Polydimethylsiloxane Sponge-Supported Metal Nanoparticles as Reusable Catalyst for Continuous Flow Reactions
Abstract
:1. Introduction
2. Experimental Part
2.1. Chemicals and Materials
2.2. PDMS Sponges
2.3. PDMS Supported Gold or Palladium Catalyst
2.4. Reduction of p-NP to p-AP
2.5. Catalyst Recycling
2.6. Continuous Flow Reaction
2.7. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Chen, J.; Lim, B.; Lee, E.P.; Xia, Y. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95. [Google Scholar] [CrossRef]
- Crespo-Quesada, M.; Yarulin, A.; Jin, M.; Xia, Y.; Kiwi-Minsker, L. Structure Sensitivity of Alkynol Hydrogenation on Shape- and Size-Controlled Palladium Nanocrystals: Which Sites Are Most Active and Selective? J. Am. Chem. Soc. 2011, 133, 12787–12794. [Google Scholar] [CrossRef]
- Dimitratos, N.; Lopez-Sanchez, J.A.; Hutchings, G.J. Selective liquid phase oxidation with supported metal nanoparticles. Chem. Sci. 2012, 3, 20–44. [Google Scholar] [CrossRef]
- Hong, J.W.; Kang, S.W.; Choi, B.-S.; Kim, D.; Lee, S.B.; Han, S.W. Controlled Synthesis of Pd–Pt Alloy Hollow Nanostructures with Enhanced Catalytic Activities for Oxygen Reduction. ACS Nano 2012, 6, 2410–2419. [Google Scholar] [CrossRef]
- Younan, X.; Hong, Y.; Campbell, C.T. Nanoparticles for Catalysis. Acc. Chem. Res. 2013, 46, 1671–1672. [Google Scholar]
- Narayanan, R.; El-Sayed, M.A. Catalysis with Transition Metal Nanoparticles in Colloidal Solution: Nanoparticle Shape Dependence and Stability. J. Phys. Chem. B 2005, 109, 12663–12676. [Google Scholar] [CrossRef]
- Mikami, Y.; Dhakshinamoorthy, A.; Alvaro, M.; García, H. Catalytic activity of unsupported gold nanoparticles. Catal. Sci. Technol. 2013, 3, 58–69. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Jin, Y.; Qiao, S.Z. Nanostructured Metal-Free Electrochemical Catalysts for Highly Efficient Oxygen Reduction. Small 2012, 8, 3550–3566. [Google Scholar] [CrossRef]
