Unravelling the Role of Synthesis Conditions on the Structure of Zinc Oxide-Reduced Graphene Oxide Nanofillers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Graphene Oxide
2.3. Solvothermal Synthesis of ZnO Particles and ZnO–rGO Composites
2.4. Nanoparticles’ Characterization
3. Results and Discussion
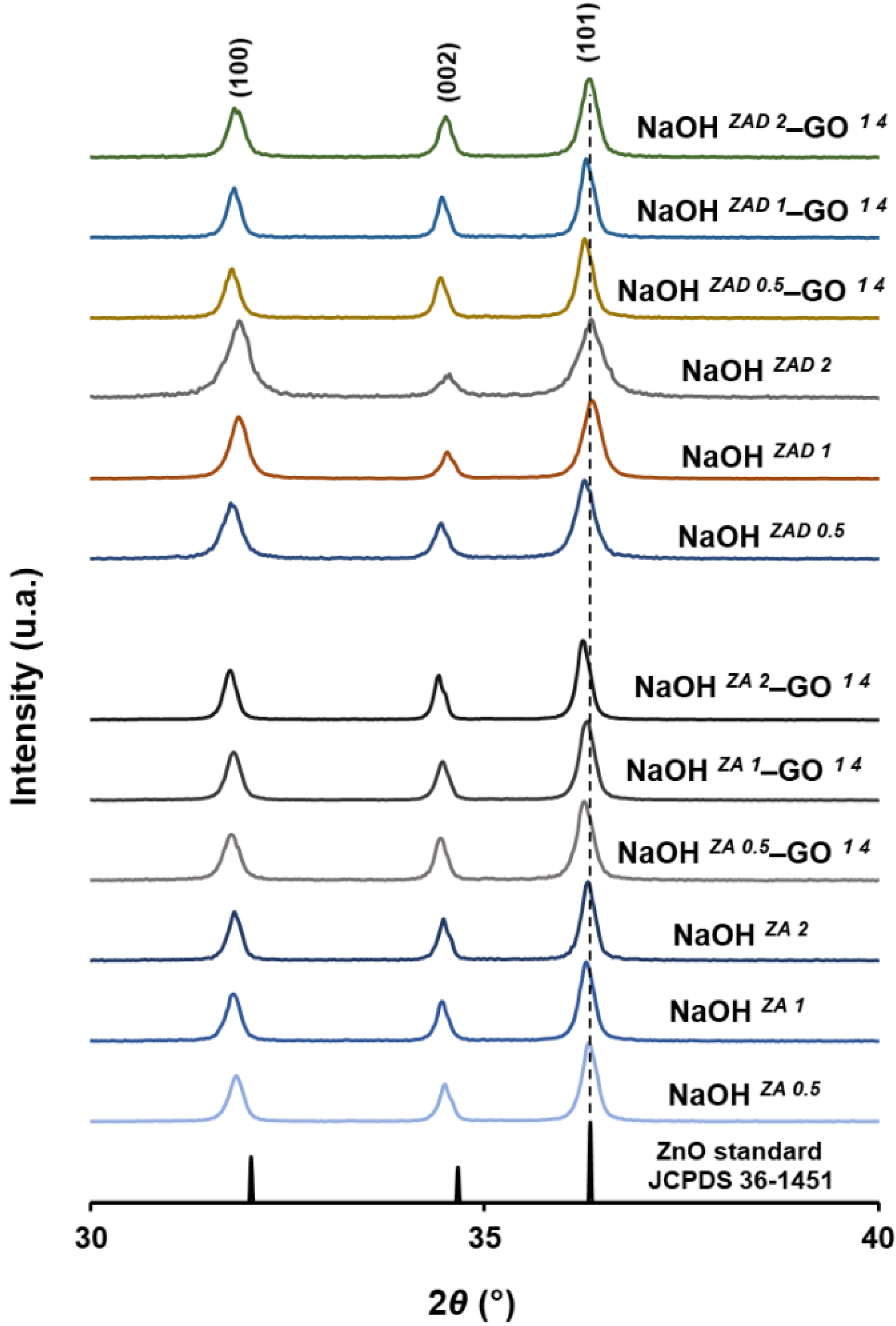
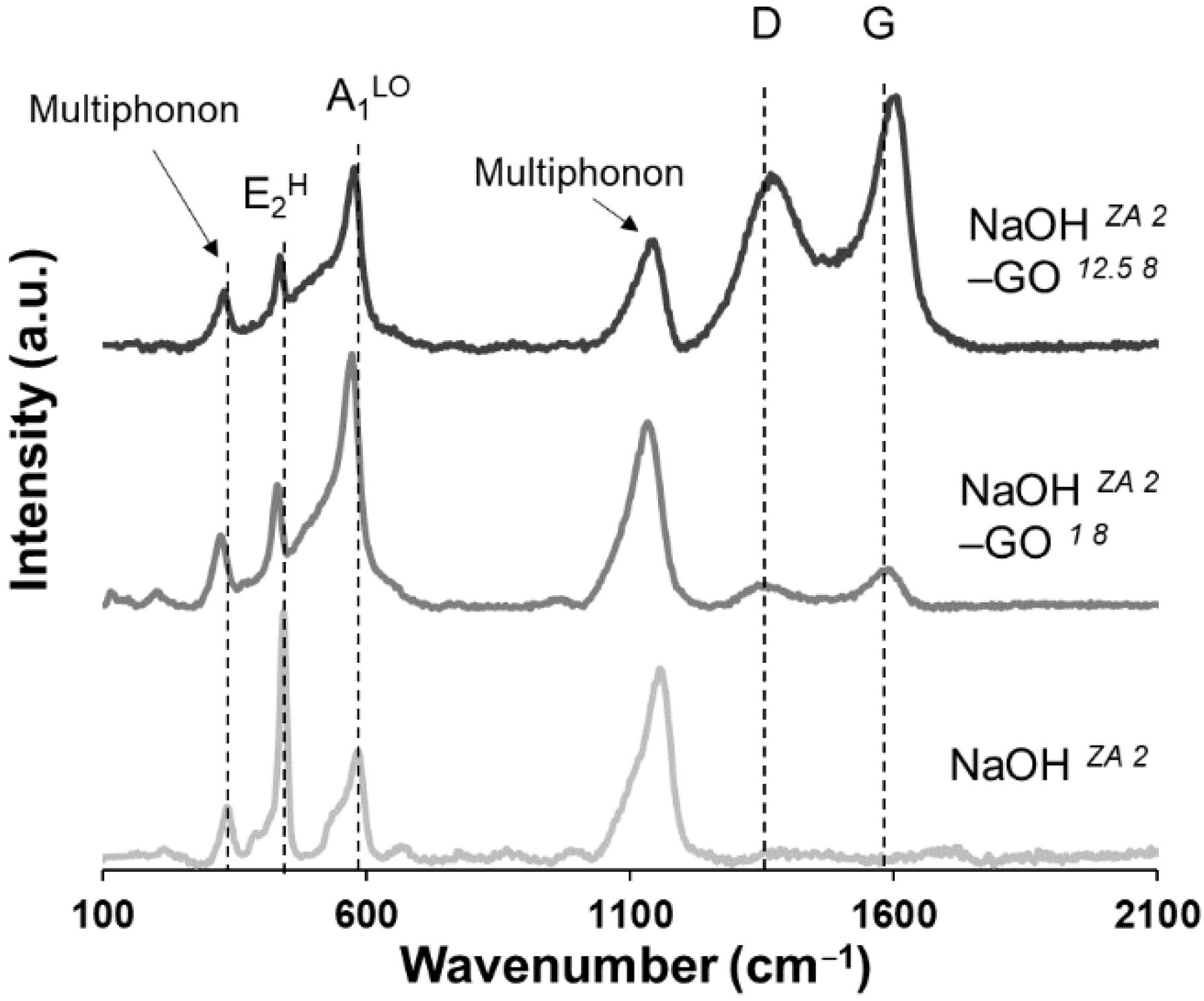
3.1. Effect of Zinc Precursor, NaOH Concentration and GO Addition on ZnO Structures
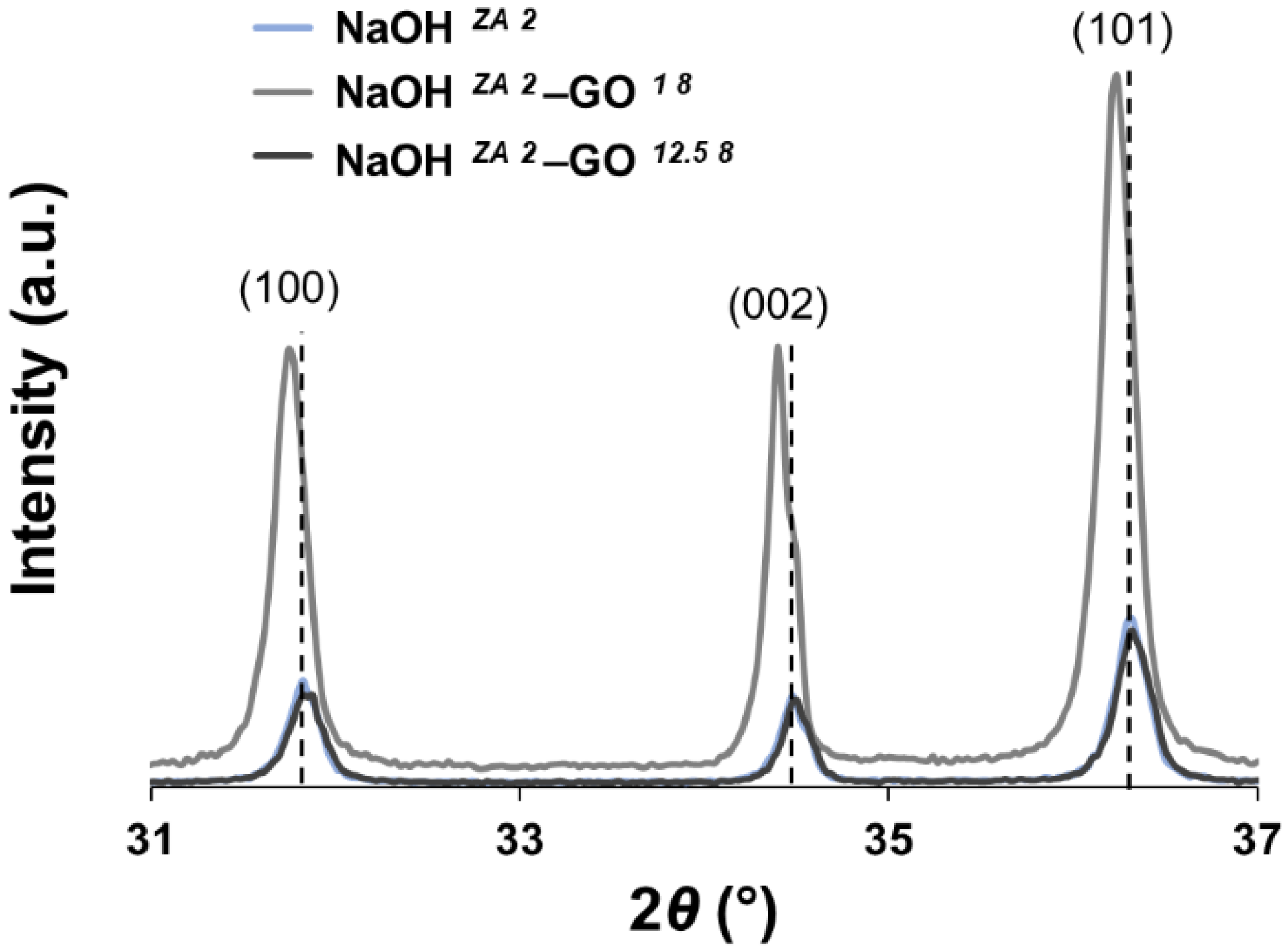
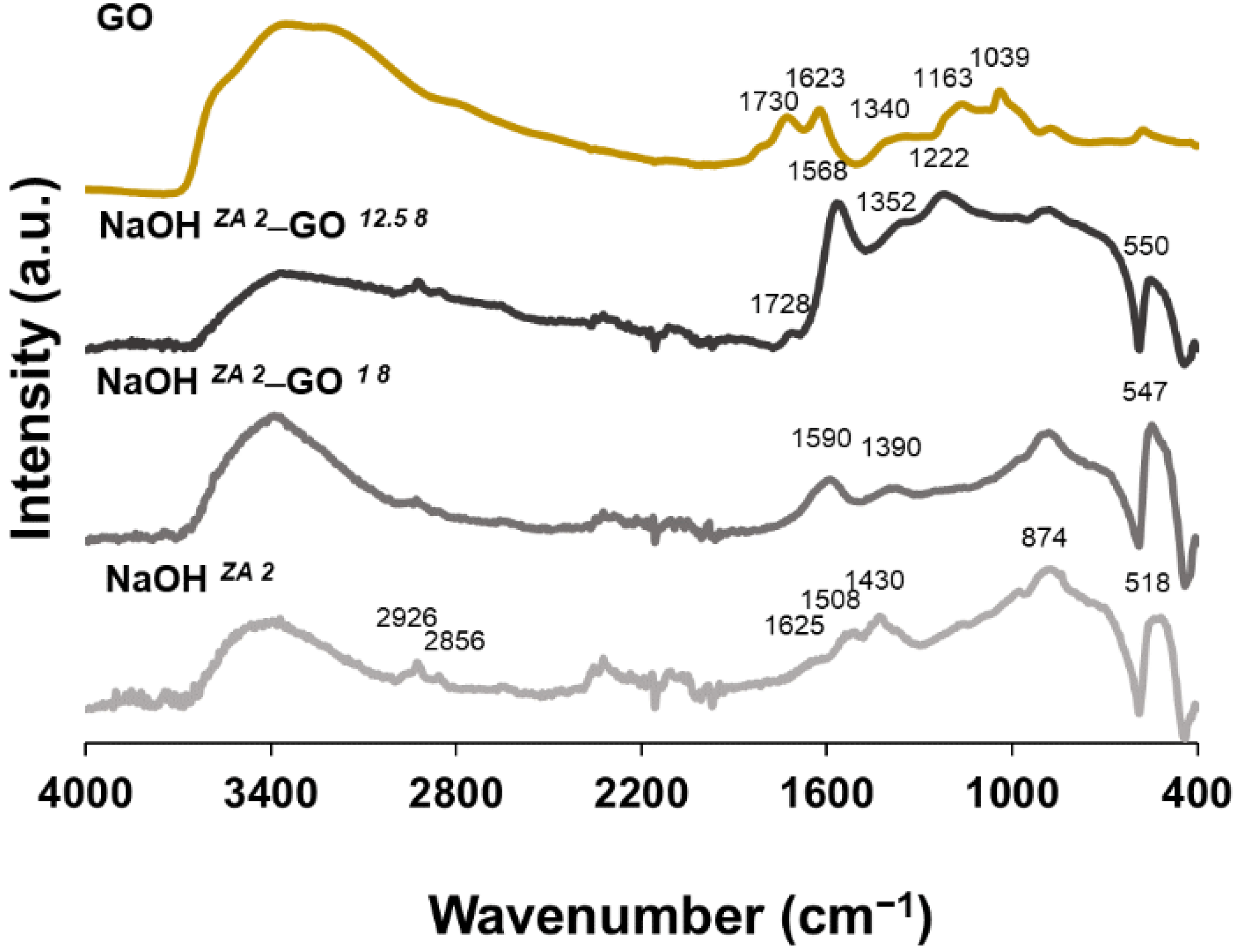
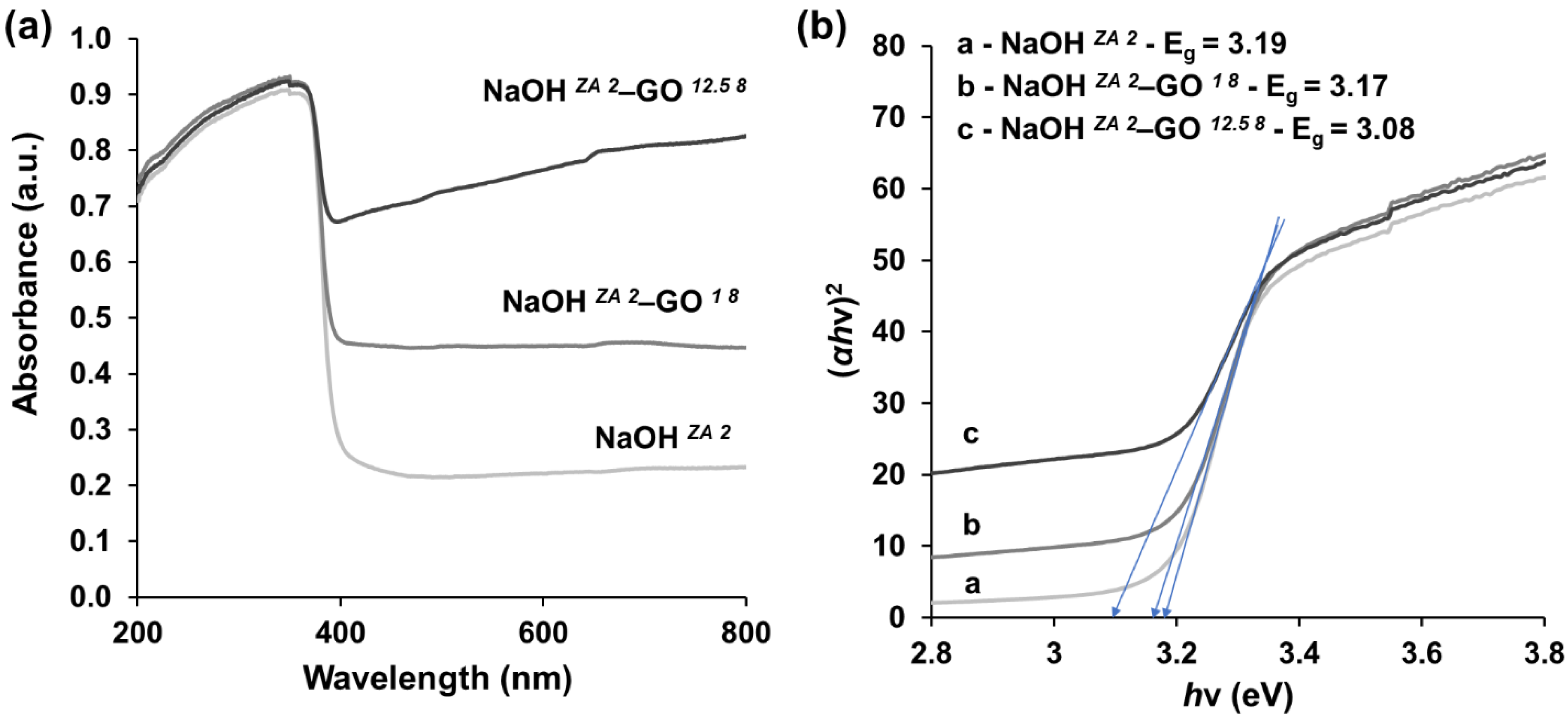
3.2. Effect of GO Concentration on ZnO Structures
3.3. Effect of pH of GO Suspension on ZnO Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, M.; Buntinx, M.; Deferme, W.; Peeters, R. (Bio)polymer/ZnO nanocomposites for packaging applications: A review of gas barrier and mechanical properties. Nanomaterials 2019, 9, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barra, A.; Santos, J.D.C.; Silva, M.R.F.; Nunes, C.; Ruiz-Hitsky, E.; Gonçalves, I.; Yildirim, S.; Ferreira, P.; Marques, P.A.A.P. Graphene Derivatives in Biopolymer-Based Composites for Food Packaging Applications. Nanomaterials 2020, 10, 2077. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Rhim, J.W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef]
- Barra, A.; Ferreira, N.M.; Martins, M.A.; Lazar, O.; Pantazi, A.; Jderu, A.A.; Neumayer, S.M.; Rodriguez, B.J.; Enăchescu, M.; Ferreira, P.; et al. Eco-friendly preparation of electrically conductive chitosan-reduced graphene oxide flexible bionanocomposites for food packaging and biological applications. Compos. Sci. Technol. 2019, 173, 53–60. [Google Scholar] [CrossRef]
- Salarbashi, D.; Mortazavi, S.A.; Noghabi, M.S.; Bazzaz, B.S.F.; Sedaghat, N.; Ramezani, M.; Shahabi-Ghahfarrokhi, I. Development of new active packaging film made from a soluble soybean polysaccharide incorporating ZnO nanoparticles. Carbohydr. Polym. 2016, 140, 220–227. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Nassiri, R.; Sheibani, S.; Ariffin, F.; Karim, A.A. Preparation and characterization of bionanocomposite films filled with nanorod-rich zinc oxide. Carbohydr. Polym. 2013, 96, 233–239. [Google Scholar] [CrossRef]
