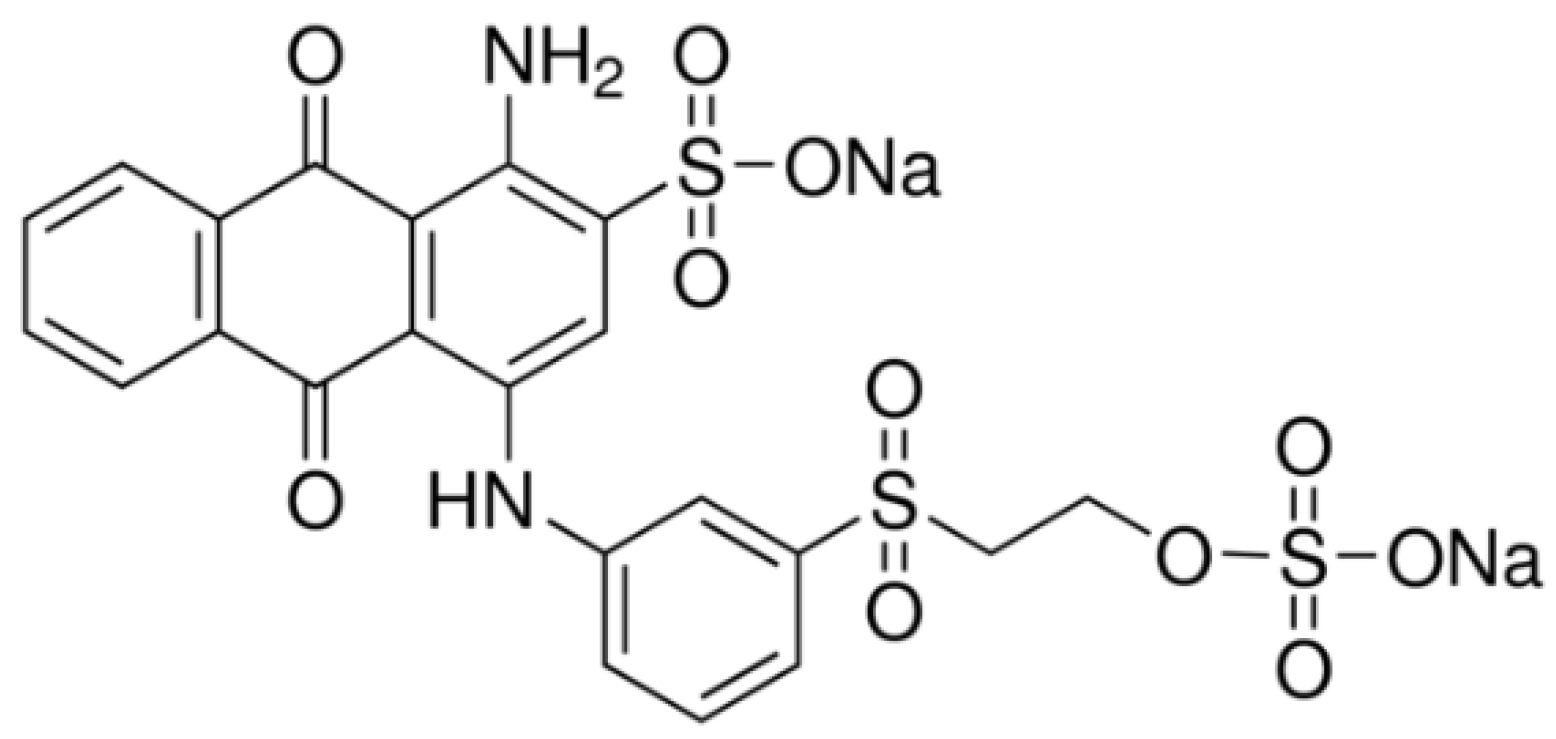
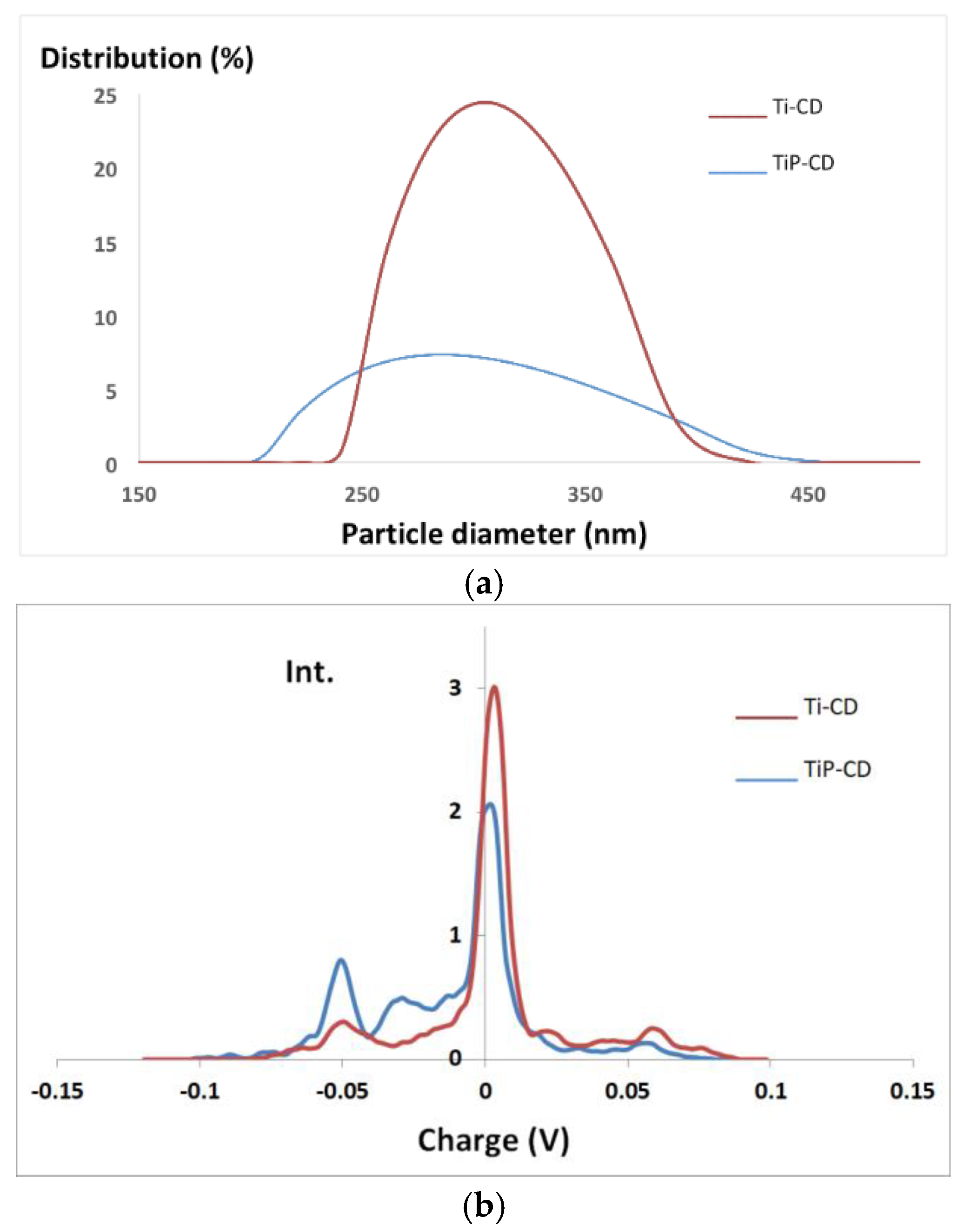
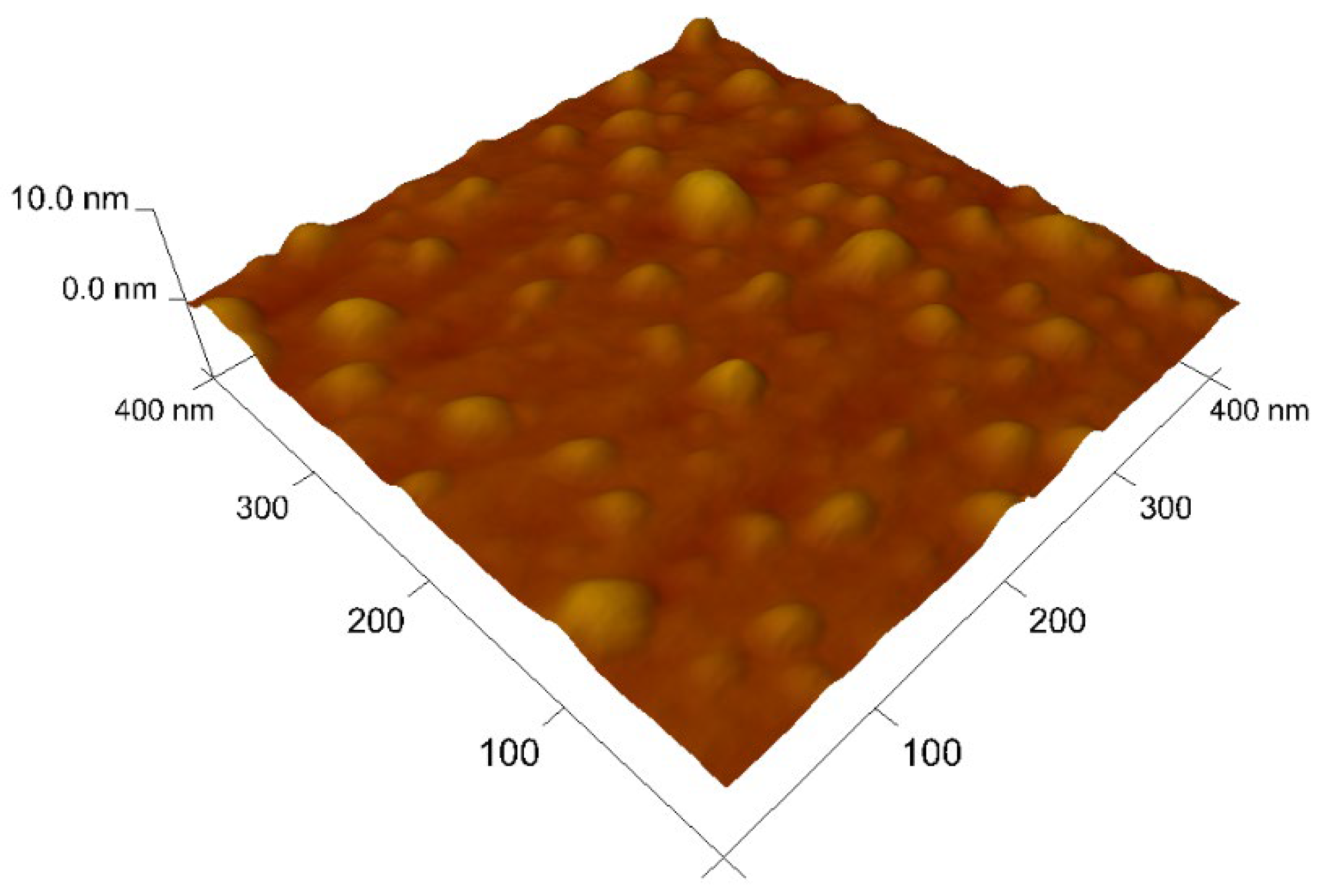
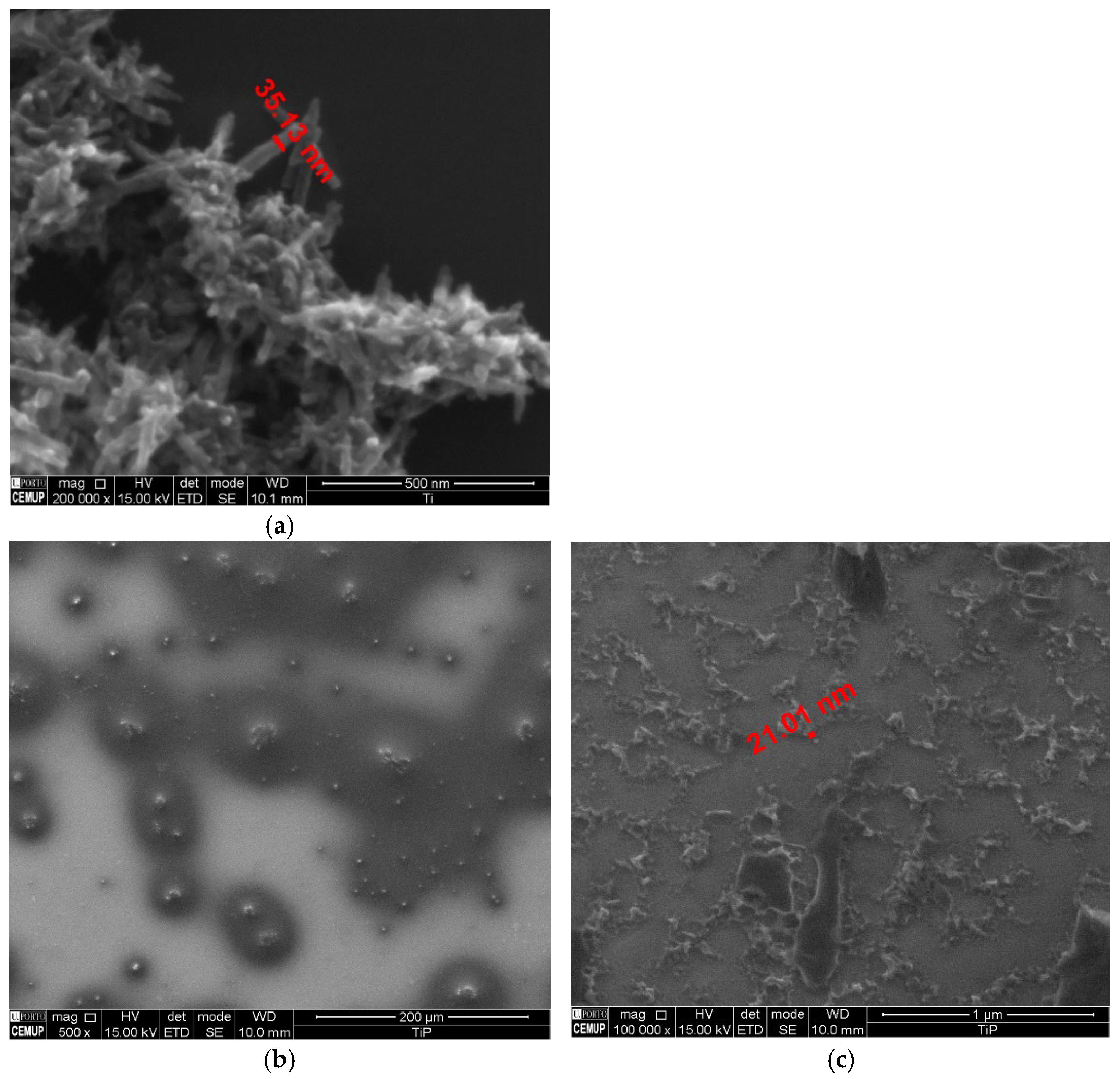

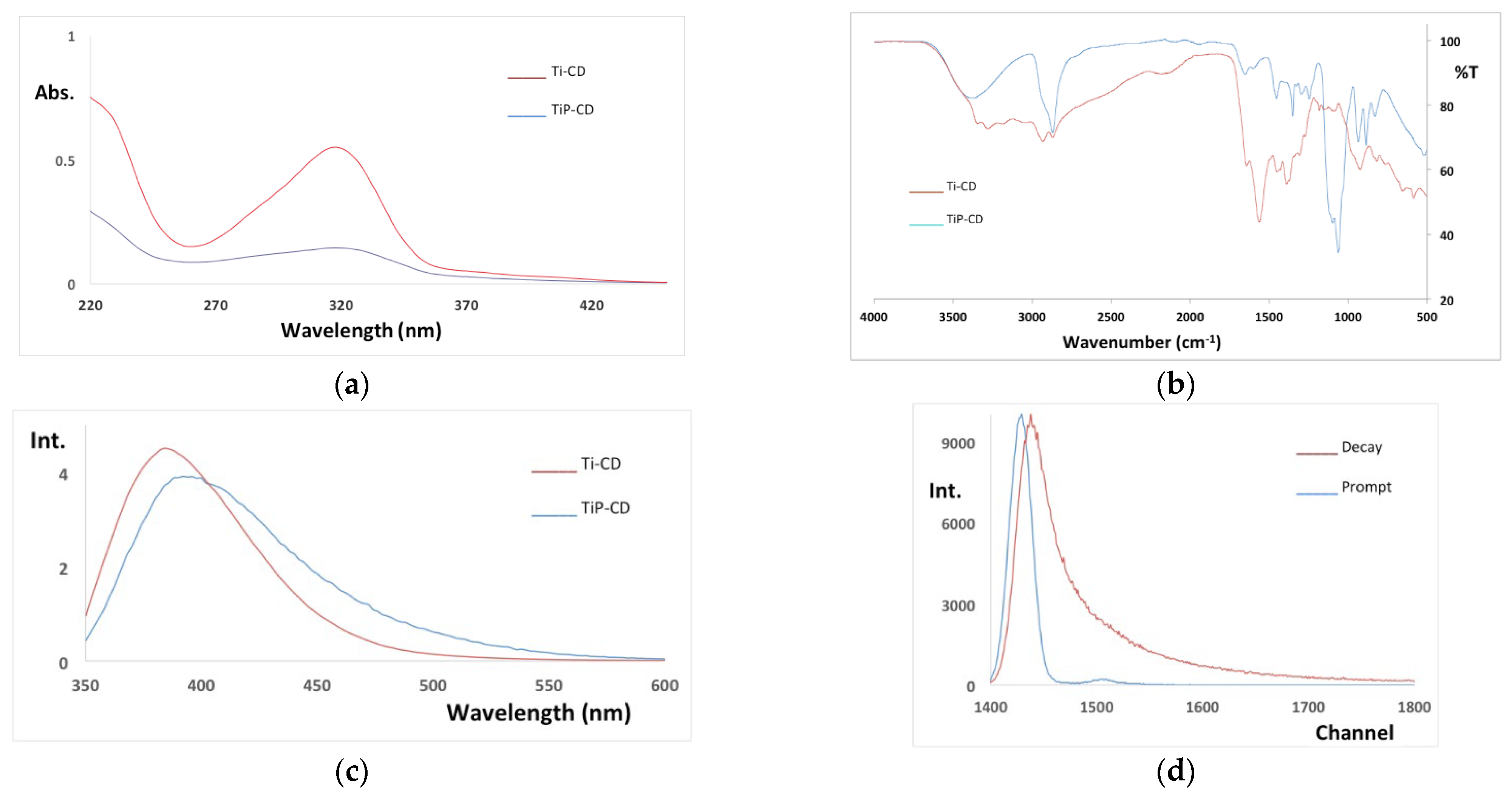
| Dye | pH | kap (min−1) | %DR |
|---|---|---|---|
| MO | 3 | 0.0294 ± 0.0108 | 63.53 ± 3.56 |
| 7 | 0.0051 ± 0.0006 | 15.23 ± 1.94 | |
| 10 | 0.0169 ± 0.0012 | 40.58 ± 0.27 | |
| O-II | 3 | 0.0129 ± 0.0003 | 33.73 ± 0.83 |
| 7 | 0.0133 ± 0.0016 | 32.53 ± 3.16 | |
| 10 | 0.0337 ± 0.0006 | 64.50 ± 2.57 | |
| RB-5 | 3 | 0.0227 ± 0.0006 | 47.37 ± 3.42 |
| 7 | 0.0235 ± 0.0007 | 48.39 ± 1.94 | |
| 10 | 0.0474 ± 0.0046 | 78.07 ± 1.03 | |