- Wu, B.; Zheng, N. Surface and interface control of noble metal nanocrystals for catalytic and electrocatalytic applications. Nano Today 2013, 8, 168–197. [Google Scholar] [CrossRef]
- Chirea, M.; Freitas, A.; Vasile, B.S.; Ghitulica, C.; Pereira, C.M.; Silva, F. Gold Nanowire Networks: Synthesis, Characterization, and Catalytic Activity. Langmuir 2011, 27, 3906–3913. [Google Scholar] [CrossRef]
- Hu, H.; Xin, J.H.; Hu, H.; Wang, X.; Miao, D.; Liu, Y. Synthesis and stabilization of metal nanocatalysts for reduction reactions—A review. J. Mater. Chem. A 2015, 3, 11157–11182. [Google Scholar] [CrossRef]
- Carregal-Romero, S.; Pérez-Juste, J.; Hervés, P.; Liz-Marzán, L.M.; Mulvaney, P. Colloidal Gold-Catalyzed Reduction of Ferrocyanate (III) by Borohydride Ions: A Model System for Redox Catalysis. Langmuir 2010, 26, 1271–1277. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Sun, L.-D.; Xu, C.-H.; Wang, J.; Yu, J.C.; Yan, C.-H. Porous Single-Crystalline Palladium Nanoparticles with High Catalytic Activities. Angew. Chem. Int. Ed. 2012, 51, 4872–4876. [Google Scholar] [CrossRef]
- Han, C.; Chen, Z.; Zhang, N.; Colmenares, J.C.; Xu, Y.-J. Hierarchically CdS Decorated 1D ZnO Nanorods-2D Graphene Hybrids: Low Temperature Synthesis and Enhanced Photocatalytic Performance. Adv. Funct. Mater. 2015, 25, 221–229. [Google Scholar] [CrossRef]
- Lee, J.-W.; Mayer-Gall, T.; Opwis, K.; Song Choong, E.; Gutmann Jochen, S.; List, B. Organotextile Catalysis. Science 2013, 341, 1225–1229. [Google Scholar] [CrossRef]
- Taladriz-Blanco, P.; Hervés, P.; Pérez-Juste, J. Supported Pd Nanoparticles for Carbon–Carbon Coupling Reactions. Top. Catal. 2013, 56, 1154–1170. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, C.; Xiao, M.; Wang, F.; Li, C.; Wang, J.; Yu, J.C. Loading Metal Nanostructures on Cotton Fabrics as Recyclable Catalysts. Small 2013, 9, 1003–1007. [Google Scholar] [CrossRef]
