High-Quality Single-Crystalline β-Ga2O3 Nanowires: Synthesis to Nonvolatile Memory Applications
Abstract
:1. Introduction
2. Experimental Methods
3. Result and Discussion
3.1. Structural and Morphological Analysis
3.2. Elemental Analysis
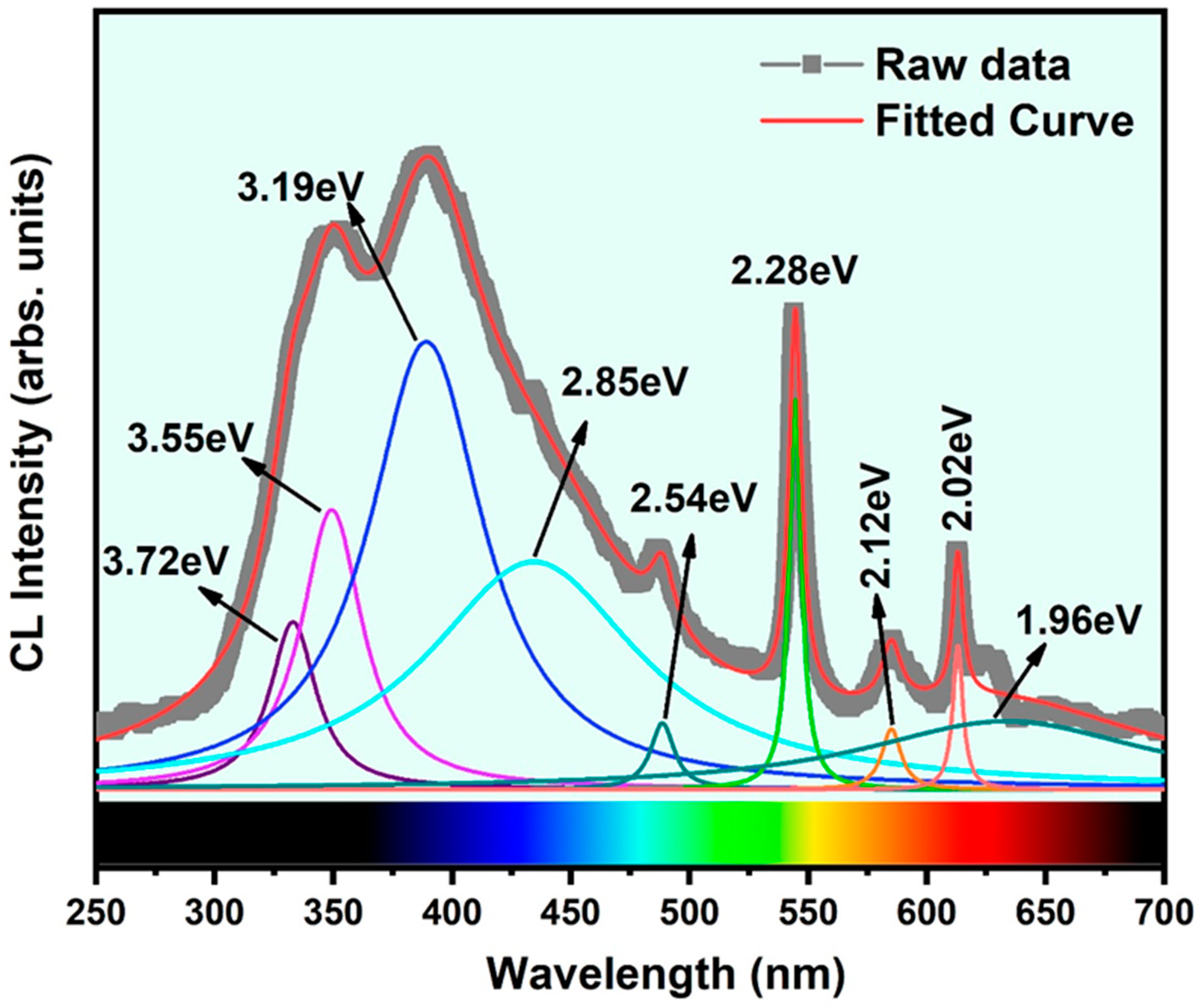
3.3. Investigation of Bonding Features and RT-Cathodoluminescence Study
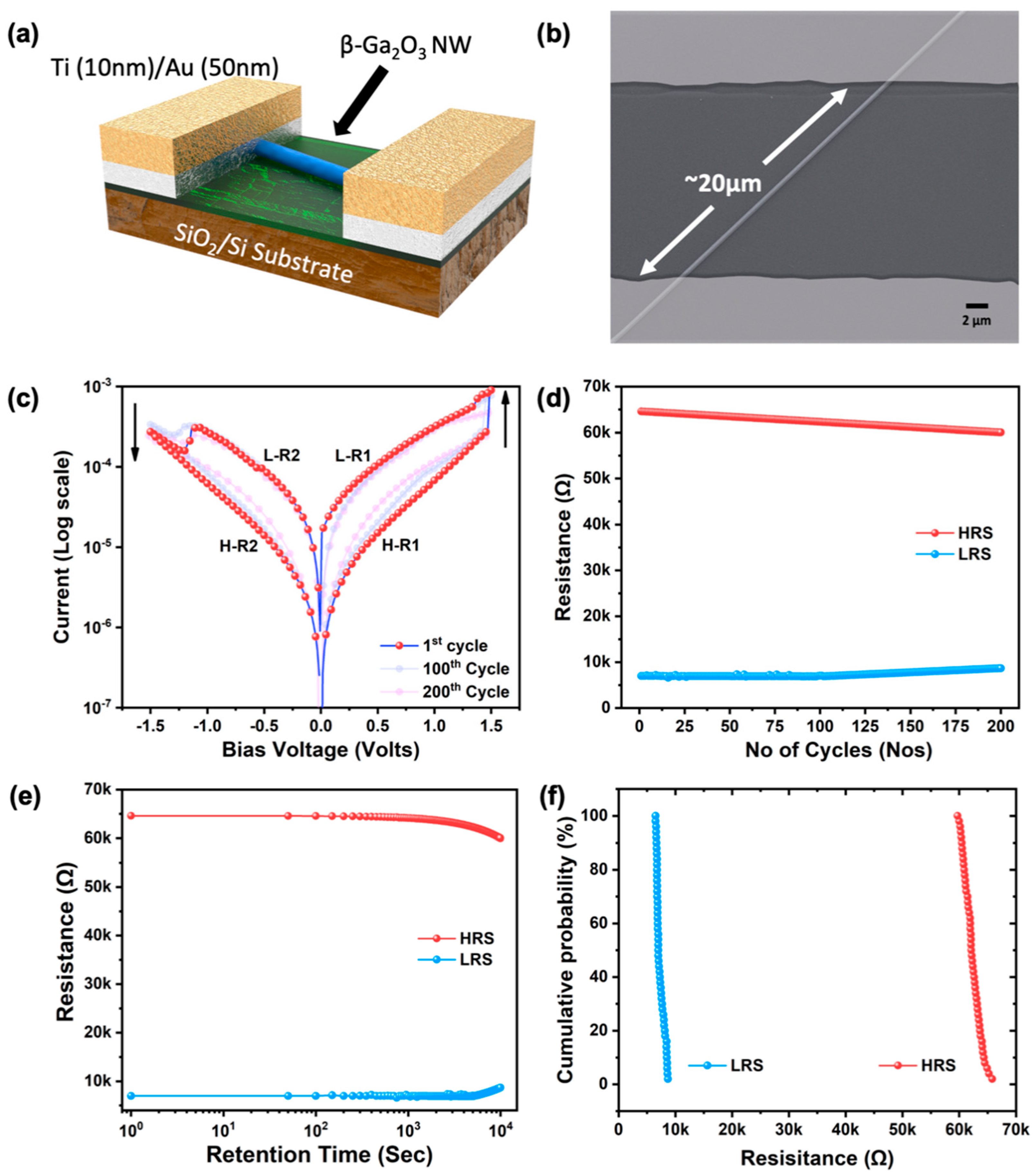
3.4. ReRAM Device Characteristics
3.5. Physical Mechanism of Resistive Switching
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ueda, N.; Hosono, H.; Waseda, R.; Kawazoe, H. Anisotropy of electrical and optical properties in β-Ga2O3 single crystals. Appl. Phys. Lett. 1997, 71, 933–935. [Google Scholar] [CrossRef]
- Oshima, Y.; Villora, E.G.; Shimamura, K. Halide vapor phase epitaxy of twin-free α-Ga2O3 on sapphire (0001) substrates. Appl. Phys. Express 2015, 8, 55501. [Google Scholar] [CrossRef]
- Oshima, Y.; Víllora, E.G.; Matsushita, Y.; Yamamoto, S.; Shimamura, K. Epitaxial growth of phase-pure ε-Ga2O3 by halide vapor phase epitaxy. J. Appl. Phys. 2015, 118, 085301. [Google Scholar] [CrossRef]
- Pearton, S.J.; Yang, J.; Cary, P.H.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A Review of Ga2O3 Materials, Processing, and Devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef] [Green Version]
- Baban, C.; Toyoda, Y.; Ogita, M. Oxygen sensing at high temperatures using Ga2O3 films. Thin Solid Films 2005, 484, 369–373. [Google Scholar] [CrossRef]
- Li, Y.; Tokizono, T.; Liao, M.; Zhong, M.; Koide, Y.; Yamada, I.; Delaunay, J.-J. Efficient Assembly of Bridged β-Ga2O3 Nanowires for Solar-Blind Photodetection. Adv. Funct. Mater. 2010, 20, 3972–3978. [Google Scholar] [CrossRef]
- Lee, J.-H.; Doan, T.A.; Park, Y.J.; Hoa, H.T.M.; Phuong, P.H.; Le, D.T.; Hung, N.H.; Tran, Q.T.; Lee, H.-S.; Ryu, J.H.; et al. Synthesis and Photocatalytic Activity of β-Ga2O3 Nanostructures for Decomposition of Formaldehyde under Deep Ultraviolet Irradiation. Catalysts 2020, 10, 1105. [Google Scholar] [CrossRef]