| RBB-R | 3 | 0.0078 ± 0.0009 | 22.20 ± 3.10 |
| 7 | 0.0034 ± 0.0003 | 9.16 ± 0.32 | |
| 10 | 0.0025 ± 0.0009 | 7.78 ± 2.82 |
| Dye | kapc (min−1) | %Inc | %DR |
|---|---|---|---|
| MO + 0.30 mM H2O2 | 0.2294 ± 0.0179 | 4398 | 99.95 ± 0.03 (30 min) |
| O-II + 0.30 mM H2O2 | 0.5921 ± 0.1465 | 4352 | 98.64 ± 0.46 (10 min) |
| RB-5 + 0.30 mM H2O2 | 0.9017 ± 0.0773 | 3737 | 96.19 ± 1.93 (4 min) |
| RBB-R + 0.30 mM H2O2 | 0.0046 ± 0.0007 | 35 | 13.87 ± 1.84 (30 min) |
| RBB-R + 3.0 mM H2O2 | 0.0091 ± 0.0016 | 168 | 23.72 ± 1.76 (30 min) |
| RBB-R + 30 mM H2O2 | 0.1169 ± 0.0121 | 3338 | 96.22 ± 1.43 (30 min) |
| Dye | kapc (min−1) | %Inc | %DR |
|---|---|---|---|