- Das, D.; Nath, B.C.; Phukon, P.; Kalita, A.; Dolui, S.K. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surf. B Biointerfaces 2013, 111, 556–560. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Zinc Oxide Nanoparticles for Food Packaging Applications. In Antimicrobial Food Pckaging; Academic Press: Cambridge, MA, USA, 2016; pp. 425–431. ISBN 9780128007235. [Google Scholar]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2020, 1–29. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Food Contact Materials; Enzymes, F.P.A. (CEF) Safety assessment of the substance zinc oxide, nanoparticles, for use in food contact materials. EFSA J. 2016, 14, 4408. [Google Scholar]
- Zhang, X.; Wang, B.; Sunarso, J.; Liu, S.; Zhi, L. Graphene nanostructures toward clean energy technology applications. Wiley Interdiscip. Rev. Energy Environ. 2012, 1, 317–336. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Z.; Owens, A.C.E.; Kulaots, I.; Chen, Y.; Kane, A.B.; Hurt, R.H. Antioxidant Chemistry of Graphene-based Materials and its Role in oxidation Protection technology. Nanoscale 2014, 6, 11744. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, A.F.; Badawy, A.A.; Mohram, M.E.; Rehim, M.H.A. Synergistic effect of zinc oxide nanorods on the photocatalytic performance and the biological activity of graphene nano sheets. Heliyon 2020, 6, e03283. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Noor, N.H.B.M.; Serrà, A.; Ibrahim, M.N.M. Advances and challenges in developing efficient graphene oxide-based ZnO photocatalysts for dye photo-oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef]
- Tomić, M.; Šetka, M.; Vojkůvka, L.; Vallejos, S. VOCs sensing by metal oxides, conductive polymers, and carbon-based materials. Nanomaterials 2021, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Bhujel, R.; Khadka, M.; Chetry, R.L.; Swain, B.P.; Biswas, J. Synthesis, characterizations, and electrochemical studies of ZnO/reduced graphene oxide nanohybrids for supercapacitor application. Mater. Today Chem. 2021, 20, 100472. [Google Scholar] [CrossRef]
- Van Tuan, P.; Phuong, T.T.; Tan, V.T.; Nguyen, S.X.; Khiem, T.N. In-situ hydrothermal fabrication and photocatalytic behavior of ZnO/reduced graphene oxide nanocomposites with varying graphene oxide concentrations. Mater. Sci. Semicond. Process. 2020, 115, 105114. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, S.; Zhu, T.; Sun, W.; Li, Q.; Wei, G.; Su, Z. Hydrothermal synthesis of zinc oxide-reduced graphene oxide nanocomposites for an electrochemical hydrazine sensor. RSC Adv. 2015, 5, 22935–22942. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef]