- Zhang, K.; Suh, J.M.; Choi, J.-W.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Recent Advances in the Nanocatalyst-Assisted NaBH4 Reduction of Nitroaromatics in Water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef] [Green Version]
- Hariprasad, E.; Radhakrishnan, T.P. Palladium Nanoparticle-Embedded Polymer Thin Film “Dip Catalyst” for Suzuki–Miyaura Reaction. ACS Catal. 2012, 2, 1179–1186. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Wacławek, S.; Černík, M.; Varma, R.S. Tree gum-based renewable materials: Sustainable applications in nanotechnology, biomedical and environmental fields. Biotechnol. Adv. 2018, 36, 1984–2016. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic Analysis of Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles Immobilized in Spherical Polyelectrolyte Brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Carregal-Romero, S.; Buurma, N.J.; Pérez-Juste, J.; Liz-Marzán, L.M.; Hervés, P. Catalysis by Au@pNIPAM Nanocomposites: Effect of the Cross-Linking Density. Chem. Mater. 2010, 22, 3051–3059. [Google Scholar] [CrossRef]
- Han, J.; Fang, P.; Jiang, W.; Li, L.; Guo, R. Ag-Nanoparticle-Loaded Mesoporous Silica: Spontaneous Formation of Ag Nanoparticles and Mesoporous Silica SBA-15 by a One-Pot Strategy and Their Catalytic Applications. Langmuir 2012, 28, 4768–4775. [Google Scholar] [CrossRef]
- Elhage, A.; Lanterna, A.E.; Scaiano, J.C. Tunable Photocatalytic Activity of Palladium-Decorated TiO2: Non-Hydrogen-Mediated Hydrogenation or Isomerization of Benzyl-Substituted Alkenes. ACS Catal. 2017, 7, 250–255. [Google Scholar] [CrossRef]
- Jin, Z.; Xiao, M.; Bao, Z.; Wang, P.; Wang, J. A General Approach to Mesoporous Metal Oxide Microspheres Loaded with Noble Metal Nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 6406–6410. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Pérez-Juste, J.; Carregal-Romero, S.; Hervés, P.; Liz-Marzán, L.M. Metallodielectric Hollow Shells: Optical and Catalytic Properties. Chem. Asian J. 2006, 1, 730–736. [Google Scholar] [CrossRef]