| MO + 0.10 mM Na2S2O8 | 0.0677 ± 0.0010 | 1227 | 87.05 ± 0.78 (30 min) |
| MO + 1.02 mM Na2S2O8 | 0.9365 ± 0.0810 | 18,263 | 98.56 ± 0.60 (5 min) |
| O-II + 0.010 mM Na2S2O8 | 0.0255 ± 0.0205 | 400 | 54.08 ± 0.42 (30 min) |
| O-II + 0.10 mM Na2S2O8 | 0.4498 ± 0.0280 | 8720 | 94.99 ± 3.39 (30 min) |
| O-II + 1.02 mM Na2S2O8 | 1.1187± 0.1949 | 21,835 | 98.86 ± 0.31 (5 min) |
| RB-5 + 0.010 mM Na2S2O8 | 0.0601 ± 0.0042 | 1078 | 80.74 ± 4.24 (30 min) |
| RB-5 + 0.10 mM Na2S2O8 | 0.5608 ± 0.0606 | 10,896 | 91.03 ± 4.68 (5 min) |
| RBB-R + 0.010 mM Na2S2O8 | 0.0017 ± 0.0049 | −50 | 5.31 ± 1.21 (30 min) |
| RBB-R + 1.02 mM Na2S2O8 | 0.0277 ± 0.0049 | 715 | 58.70 ± 7.36 (30 min) |