- Ohta, H.; Nomura, K.; Hiramatsu, H.; Ueda, K.; Kamiya, T.; Hirano, M.; Hosono, H. Frontier of transparent oxide semiconductors. Solid-State Electron. 2003, 47, 2261–2267. [Google Scholar] [CrossRef]
- Stepanov, S.I.; Nikolaev, V.I.; Bougrov, V.E.; Romanov, A.E. Gallium Oxide: Properties and Application—A Review. Rev. Adv. Mater. Sci. 2016, 44, 63–86. [Google Scholar]
- Bayraktaroglu, B. Assessment of Gallium Oxide Technology; Report AFRL-RY-WP-TR-2017-0167; Air Force Research Lab: Dayton, OH, USA, 2017. [Google Scholar]
- Alhalaili, B.; Vidu, R.; Mao, H.; Islam, M.S. Comparative Study of Growth Morphologies of Ga2O3 Nanowires on Different Substrates. Nanomaterials 2020, 10, 1920. [Google Scholar] [CrossRef]
- Suzuki, N.; Ohira, S.; Tanaka, M.; Sugawara, T.; Nakajima, K.; Shishido, T. Fabrication and Characterization of Transparent Conductive Sn-Doped β-Ga2O3 Single Crystal. Phys. Status Solidi 2007, 4, 2310. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Li, Q.; Qu, C.; Zhang, L.; Li, T. Structural and optical properties of N-doped β-Ga2O3 films deposited by RF magnetron sputtering. Phys. B Condens. Matter 2011, 406, 3079–3082. [Google Scholar] [CrossRef]
- Liu, L.L.; Li, M.K.; Yu, D.Q.; Zhang, J.; Zhang, H.; Qian, C.; Yang, Z. Fabrication and characteristics of N-doped β-Ga2O3 nanowires. Appl. Phys. A 2010, 98, 831–835. [Google Scholar] [CrossRef]
- Chang, P.-C.; Fan, Z.; Tseng, W.-Y.; Rajagopal, A.; Lu, J.G. β-Ga2O3 nanowires: Synthesis, characterization, and p-channel field-effect transistor. Appl. Phys. Lett. 2005, 87, 222102. [Google Scholar] [CrossRef]