- Zhou, N.; Polavarapu, L.; Gao, N.; Pan, Y.; Yuan, P.; Wang, Q.; Xu, Q.-H. TiO2 coated Au/Ag nanorods with enhanced photocatalytic activity under visible light irradiation. Nanoscale 2013, 5, 4236–4241. [Google Scholar] [CrossRef]
- Shang, L.; Bian, T.; Zhang, B.; Zhang, D.; Wu, L.-Z.; Tung, C.-H.; Yin, Y.; Zhang, T. Graphene-Supported Ultrafine Metal Nanoparticles Encapsulated by Mesoporous Silica: Robust Catalysts for Oxidation and Reduction Reactions. Angew. Chem. Int. Ed. 2014, 53, 250–254. [Google Scholar] [CrossRef]
- Ye, W.; Yu, J.; Zhou, Y.; Gao, D.; Wang, D.; Wang, C.; Xue, D. Green synthesis of Pt–Au dendrimer-like nanoparticles supported on polydopamine-functionalized graphene and their high performance toward 4- nitrophenol reduction. Appl. Catal. B Environ. 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Pan, X.; Zhang, N.; Xu, Y.-J. A facile one-step way to anchor noble metal (Au, Ag, Pd) nanoparticles on a reduced graphene oxide mat with catalytic activity for selective reduction of nitroaromatic compounds. CrystEngComm 2013, 15, 6819–6828. [Google Scholar] [CrossRef]
- Zhang, K.; Hong, K.; Suh, J.M.; Lee, T.H.; Kwon, O.; Shokouhimehr, M.; Jang, H.W. Facile synthesis of monodispersed Pd nanocatalysts decorated on graphene oxide for reduction of nitroaromatics in aqueous solution. Res. Chem. Intermed. 2019, 45, 599–611. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Z.; Na, C. Hierarchical Carbon Nanotube Membrane-Supported Gold Nanoparticles for Rapid Catalytic Reduction of p-Nitrophenol. ACS Sustain. Chem. Eng. 2013, 1, 746–752. [Google Scholar] [CrossRef]
- Sanles-Sobrido, M.; Correa-Duarte, M.A.; Carregal-Romero, S.; Rodríguez-González, B.; Álvarez-Puebla, R.A.; Hervés, P.; Liz-Marzán, L.M. Highly Catalytic Single-Crystal Dendritic Pt Nanostructures Supported on Carbon Nanotubes. Chem. Mater. 2009, 21, 1531–1535. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Catalysis by metal nanoparticles embedded on metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 5262–5284. [Google Scholar] [CrossRef]