| RBB-R + 2.04 mM Na2S2O8 | 0.0531 ± 0.0108 | 1462 | 80.39 ± 5.93 (30 min) |
| RBB-R + 4.07 mM Na2S2O8 | 0.0712 ± 0.0024 | 1994 | 81.59 ± 4.87 (30 min) |
| RBB-R + 8.15 mM Na2S2O8 | 0.0960 ± 0.0056 | 2724 | 95.25 ± 1.21 (30 min) |
| Dye | kapc (min−1) | %Inc | %DR |
|---|---|---|---|
| MO + 0.050 g/L TiO2 | 0.0220 ± 0.0032 | 331 | 48.92 ± 5.28 (30 min) |
| O-II + 0.050 g/L TiO2 | 0.0409 ± 0.0022 | 208 | 68.69 ± 1.96 (30 min) |
| RB-5 + 0.050 g/L TiO2 | 0.0591 ± 0.0085 | 151 | 66.12 ± 2.22 (30 min) |
| RBB-R + 0.050 g/L TiO2 | 0.0035 ± 0.0009 | 3 | 8.87 ± 1.29 (30 min) |
| kapN (min−1) | %IncN | %DR | |
|---|---|---|---|
| RBB-R + TiP-CD | 0.0029 ± 0.0003 | −14 | 8.02 ± 0.88 |
