Simple Determination of Gold Nanocrystal Dimensions by Analytical Ultracentrifugation via Surface Ligand-Solvent Density Matching
Abstract
:1. Introduction
- NC@SL density in solution: In an AUC experiment, the sedimentation (sNC@SL@SS) and diffusion (DNC@SL@SS) of the entire NC@SL@SS colloids over time and under an applied centrifugal field are observed. Although sNC@SL@SS and DNC@SL@SS can be successfully retrieved from the AUC data, their translation into NC mass values still demands a priori knowledge of the density in the solution of the NC@SL (i.e., the partial specific volume of the NC@SL in solution). As this information is typically unknown unless other characterisation techniques are utilised to obtain it, the use of AUC for investigating colloidal NCs in detail becomes challenging.
- Density of the NC and SL, and mass of SL. In general, the adsorption of SLs has a strong impact on the sedimentation behaviour of NCs, owing to their low density (e.g., ca. 1.1–1.5 g/cm3 for most organic ligands vs. 4–20 g/cm3 for many NCs such as noble metals, metal oxides, and sulfides). Because the NCs and the SL sediment together as an assembly, the lower density of the organic shell drags the sedimentation of the NCs (i.e., different buoyancy). The magnitude of such an effect depends on the density and relative mass of both the NC and the SL shell [11,12,29,30]. In general, the inorganic NC density in solution is the same as in the dry state, and the bulk density is used. In the case of SL, the partial specific volume of the free SL is typically used. However, a priori knowledge of SL mass is completely unknown in most cases, which becomes one of the major obstacles toward using AUC to characterise NCs.
2. Materials and Methods
2.1. Chemicals
2.2. n-decanol/CTAB Solution
2.3. Synthesis of 9 nm AuNCs
2.4. Synthesis of 13 and 17 nm AuNCs
2.5. Synthesis of AuNCs with Dimensions Below 3 nm
2.6. Transmission Electron Microscopy
2.7. UV/Vis/NIR Spectra
2.8. Dynamic Light Scattering
2.9. Density and Viscosity of the CTAB and n-decanol/CTAB solutions
2.10. Multiwavelength Analytical Ultracentrifugation
3. Results
3.1. Analysis of Colloid Sedimentation Behaviour: A Priori Knowledge
3.2. Forces during Sedimentation Experiments: The Density-Matching Approach
3.3. AUC Characterisation of CTAB Stabilized AuNC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fornasieri, G.; Bleuzen, A. Controlled Synthesis of Photomagnetic Nanoparticles of a Prussian Blue Analogue in a Silica Xerogel. Angew. Chem. Int. Ed. 2008, 47, 7750–7752. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.-C.; Feldmann, C. Polyol Synthesis of Nanoparticles: Status and Options Regarding Metals, Oxides, Chalcogenides, and Non-Metal Elements. Green Chem. 2015, 17, 4107–4132. [Google Scholar] [CrossRef]
- Manoharan, V.N. Colloidal Matter: Packing, Geometry, and Entropy. Science 2015, 349. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Gilroy, K.D.; Peng, H.-C.; Xia, X. Seed-Mediated Growth of Colloidal Metal Nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 60–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, Q.; Wu, X.-J.; Li, L.; Liu, J.; Zhang, H. Wet-Chemical Synthesis and Applications of Semiconductor Nanomaterial-Based Epitaxial Heterostructures. Nano Micro Lett. 2019, 11, 86. [Google Scholar] [CrossRef] [Green Version]
- Segets, D.; Marczak, R.; Schäfer, S.; Paula, C.; Gnichwitz, J.-F.; Hirsch, A.; Peukert, W. Experimental and Theoretical Studies of the Colloidal Stability of Nanoparticles−A General Interpretation Based on Stability Maps. ACS Nano 2011, 5, 4658–4669. [Google Scholar] [CrossRef]