- Xiang, Z.; Chen, Y.; Liu, Q.; Lu, F. A highly recyclable dip-catalyst produced from palladium nanoparticle-embedded bacterial cellulose and plant fibers. Green Chem. 2018, 20, 1085–1094. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Xu, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Wang, B.; Sui, X. Cellulose Sponge Supported Palladium Nanoparticles as Recyclable Cross-Coupling Catalysts. ACS Appl. Mater. Interfaces 2017, 9, 17155–17162. [Google Scholar] [CrossRef]
- Zheng, G.; Kaefer, K.; Mourdikoudis, S.; Polavarapu, L.; Vaz, B.; Cartmell, S.E.; Bouleghlimat, A.; Buurma, N.J.; Yate, L.; de Lera, Á.R.; et al. Palladium Nanoparticle-Loaded Cellulose Paper: A Highly Efficient, Robust, and Recyclable Self-Assembled Composite Catalytic System. J. Phys. Chem. Lett. 2015, 6, 230–238. [Google Scholar] [CrossRef]
- Zheng, G.; Polavarapu, L.; Liz-Marzán, L.M.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold nanoparticle-loaded filter paper: A recyclable dip-catalyst for real-time reaction monitoring by surface enhanced Raman scattering. Chem. Commun. 2015, 51, 4572–4575. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Gao, J.; Nag, A.; Rahaman, A. Integration of Different Graphene Nanostructures with PDMS to Form Wearable Sensors. Nanomaterials 2022, 12, 950. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, T.; Liu, Y.; Huang, Y.; Liu, Z.; Liu, Y.; Yang, B.; Zhou, X.; Zhang, J. Polydimethylsiloxane Sponge-Supported Nanometer Gold: Highly Efficient Recyclable Catalyst for Cross-Dehydrogenative Coupling in Water. ChemSusChem 2018, 11, 3586–3590. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, W.; Huang, Y.; Li, X.; Liu, Y.; Yang, B.; He, C.; Zhou, X.; Zhang, J. Bifunctional organic sponge photocatalyst for efficient cross-dehydrogenative coupling of tertiary amines to ketones. Chem. Commun. 2017, 53, 12536–12539. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, Y.; Huang, Y.; Zhang, T.; Liu, Y.; Yang, B.; He, C.; Zhou, X.; Zhang, J. Organic sponge photocatalysis. Green Chem. 2017, 19, 2925–2930. [Google Scholar] [CrossRef]