- Slesazeck, S.; Mikolajick, T. Nanoscale resistive switching memory devices: A review. Nanotechnology 2019, 30, 352003. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Balraj, B.; Chung, P.-F.; Sivakumar, C.; Lee, W.-J.; Ho, M.-S. Impact of lattice plane orientation in TiO2 based resistive switching memory: A computational approach. Appl. Phys. Lett. 2021, 118, 083502. [Google Scholar] [CrossRef]
- Bharathi, M.; Balraj, B.; Sivakumar, C.; Wang, Z.; Shuai, J.; Ho, M.-S.; Guo, D. Effect of Ag doping on bipolar switching operation in molybdenum trioxide (MoO3) nanostructures for non-volatile memory. J. Alloys Compd. 2021, 862, 158035. [Google Scholar] [CrossRef]
- Chiu, F.-C. A Review on Conduction Mechanisms in Dielectric Films. Adv. Mater. Sci. Eng. 2014, 2014, 578168. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-C.; Chang, K.-C.; Tsai, T.-M.; Chu, T.-J.; Sze, S.M. Resistance random access memory. Mater. Today 2016, 19, 254–264. [Google Scholar] [CrossRef]
- Makarov, A.; Sverdlov, V.; Selberherr, S. Stochastic modeling of bipolar resistive switching in metal-oxide based memory by Monte Carlo technique. J. Comput. Electron. 2010, 9, 146–152. [Google Scholar] [CrossRef]
- Weng, T.-F.; Ho, M.-S.; Sivakumar, C.; Balraj, B.; Chung, P.-F. VLS growth of pure and Au decorated β-Ga2O3 nanowires for room temperature CO gas sensor and resistive memory applications. Appl. Surf. Sci. 2020, 533, 147476. [Google Scholar] [CrossRef]
- Sutter, E.; Idrobo, J.C.; Sutter, P. Synthesis and optoelectronic properties of ultrathin Ga2O3 nanowires. J. Mater. Chem. C 2020, 8, 11555. [Google Scholar] [CrossRef]
- Wong, H.-S.P.; Lee, H.-Y.; Yu, S.; Chen, Y.-S.; Wu, Y.; Chen, P.-S.; Lee, B.; Chen, F.T.; Tsa, M.-J. Metal–Oxide RRAM. Proc. IEEE 2012, 100, 1951–1970. [Google Scholar] [CrossRef]
- Ielmini, D.; Cagli, C.; Nardi, F.; Zhang, Y. Nanowire-based resistive switching memories: Devices, operation and scaling. J. Phys. D Appl. Phys. 2013, 46. [Google Scholar] [CrossRef]