- Ong, Q.; Luo, Z.; Stellacci, F. Characterization of Ligand Shell for Mixed-Ligand Coated Gold Nanoparticles. Acc. Chem. Res. 2017, 50, 1911–1919. [Google Scholar] [CrossRef]
- Svedberg, T.; Rinde, H. The Ultra-Centrifuge, A New Instrument for the Determination of Size And Distribution of Size of Particle in a Microscopic Colloids. J. Am. Chem. Soc. 1924, 46, 2677–2693. [Google Scholar] [CrossRef]
- Schuck, P. Size-Distribution Analysis of Macromolecules by Sedimentation Velocity Ultracentrifugation and Lamm Equation Modeling. Biophys. J. 2000, 78, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Demeler, B.; Brookes, E.; Nagel-Steger, L. Analysis of Heterogeneity in Molecular Weight and Shape by Analytical Ultracentrifugation Using Parallel Distributed Computing. Meth. Enzymol. 2009, 454, 87–113. [Google Scholar] [CrossRef]
- Planken, K.L.; Cölfen, H. Analytical Ultracentrifugation of Colloids. Nanoscale 2010, 2, 1849–1869. [CrossRef]
- Karabudak, E.; Brookes, E.; Lesnyak, V.; Gaponik, N.; Eychmüller, A.; Walter, J.; Segets, D.; Peukert, W.; Wohlleben, W.; Demeler, B.; et al. Simultaneous Identification of Spectral Properties and Sizes of Multiple Particles in Solution with Subnanometer Resolution. Angew. Chem. Int. Ed. 2016, 55, 11770–11774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cölfen, H.; Pauck, T. Determination of Particle Size Distributions with Angström Resolution. Colloid Polym Sci 1997, 275, 175–180. [Google Scholar] [CrossRef]
- Cölfen, H.; Schnablegger, H.; Fischer, A.; Jentoft, F.C.; Weinberg, G.; Schlögl, R. Particle Growth Kinetics in Zirconium Sulfate Aqueous Solutions Followed by Dynamic Light Scattering and Analytical Ultracentrifugation: Implications for Thin Film Deposition. Langmuir 2002, 18, 3500–3509. [Google Scholar] [CrossRef]
- Arthur, K.K.; Gabrielson, J.P.; Kendrick, B.S.; Stoner, M.R. Detection of Protein Aggregates by Sedimentation Velocity Analytical Ultracentrifugation (SV-AUC): Sources of Variability and Their Relative Importance. J. Pharm. Sci. 2009, 98, 3522–3539. [Google Scholar] [CrossRef]
- Zook, J.M.; Rastogi, V.; MacCuspie, R.I.; Keene, A.M.; Fagan, J. Measuring Agglomerate Size Distribution and Dependence of Localized Surface Plasmon Resonance Absorbance on Gold Nanoparticle Agglomerate Size Using Analytical Ultracentrifugation. ACS Nano 2011, 5, 8070–8079. [Google Scholar] [CrossRef]
- Schuck, P.; Perugini, M.A.; Gonzales, N.R.; Howlett, G.J.; Schubert, D. Size-Distribution Analysis of Proteins by Analytical Ultracentrifugation: Strategies and Application to Model Systems. Biophys. J. 2002, 82, 1096–1111. [Google Scholar] [CrossRef] [Green Version]
- Lebowitz, J.; Lewis, M.S.; Schuck, P. Modern Analytical Ultracentrifugation in Protein Science: A Tutorial Review. Protein Sci. 2002, 11, 2067–2079. [Google Scholar] [CrossRef] [Green Version]
- Ortega, Á.; de la Torre, J.I.G. Hydrodynamic Properties of Rodlike and Disklike Particles in Dilute Solution. J. Chem. Phys. 2003, 119, 9914. [Google Scholar] [CrossRef] [Green Version]
- Harding, S.E. Challenges for the Modern Analytical Ultracentrifuge Analysis of Polysaccharides. Carbohydr. Res. 2005, 340, 811–826. [Google Scholar] [CrossRef]
- Liu, J.; Andya, J.D.; Shire, S.J. A Critical Review of Analytical Ultracentrifugation and Field Flow Fractionation Methods for Measuring Protein Aggregation. AAPS J. 2006, 8, E580–E589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howlett, G.J.; Minton, A.P.; Rivas, G. Analytical Ultracentrifugation for the Study of Protein Association and Assembly. Curr Opin. Chem. Biol. 2006, 10, 430–436. [Google Scholar] [CrossRef]