- Hu, C.; Lu, T.; Chen, F.; Zhang, R. A brief review of graphene–metal oxide composites synthesis and applications in photocatalysis. J. Chin. Adv. Mater. Soc. 2013, 1, 21–39. [Google Scholar] [CrossRef]
- Barra, A.; Lazăr, O.; Pantazi, A.; Hortigüela, M.J.; Otero-Irurueta, G.; Enăchescu, M.; Ruiz-Hitzky, E.; Nunes, C.; Ferreira, P. Joining caffeic acid and hydrothermal treatment to produce environmentally benign highly reduced graphene oxide. Nanomaterials 2021, 11, 732. [Google Scholar] [CrossRef]
- Xu, S.; Fu, L.; Pham, T.S.H.; Yu, A.; Han, F.; Chen, L. Preparation of ZnO flower/reduced graphene oxide composite with enhanced photocatalytic performance under sunlight. Ceram. Int. 2015, 41, 4007–4013. [Google Scholar] [CrossRef]
- Ezeigwe, E.R.; Tan, M.T.T.; Khiew, P.S.; Siong, C.W. One-step green synthesis of graphene/ZnO nanocomposites for electrochemical capacitors. Ceram. Int. 2015, 41, 715–724. [Google Scholar] [CrossRef]
- González, S.C.E.; Bolaina-Lorenzo, E.; Pérez-Trujillo, J.J.; Puente-Urbina, B.A.; Rodríguez-Fernández, O.; Fonseca-García, A.; Betancourt-Galindo, R. Antibacterial and anticancer activity of ZnO with different morphologies: A comparative study. 3 Biotech 2021, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.D.; Kumbhakar, P.; Pal, S.; Khannam, S.K.; Kumbhakar, P. Investigating morphology-dependent antibacterial property of ZnO nanoparticles and developing an insight into oxidative stress generation and cellular response. Biologia 2021, 76, 1339–1348. [Google Scholar] [CrossRef]
- Stanković, A.; Dimitrijević, S.; Uskoković, D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothemally synthesized using different surface stabilizing agents. Colloids Surf. B Biointerfaces 2013, 102, 21–28. [Google Scholar] [CrossRef]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B Biol. 2013, 120, 66–73. [Google Scholar] [CrossRef]
- Mohd Bakhori, S.K.; Mahmud, S.; Ling, C.A.; Sirelkhatim, A.H.; Hasan, H.; Mohamad, D.; Masudi, S.M.; Seeni, A.; Abd Rahman, R. In-vitro efficacy of different morphology zinc oxide nanopowders on Streptococcus sobrinus and Streptococcus mutans. Mater. Sci. Eng. C 2017, 78, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO-nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Danial, E.N.; Hjiri, M.; Abdel-wahab, M.S.; Alonizan, N.H.; El Mir, L.; Aida, M.S. Antibacterial activity of In-doped ZnO nanoparticles. Inorg. Chem. Commun. 2020, 122, 108281. [Google Scholar] [CrossRef]