- Hickman, R.; Walker, E.; Chowdhury, S. TiO2-PDMS composite sponge for adsorption and solar mediated photodegradation of dye pollutants. J. Water Process Eng. 2018, 24, 74–82. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, W.; Huang, Y.; Zhang, T.; Yang, B.; Liu, Y.; Zhou, X.; Zhang, J. Polydimethylsiloxane sponge supported DMAP on polymer brushes: Highly efficient recyclable base catalyst and ligand in water. J. Catal. 2018, 367, 264–268. [Google Scholar] [CrossRef]
- Elhage, A.; Wang, B.; Marina, N.; Marin, M.L.; Cruz, M.; Lanterna, A.E.; Scaiano, J.C. Glass wool: A novel support for heterogeneous catalysis. Chem. Sci. 2018, 9, 6844–6852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Ji, W.; Yao, L.; Wang, Y.; Khezri, B.; Webster, R.D.; Chen, H. Strategy for Nano-Catalysis in a Fixed-Bed System. Adv. Mater. 2014, 26, 4151–4155. [Google Scholar] [CrossRef] [PubMed]
- Pardieu, E.; Chau, N.T.T.; Dintzer, T.; Romero, T.; Favier, D.; Roland, T.; Edouard, D.; Jierry, L.; Ritleng, V. Polydopamine-coated open cell polyurethane foams as an inexpensive, flexible yet robust catalyst support: A proof of concept. Chem. Commun. 2016, 52, 4691–4693. [Google Scholar] [CrossRef]
- Frindy, S.; Primo, A.; Lahcini, M.; Bousmina, M.; Garcia, H.; El Kadib, A. Pd embedded in chitosan microspheres as tunable soft-materials for Sonogashira cross-coupling in water–ethanol mixture. Green Chem. 2015, 17, 1893–1898. [Google Scholar] [CrossRef]
- Sahoo, L.; Mondal, S.; Beena, N.C.; Gloskovskii, A.; Manju, U.; Topwal, D.; Gautam, U.K. 3D Porous Polymeric-Foam-Supported Pd Nanocrystal as a Highly Efficient and Recyclable Catalyst for Organic Transformations. ACS Appl. Mater. Interfaces 2021, 13, 10120–10130. [Google Scholar] [CrossRef]