- Domènech-Gil, G.; Peiró, I.; López-Aymerich, E.; Moreno, M.; Pellegrino, P.; Gràcia, I.; Cané, C.; Barth, S.; Romano-Rodríguez, A. Room Temperature Humidity Sensor Based on Single β-Ga2O3 Nanowires. Proceedings 2018, 2, 958. [Google Scholar] [CrossRef] [Green Version]
- Alhalaili, B.; Mao, H.; Islam, S. Ga2O3 Nanowire Synthesis and Device Applications, Novel Nanomaterials—Synthesis and Applications; George, Z.K., Athanasios, C.M., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- Sharma, S.; Sunkara, M.K. Direct Synthesis of Gallium Oxide Tubes, Nanowires, and Nanopaintbrushes. J. Am. Chem. Soc. 2002, 124, 12288–12293. [Google Scholar] [CrossRef]
- Guo, D.L.; Huang, X.; Xing, G.Z.; Zhang, Z.; Li, G.P.; He, M.; Zhang, H.; Chen, H.; Wu, T. Metal-layer-assisted coalescence of Au nanoparticles and its effect on diameter control in va-por-liquid-solid growth of oxide nanowires. Phys. Rev. B 2011, 83, 045403. [Google Scholar] [CrossRef]
- Szörenyi, T.; Laude, L.D.; Bertoti, I.; Kantor, Z.; Geretovszky, Z. Excimer laser processing of indium-tin-oxide films: An optical investigation. J. Appl. Phys. 1995, 78, 6211. [Google Scholar] [CrossRef]
- Liao, Y.; Jiao, S.; Li, S.; Wang, J.; Wang, D.; Gao, S.; Yu, Q.; Li, H. Effect of deposition pressure on the structural and optical properties of Ga2O3films obtained by thermal post-crystallization. CrystEngComm 2017, 20, 133–139. [Google Scholar] [CrossRef]
- Wang, C.; Cui, X.; Liu, J.; Zhou, X.; Cheng, X.; Sun, P.; Hu, X.; Li, X.; Zheng, J.; Lu, G. Design of Superior Ethanol Gas Sensor Based on Al-Doped NiO Nanorod-Flowers. ACS Sens. 2015, 1, 131–136. [Google Scholar] [CrossRef]
- Ho, C.-H.; Tseng, C.-Y.; Tien, L.-C. Thermoreflectance characterization of β-Ga2O3 thin-film nanostrips. Opt. Express 2010, 18, 16360–16369. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Muralidharan, S.; Pronin, N.; Karim, R.; White, S.M.; Asel, T.; Foster, G.; Krishnamoorthy, S.; Rajan, S.; Cao, L.R.; et al. Optical signatures of deep level defects in Ga2O3. Appl. Phys. Lett. 2018, 112, 242102. [Google Scholar] [CrossRef]