- Jan, T.; Iqbal, J.; Ismail, M.; Mahmood, A. Synthesis of highly efficient antibacterial agent Ag doped ZnO nanorods: Structural, Raman and optical properties. J. Appl. Phys. 2014, 115, 154308. [Google Scholar] [CrossRef]
- Asok, A.; Ghosh, S.; More, P.A.; Chopade, B.A.; Gandhi, M.N.; Kulkarni, A.R. Surface defect rich ZnO quantum dots as antioxidants inhibiting α-amylase and α-glucosidase: A potential anti-diabetic nanomedicine. J. Mater. Chem. B 2015, 3, 4597–4606. [Google Scholar] [CrossRef] [Green Version]
- Ozel, E.; Tuncolu, I.G.; Aciksari, C.; Suvaci, E. Effect of Precursor Type on Zinc Oxide Formation and Morphology Development during Hydrothermal Synthesis. Hittite J. Sci. Eng. 2016, 3, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Rayerfrancis, A.; Balaji Bhargav, P.; Ahmed, N.; Chandra, B.; Dhara, S. Effect of pH on the morphology of ZnO nanostructures and its influence on structural and optical properties. Phys. B Condens. Matter 2015, 457, 96–102. [Google Scholar] [CrossRef]
- Kumar, S.; Sahare, P.D. Observation of band gap and surface defects of ZnO nanoparticles synthesized via hydrothermal route at different reaction temperature. Opt. Commun. 2012, 285, 5210–5216. [Google Scholar] [CrossRef]
- Hussain, S.; Liu, T.; Kashif, M.; Lin, L.; Wu, S.; Guo, W.; Zeng, W.; Hashim, U. Effects of reaction time on the morphological, structural, and gas sensing properties of ZnO nanostructures. Mater. Sci. Semicond. Process. 2014, 18, 52–58. [Google Scholar] [CrossRef]
- Rodwihok, C.; Wongratanaphisan, D.; Ngo, Y.L.T.; Khandelwal, M.; Hur, S.H.; Chung, J.S. Effect of GO additive in ZnO/rGO nanocomposites with enhanced photosensitivity and photocatalytic activity. Nanomaterials 2019, 9, 1141. [Google Scholar] [CrossRef] [Green Version]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Mohammadi, K.; Safa, S.; Moghaddam, S.S.; Rabbani, M.; Azimirad, R. Photocatalytic activity of one-pot synthesized reduced graphene oxide – zinc oxide nanocomposites. J. Nano Res. 2015, 37, 74–84. [Google Scholar] [CrossRef]