- Berkowitz, S.A.; Engen, J.R.; Mazzeo, J.R.; Jones, G.B. Analytical Tools for Characterizing Biopharmaceuticals and the Implications for Biosimilars. Nat. Rev. Drug Discov. 2012, 11, 527–540. [Google Scholar] [CrossRef] [Green Version]
- Wright, R.T.; Hayes, D.B.; Stafford, W.F.; Sherwood, P.J.; Correia, J.J. Characterization of Therapeutic Antibodies in the Presence of Human Serum Proteins by AU-FDS Analytical Ultracentrifugation. Anal. Biochem. 2018, 550, 72–83. [Google Scholar] [CrossRef]
- Mitra, S.; Demeler, B. Probing RNA–Protein Interactions and RNA Compaction by Sedimentation Velocity Analytical Ultracentrifugation. RNA Spectrosc. 2020, 281–317. [Google Scholar] [CrossRef]
- Demeler, B.; Nguyen, T.-L.; Gorbet, G.E.; Schirf, V.; Brookes, E.H.; Mulvaney, P.; El-Ballouli, A.O.; Pan, J.; Bakr, O.M.; Demeler, A.K.; et al. Characterization of Size, Anisotropy, and Density Heterogeneity of Nanoparticles by Sedimentation Velocity. Anal. Chem. 2014, 86, 7688–7695. [Google Scholar] [CrossRef] [Green Version]
- Walter, J.; Löhr, K.; Karabudak, E.; Reis, W.; Mikhael, J.; Peukert, W.; Wohlleben, W.; Cölfen, H. Multidimensional Analysis of Nanoparticles with Highly Disperse Properties Using Multiwavelength Analytical Ultracentrifugation. ACS Nano 2014, 8, 8871–8886. [Google Scholar] [CrossRef]
- Pearson, J.; Walter, J.; Peukert, W.; Cölfen, H. Advanced Multiwavelength Detection in Analytical Ultracentrifugation. Anal. Chem. 2018, 90, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.P.; Kim, J.Y.; Qian, H.; Jin, R.; Mehenni, H.; Stellacci, F.; Bakr, O.M. Determination of Nanoparticle Size Distribution Together with Density or Molecular Weight by 2D Analytical Ultracentrifugation. Nat. Commun 2011, 2, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.M.; Cölfen, H. High-Resolution Analysis of Small Silver Clusters by Analytical Ultracentrifugation. J. Phys. Chem. Lett. 2019, 10, 6558–6564. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Schwahn, D.; Cölfen, H. On the Role of Block Copolymer Additives for Calcium Carbonate Crystallization: Small Angle Neutron Scattering Investigation by Applying Contrast Variation. J. Chem. Phys. 2004, 120, 9410–9423. [Google Scholar] [CrossRef] [Green Version]
- Patil, R.S.; Shaikh, V.R.; Patil, P.D.; Borse, A.U.; Patil, K.J. Volumetric Properties of Alkyltrimethylammonium Bromides in Aqueous Solutions. J. Chem. Eng. Data 2016, 61, 195–206. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhong, X.; Li, Z.; Xia, Y. Successive, Seed-Mediated Growth for the Synthesis of Single-Crystal Gold Nanospheres with Uniform Diameters Controlled in the Range of 5–150 nm. Part. Part. Syst Charact 2014, 31, 266–273. [Google Scholar] [CrossRef]
- González-Rubio, G.; Kumar, V.; Llombart, P.; Díaz-Núñez, P.; Bladt, E.; Altantzis, T.; Bals, S.; Peña-Rodríguez, O.; Noya, E.G.; MacDowell, L.G.; et al. Disconnecting Symmetry Breaking from Seeded Growth for the Reproducible Synthesis of High Quality Gold Nanorods. ACS Nano 2019, 13, 4424–4435. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Hsiao, M.-S.; Koerner, H.; Jawaid, A.; Che, J.; Vaia, R.A. Optimizing Seed Aging for Single Crystal Gold Nanorod Growth: The Critical Role of Gold Nanocluster Crystal Structure. J. Phys. Chem. C 2016, 120, 28235–28245. [Google Scholar] [CrossRef]