- Zamoryanskaya, M.V.; Sokolov, V.I. Cathodoluminescence study of silicon oxide-silicon interface. Semiconductors 2007, 41, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Zahoor, F.; Zulkifli, T.Z.A.; Khanday, F.A. Resistive Random Access Memory (RRAM): An Over-view of Materials, Switching Mechanism, Performance, Multilevel Cell (mlc) Storage, Modeling, and Applications. Nanoscale Res. Lett. 2020, 15, 90. [Google Scholar] [CrossRef]
- Min, K.; Jung, D.; Kwon, Y. Investigation of switching uniformity in resistive memory via finite element simulation of conductive-filament formation. Sci. Rep. 2021, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Das, N.C.; Oh, S.-I.; Rani, J.R.; Hong, S.-M.; Jang, J.-H. Multilevel Bipolar Electroforming-Free Resistive Switching Memory Based on Silicon Oxynitride. Appl. Sci. 2020, 10, 3506. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Chou, L.-J. Bipolar Resistive Switching of Single Gold-in-Ga2O3 Nanowire. Nano Lett. 2012, 12, 4247–4253. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivakumar, C.; Tsai, G.-H.; Chung, P.-F.; Balraj, B.; Lin, Y.-F.; Ho, M.-S. High-Quality Single-Crystalline β-Ga2O3 Nanowires: Synthesis to Nonvolatile Memory Applications. Nanomaterials 2021, 11, 2013. https://doi.org/10.3390/nano11082013
Sivakumar C, Tsai G-H, Chung P-F, Balraj B, Lin Y-F, Ho M-S. High-Quality Single-Crystalline β-Ga2O3 Nanowires: Synthesis to Nonvolatile Memory Applications. Nanomaterials. 2021; 11(8):2013. https://doi.org/10.3390/nano11082013
Chicago/Turabian StyleSivakumar, Chandrasekar, Gang-Han Tsai, Pei-Fang Chung, Babu Balraj, Yen-Fu Lin, and Mon-Shu Ho. 2021. "High-Quality Single-Crystalline β-Ga2O3 Nanowires: Synthesis to Nonvolatile Memory Applications" Nanomaterials 11, no. 8: 2013. https://doi.org/10.3390/nano11082013