- Uthirakumar, P.; Hong, C.H. Effect of annealing temperature and pH on morphology and optical property of highly dispersible ZnO nanoparticles. Mater. Charact. 2009, 60, 1305–1310. [Google Scholar] [CrossRef]
- Chand, P.; Gaur, A.; Kumar, A. Structural and optical properties of ZnO nanoparticles synthesized at different pH values. J. Alloys Compd. 2012, 539, 174–178. [Google Scholar] [CrossRef]
- Wang, M.; Ren, F.; Zhou, J.; Cai, G.; Cai, L.; Hu, Y.; Wang, D.; Liu, Y.; Guo, L.; Shen, S. N Doping to ZnO Nanorods for Photoelectrochemical Water Splitting under Visible Light: Engineered Impurity Distribution and Terraced Band Structure. Sci. Rep. 2015, 5, 12925. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, B.P.; Zhao, J.X.; Ge, Z.H.; Zhao, X.K.; Zou, L. ZnO/carbon quantum dots heterostructure with enhanced photocatalytic properties. Appl. Surf. Sci. 2013, 279, 367–373. [Google Scholar] [CrossRef]
- Yogamalar, N.R.; Bose, A.C. Tuning the aspect ratio of hydrothermally grown ZnO by choice of precursor. J. Solid State Chem. 2011, 184, 12–20. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Song, X.; Wei, Y.; Battaglia, V.S. Facile hydrothermal fabrication of ZnO–graphene hybrid anode materials with excellent lithium storage properties. Sustain. Energy Fuels 2017, 1, 767–779. [Google Scholar] [CrossRef]
- Sreejesh, M.; Dhanush, S.; Rossignol, F.; Nagaraja, H.S. Microwave assisted synthesis of rGO/ZnO composites for non-enzymatic glucose sensing and supercapacitor applications. Ceram. Int. 2017, 43, 4895–4903. [Google Scholar] [CrossRef]
- Piovesana, S.; Iglesias, D.; Melle-Franco, M.; Kralj, S.; Cavaliere, C.; Melchionna, M.; Laganà, A.; Capriotti, A.L.; Marchesan, S. Carbon nanostructure morphology templates nanocomposites for phosphoproteomics. Nano Res. 2020, 13, 380–388. [Google Scholar] [CrossRef]
- Gong, X.; Liu, G.; Li, Y.; Yu, D.Y.W.; Teoh, W.Y. Functionalized-graphene composites: Fabrication and applications in sustainable energy and environment. Chem. Mater. 2016, 28, 8082–8118. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, J.; Zhu, C.; Sheng, Y.; Yan, Z.; Wang, Y.; Feng, B. The visible light catalytic properties of carbon quantum dots/ZnO nanoflowers composites. J. Mater. Sci. Mater. Electron. 2015, 26, 2861–2866. [Google Scholar] [CrossRef]
- Bozetine, H.; Wang, Q.; Barras, A.; Li, M.; Hadjersi, T.; Szunerits, S.; Boukherroub, R. Green chemistry approach for the synthesis of ZnO–carbon dots nanocomposites with good photocatalytic properties under visible light. J. Colloid Interface Sci. 2016, 465, 286–294. [Google Scholar] [CrossRef]