- Arora, N.; Mehta, A.; Mishra, A.; Basu, S. 4-Nitrophenol reduction catalysed by Au-Ag bimetallic nanoparticles supported on LDH: Homogeneous vs. heterogeneous catalysis. Appl. Clay Sci. 2018, 151, 1–9. [Google Scholar] [CrossRef]
- Hervés, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lu, Y.; Ballauff, M. Catalysis by metallic nanoparticles in aqueous solution: Model reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kwon, T.-H.; Im, H.; Moon, D.-I.; Baek, D.J.; Seol, M.-L.; Duarte, J.P.; Choi, Y.-K. A Polydimethylsiloxane (PDMS) Sponge for the Selective Absorption of Oil from Water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef]
- Wunder, S.; Lu, Y.; Albrecht, M.; Ballauff, M. Catalytic Activity of Faceted Gold Nanoparticles Studied by a Model Reaction: Evidence for Substrate-Induced Surface Restructuring. ACS Catal. 2011, 1, 908–916. [Google Scholar] [CrossRef]
- Grzeschik, R.; Schäfer, D.; Holtum, T.; Küpper, S.; Hoffmann, A.; Schlücker, S. On the Overlooked Critical Role of the pH Value on the Kinetics of the 4-Nitrophenol NaBH4-Reduction Catalyzed by Noble-Metal Nanoparticles (Pt, Pd, and Au). J. Phys. Chem. C 2020, 124, 2939–2944. [Google Scholar] [CrossRef]
- Strachan, J.; Barnett, C.; Masters, A.F.; Maschmeyer, T. 4-Nitrophenol Reduction: Probing the Putative Mechanism of the Model Reaction. ACS Catal. 2020, 10, 5516–5521. [Google Scholar] [CrossRef]
- Liu, J.; Hao, J.; Hu, C.; He, B.; Xi, J.; Xiao, J.; Wang, S.; Bai, Z. Palladium Nanoparticles Anchored on Amine-Functionalized Silica Nanotubes as a Highly Effective Catalyst. J. Phys. Chem. C 2018, 122, 2696–2703. [Google Scholar] [CrossRef]