| RBB-R + Ti-CD | 0.0023 ± 0.0003 | −32 | 7.17 ± 0.71 |
| RBB-R + 0.30 mM H2O2 + TiP-CD | 0.0071 ± 0.0021 | 54 | 17.83 ± 5.29 |
| RBB-R + 3.0 mM H2O2 + TiP-CD | 0.0330 ± 0.0087 | 263 | 57.91 ± 8.91 |
| RBB-R + 3.0 mM H2O2 + Ti-CD | 0.0079 ± 0.0013 | 1.3 | 21.12 ± 3.51 |
| RBB-R + 0.010 mM Na2S2O8 + TiP-CD | 0.0021 ± 0.0001 | 24 | 6.18 ± 0.40 |
| RBB-R + 1.02 mM Na2S2O8 + TiP-CD | 0.0345 ± 0.0032 | 25 | 63.35 ± 1.99 |
| RBB-R + 1.02 mM Na2S2O8 + Ti-CD | 0.0236 ± 0.0014 | 6 | 52.59 ± 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, I.M.F.; Cardoso, R.M.F.; Pinto da Silva, L.; Esteves da Silva, J.C.G. UV-Based Advanced Oxidation Processes of Remazol Brilliant Blue R Dye Catalyzed by Carbon Dots. Nanomaterials 2022, 12, 2116. https://doi.org/10.3390/nano12122116
Cardoso IMF, Cardoso RMF, Pinto da Silva L, Esteves da Silva JCG. UV-Based Advanced Oxidation Processes of Remazol Brilliant Blue R Dye Catalyzed by Carbon Dots. Nanomaterials. 2022; 12(12):2116. https://doi.org/10.3390/nano12122116
Chicago/Turabian StyleCardoso, Inês M. F., Rita M. F. Cardoso, Luís Pinto da Silva, and Joaquim C. G. Esteves da Silva. 2022. "UV-Based Advanced Oxidation Processes of Remazol Brilliant Blue R Dye Catalyzed by Carbon Dots" Nanomaterials 12, no. 12: 2116. https://doi.org/10.3390/nano12122116