- Aboorvakani, R.; Vethanathan, S.J.K.; Madhu, K.U. Influence of Zn concentration on zinc oxide nanoparticles and their anti-corrosion property. J. Alloys Compd. 2020, 834, 155078. [Google Scholar] [CrossRef]
- Kavitha, M.K.; John, H.; Gopinath, P.; Philip, R. Synthesis of reduced graphene oxide-ZnO hybrid with enhanced optical limiting properties. J. Mater. Chem. C 2013, 1, 3669–3676. [Google Scholar] [CrossRef]
- Sandhya, P.K.; Jose, J.; Sreekala, M.S.; Padmanabhan, M.; Kalarikkal, N.; Thomas, S. Reduced graphene oxide and ZnO decorated graphene for biomedical applications. Ceram. Int. 2018, 44, 15092–15098. [Google Scholar] [CrossRef]
- Tien, H.N.; Luan, V.H.; Hoa, L.T.; Khoa, N.T.; Hahn, S.H.; Chung, J.S.; Shin, E.W.; Hur, S.H. One-pot synthesis of a reduced graphene oxide-zinc oxide sphere composite and its use as a visible light photocatalyst. Chem. Eng. J. 2013, 229, 126–133. [Google Scholar] [CrossRef]
- Silva, M.R.F.; Lourenço, M.A.O.; Tobaldi, D.M.; da Silva, C.F.; Seabra, M.P.; Ferreira, P. Carbon-modified titanium oxide materials for photocatalytic water and air decontamination. Chem. Eng. J. 2020, 387, 124099. [Google Scholar] [CrossRef]
- Prabhu, S.; Megala, S.; Harish, S.; Navaneethan, M.; Maadeswaran, P.; Sohila, S.; Ramesh, R. Enhanced photocatalytic activities of ZnO dumbbell/reduced graphene oxide nanocomposites for degradation of organic pollutants via efficient charge separation pathway. Appl. Surf. Sci. 2019, 487, 1279–1288. [Google Scholar] [CrossRef]
- Tien, H.N.; Luan, V.H.; Lee, T.K.; Kong, B.; Chung, J.S.; Kim, E.J.; Hur, S.H. Enhanced solvothermal reduction of graphene oxide in a mixed solution of sulfuric acid and organic solvent. Chem. Eng. J. 2012, 211–212, 97–103. [Google Scholar] [CrossRef]
- Anand, K.; Singh, O.; Singh, M.P.; Kaur, J.; Singh, R.C. Hydrogen sensor based on graphene/ZnO nanocomposite. Sensors Actuators B. Chem. 2014, 195, 409–415. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, X.; Yang, C.; Cao, M.; Ma, M.; Liu, R. ZnO microspheres-reduced graphene oxide nanocomposite for photocatalytic degradation of methylene blue dye. Appl. Surf. Sci. 2017, 392, 196–203. [Google Scholar] [CrossRef]