- Dong, F.; Guo, W.; Park, S.-K.; Ha, C.-S. Controlled synthesis of novel cyanopropyl polysilsesquioxane hollow spheres loaded with highly dispersed Au nanoparticles for catalytic applications. Chem. Commun. 2012, 48, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhang, Q.; Zhang, T.; Yin, Y. Core–Satellite Nanocomposite Catalysts Protected by a Porous Silica Shell: Controllable Reactivity, High Stability, and Magnetic Recyclability. Angew. Chem. Int. Ed. 2008, 47, 8924–8928. [Google Scholar] [CrossRef] [PubMed]
- Biondi, I.; Laurenczy, G.; Dyson, P.J. Synthesis of Gold Nanoparticle Catalysts Based on a New Water-Soluble Ionic Polymer. Inorg. Chem. 2011, 50, 8038–8045. [Google Scholar] [CrossRef]
- Larm, N.E.; Bhawawet, N.; Thon, J.A.; Baker, G.A. Best practices for reporting nanocatalytic performance: Lessons learned from nitroarene reduction as a model reaction. New J. Chem. 2019, 43, 17932–17936. [Google Scholar] [CrossRef]

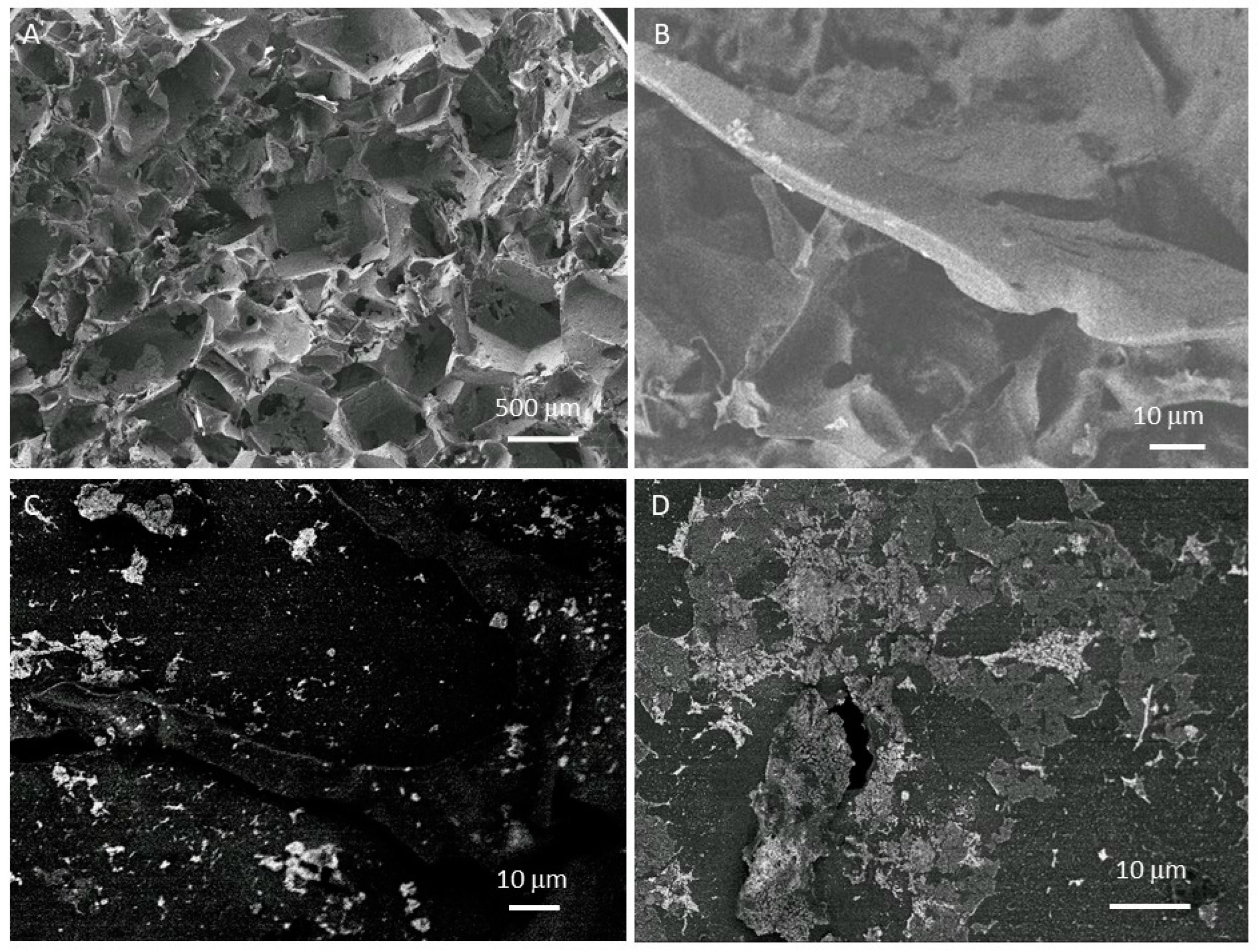


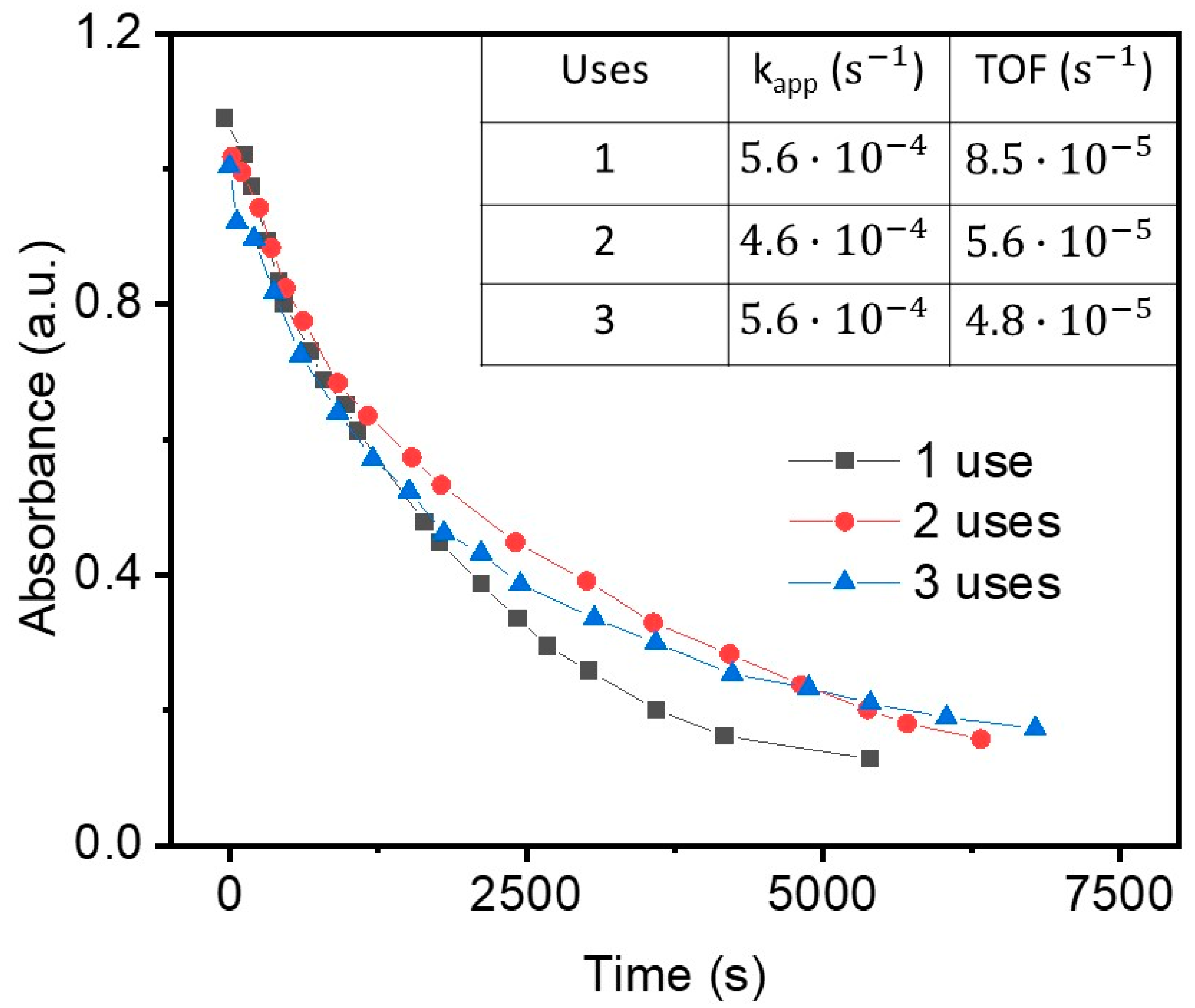
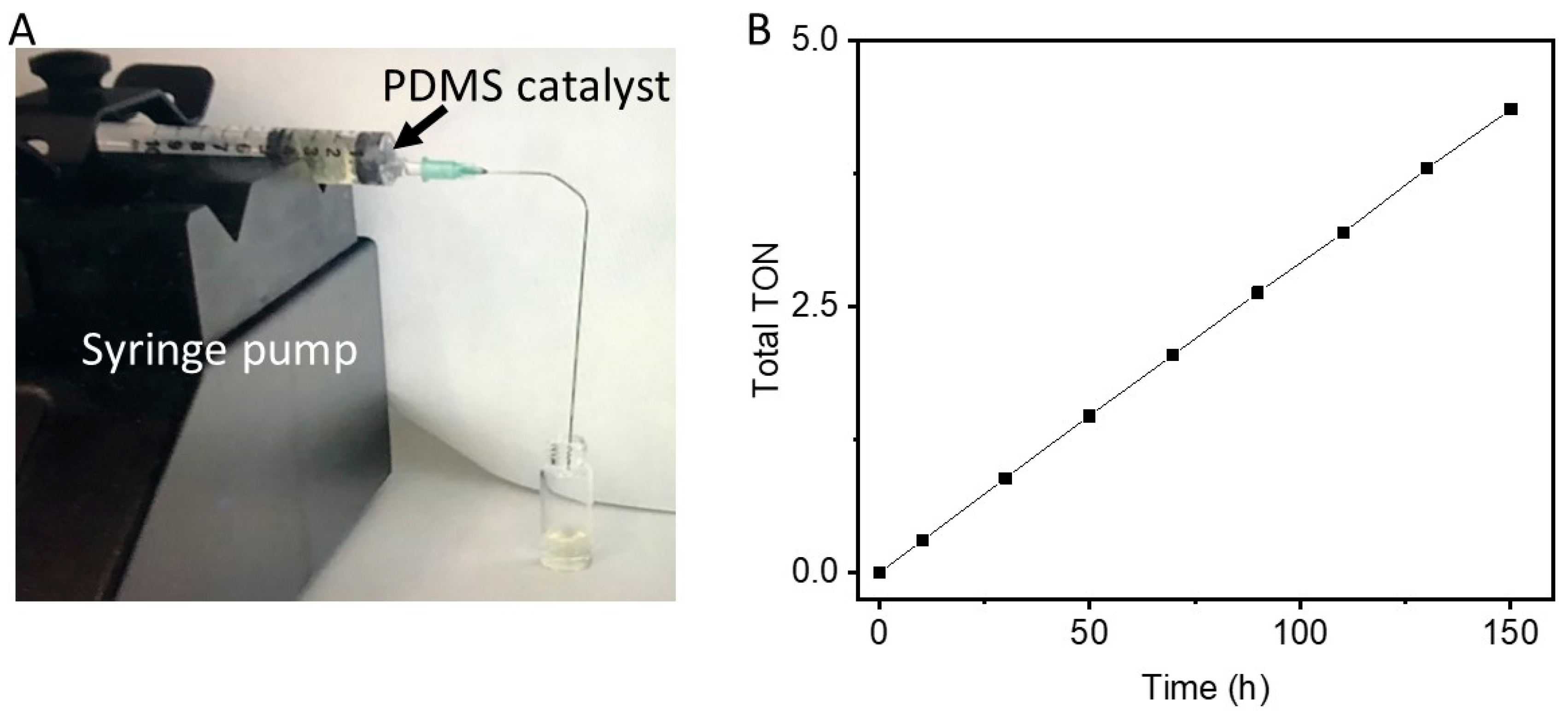
| Gold | Palladium | |||
|---|---|---|---|---|
| kapp/s−1 | TOF/s−1 | kapp/s−1 | TOF/s−1 | |
| 1 sponge | 1.9 × 10−4 | 7.0 × 10−5 | 3.3 × 10−4 | 1.03 × 10−4 |
| 2 sponge | 3.8 × 10−4 | 6.1 × 10−5 | 5.6 × 10−4 | 0.85 × 10−4 |
| 3 sponge | 6.1 × 10−4 | 6.0 × 10−5 | 10.8 × 10−4 | 1.10 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Graña, S.; Pita, M.; Humada-Iglesias, P.; Pérez-Juste, J.; Hervés, P. Polydimethylsiloxane Sponge-Supported Metal Nanoparticles as Reusable Catalyst for Continuous Flow Reactions. Nanomaterials 2022, 12, 2081. https://doi.org/10.3390/nano12122081
Gómez-Graña S, Pita M, Humada-Iglesias P, Pérez-Juste J, Hervés P. Polydimethylsiloxane Sponge-Supported Metal Nanoparticles as Reusable Catalyst for Continuous Flow Reactions. Nanomaterials. 2022; 12(12):2081. https://doi.org/10.3390/nano12122081
Chicago/Turabian StyleGómez-Graña, Sergio, Marta Pita, Paula Humada-Iglesias, Jorge Pérez-Juste, and Pablo Hervés. 2022. "Polydimethylsiloxane Sponge-Supported Metal Nanoparticles as Reusable Catalyst for Continuous Flow Reactions" Nanomaterials 12, no. 12: 2081. https://doi.org/10.3390/nano12122081