- Lv, R.; Wang, X.; Lv, W.; Xu, Y.; Ge, Y.; He, H.; Li, G.; Wu, X.; Li, X.; Li, Q. Facile synthesis of ZnO nanorods grown on graphene sheets and its enhanced photocatalytic efficiency. J. Chem. Technol. Biotechnol. 2015, 90, 550–558. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Suresh, R.; Rajalakshmi, S.; Raja, S.; Sundaravadivel, E.; Gayathri, M.; Sridharan, M. One-step synthesis, characterisation, photocatalytic and bio-medical applications of ZnO nanoplates. Mater. Technol. 2020, 35, 112–124. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, N.; Wei, Y.; Zhang, G. An in situ gelatin-assisted hydrothermal synthesis of ZnO-reduced graphene oxide composites with enhanced photocatalytic performance under ultraviolet and visible light. RSC Adv. 2014, 4, 7933–7943. [Google Scholar] [CrossRef]
- Beura, R.; Thangadurai, P. Structural, optical and photocatalytic properties of graphene-ZnO nanocomposites for varied compositions. J. Phys. Chem. Solids 2017, 102, 168–177. [Google Scholar] [CrossRef]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012, 22, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Romeiro, F.C.; Rodrigues, M.A.; Silva, L.A.J.; Catto, A.C.; da Silva, L.F.; Longo, E.; Nossol, E.; Lima, R.C. rGO-ZnO nanocomposites for high electrocatalytic effect on water oxidation obtained by microwave-hydrothermal method. Appl. Surf. Sci. 2017, 423, 743–751. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Juneja, S.; Palsaniya, S.; Manna, A.K.; Soni, R.K.; Bhattacharya, J. Evidence of oxygen defects mediated enhanced photocatalytic and antibacterial performance of ZnO nanorods. Colloids Surf. B Biointerfaces 2019, 184, 110541. [Google Scholar] [CrossRef]
- Kumar, Y.; Sahai, A.; Olive-Méndez, S.F.; Goswami, N.; Agarwal, V. Morphological transformations in cobalt doped zinc oxide nanostructures: Effect of doping concentration. Ceram. Int. 2016, 42, 5184–5194. [Google Scholar] [CrossRef]
- Reddy, T.N.; Manna, J.; Rana, R.K. Polyamine Mediated Interfacial Assembly of rGO- ZnO Nanostructures: A Bio-Inspired Approach and Enhanced Photocatalytic Properties. ACS Appl. Mater. Interfaces 2015, 7, 19684–19690. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Precursor | NaOH (M) | (GO) (mg/mL) | pH of GO | Sample ID |
|---|---|---|---|---|
| ZA (Zn(CH3COO)2) | 0.5 | 1.0 | 4 | NaOH ZA 0.5–GO 14 |
| - | - | NaOH ZA 0.5 | ||
| 1.0 | 1.0 | 4 | NaOH ZA 1–GO 14 | |
| - | - | NaOH ZA 1 | ||
| 2.0 | 1.0 | 4 | NaOH ZA 2–GO 14 | |
| 8 | NaOH ZA 2–GO 18 | |||
| 12.5 | 1 | NaOH ZA 2–GO 12.51 | ||
| 8 | NaOH ZA 2–GO 12.58 | |||
| 12 | NaOH ZA 2–GO 12.512 | |||
| - | - | NaOH ZA 2 | ||
| ZAD (Zn(CH3COO)2·2H2O) | 0.5 | 1.0 | 4 | NaOH ZAD 0.5–GO 14 |
| - | - | NaOH ZAD 0.5 | ||
| 1.0 | 1.0 | 4 | NaOH ZAD 1–GO 14 | |
| - | - | NaOH ZAD 1 | ||
| 2.0 | 1.0 | 4 | NaOH ZAD 2–GO 14 | |
| - | - | NaOH ZAD 2 |
| 2θ (°) | Lattice Parameters (Å) | FWHM | ||
|---|---|---|---|---|
| a = b | c | |||
| NaOH ZA 0.5 | 36.3157 | 3.2403 | 5.1927 | 0.2442 |
| NaOH ZA 1 | 36.2894 | 3.2456 | 5.2003 | 0.2408 |
| NaOH ZA 2 | 36.3157 | 3.2429 | 5.1965 | 0.2258 |
| NaOH ZA 0.5–GO 14 | 36.2632 | 3.2482 | 5.2003 | 0.2657 |
| NaOH ZA 1–GO 14 | 36.3157 | 3.2429 | 5.1965 | 0.2422 |
| NaOH ZA 2–GO 14 | 36.242 | 3.2482 | 5.2042 | 0.2139 |
| NaOH ZAD 0.5 | 36.2632 | 3.2482 | 5.2003 | 0.3108 |
| NaOH ZAD 1 | 36.3733 | 3.2377 | 5.1888 | 0.3022 |
| NaOH ZAD 2 | 36.3719 | 3.2377 | 5.1850 | 0.4304 |
| NaOH ZAD 0.5–GO 14 | 36.2632 | 3.2456 | 5.2003 | 0.2503 |
| NaOH ZAD 1–GO 14 | 36.2894 | 3.2429 | 5.2003 | 0.2320 |
| NaOH ZAD 2–GO 14 | 36.3419 | 3.2429 | 5.1926 | 0.2685 |
| FWHM | Lattice Parameters (Å) | BET (m2/g) | ||
|---|---|---|---|---|
| a = b | c | |||
| NaOH ZA 2 | 0.22583 | 3.243 | 5.193 | 7 |
| NaOH ZA 2–GO 18 | 0.23691 | 3.251 | 5.208 | 11 |
| NaOH ZA 2–GO 12.58 | 0.25299 | 3.243 | 5.193 | 15 |
| E2H FWHM | E2H/A1LO | FWHM | ID/IG | ||
|---|---|---|---|---|---|
| D | G | ||||
| NaOH ZA 2–GO 12.51 | 17.758 | 0.956 | 165 | 90 | 1.32 |
| NaOH ZA 2–GO 12.58 | 23.027 | 0.525 | 165 | 88 | 1.36 |
| NaOH ZA 2–GO 12.512 | 32.696 | 0.482 | 170 | 88 | 1.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, Z.; Nunes, C.; Ferreira, P. Unravelling the Role of Synthesis Conditions on the Structure of Zinc Oxide-Reduced Graphene Oxide Nanofillers. Nanomaterials 2021, 11, 2149. https://doi.org/10.3390/nano11082149
Alves Z, Nunes C, Ferreira P. Unravelling the Role of Synthesis Conditions on the Structure of Zinc Oxide-Reduced Graphene Oxide Nanofillers. Nanomaterials. 2021; 11(8):2149. https://doi.org/10.3390/nano11082149
Chicago/Turabian StyleAlves, Zélia, Cláudia Nunes, and Paula Ferreira. 2021. "Unravelling the Role of Synthesis Conditions on the Structure of Zinc Oxide-Reduced Graphene Oxide Nanofillers" Nanomaterials 11, no. 8: 2149. https://doi.org/10.3390/nano11082149