- Bhattacharyya, S.K.; Maciejewska, P.; Börger, L.; Stadler, M.; Gülsün, A.M.; Cicek, H.B.; Cölfen, H. Development of a Fast Fiber Based UV-Vis Multiwavelength Detector Foran Ultracentrifuge. In Analytical Ultracentrifugation VIII; Wandrey, C., Cölfen, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 9–22. [Google Scholar]
- Strauss, H.M.; Karabudak, E.; Bhattacharyya, S.; Kretzschmar, A.; Wohlleben, W.; Cölfen, H. Performance of a Fast Fiber Based UV/Vis Multiwavelength Detector for the Analytical Ultracentrifuge. Colloid Polym. Sci. 2008, 286, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, J.Z.; Krause, F.; Haffke, D.; Demeler, B.; Schilling, K.; Cölfen, H. Chapter One—Next-Generation AUC Adds a Spectral Dimension: Development of Multiwavelength Detectors for the Analytical Ultracentrifuge. In Methods in Enzymology; Cole, J.L., Ed.; Analytical Ultracentrifugation; Academic Press: Cambridge, MA, USA, 2015; Volume 562, pp. 1–26. [Google Scholar]
- Demeler, B.; Gorbet, G.E. Analytical Ultracentrifugation Data Analysis with UltraScan-III. In Analytical Ultracentrifugation: Instrumentation, Software, and Applications; Uchiyama, S., Arisaka, F., Stafford, W.F., Laue, T., Eds.; Springer: Tokyo, Japan, 2016; pp. 119–143. ISBN 978-4-431-55985-6. [Google Scholar]
- Gorbet, G.; Devlin, T.; Hernandez Uribe, B.I.; Demeler, A.K.; Lindsey, Z.L.; Ganji, S.; Breton, S.; Weise-Cross, L.; Lafer, E.M.; Brookes, E.H.; et al. A Parametrically Constrained Optimization Method for Fitting Sedimentation Velocity Experiments. Biophys. J. 2014, 106, 1741–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamm, O. The Theory and Method of Ultra Centrifuging. Z. Phys. Chem. A Chem. T. 1929, 143, 177–190. [Google Scholar]
- Mächtle, W.; Börger, L. Analytical Ultracentrifugation of Polymers and Nanoparticles; Springer Laboratory; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 978-3-540-23432-6. [Google Scholar]
- Miller, C.C.; Walker, J. The Stokes-Einstein Law for Diffusion in Solution. Proc. Roy. Soc. A Math. Phys. 1924, 106, 724–749. [Google Scholar] [CrossRef]
- Dam, J.; Schuck, P. Calculating Sedimentation Coefficient Distributions by Direct Modeling of Sedimentation Velocity Concentration Profiles. In Methods in Enzymology; Numerical Computer Methods, Part E; Academic Press: Cambridge, MA, USA, 2004; Volume 384, pp. 185–212. [Google Scholar]
- Gokarn, Y.R.; McLean, M.; Laue, T.M. Effect of PEGylation on Protein Hydrodynamics. Mol. Pharm. 2012, 9, 762–773. [Google Scholar] [CrossRef]
- Squire, P.G.; Himmel, M.E. Hydrodynamics and Protein Hydration. Arch. Biochem. 1979, 196, 165–177. [Google Scholar] [CrossRef]
- Meena, S.K.; Celiksoy, S.; Schäfer, P.; Henkel, A.; Sönnichsen, C.; Sulpizi, M. The Role of Halide Ions in the Anisotropic Growth of Gold Nanoparticles: A Microscopic, Atomistic Perspective. Phys. Chem. Chem. Phys. 2016, 18, 13246–13254. [Google Scholar] [CrossRef] [Green Version]
- González-Rubio, G.; Díaz-Núñez, P.; Rivera, A.; Prada, A.; Tardajos, G.; González-Izquierdo, J.; Bañares, L.; Llombart, P.; Macdowell, L.G.; Palafox, M.A.; et al. Femtosecond Laser Reshaping Yields Gold Nanorods with Ultranarrow Surface Plasmon Resonances. Science 2017, 358, 640–644. [Google Scholar] [CrossRef]
- Hendel, T.; Wuithschick, M.; Kettemann, F.; Birnbaum, A.; Rademann, K.; Polte, J. In Situ Determination of Colloidal Gold Concentrations with UV–Vis Spectroscopy: Limitations and Perspectives. Anal. Chem. 2014, 86, 11115–11124. [Google Scholar] [CrossRef] [PubMed]
- Myroshnychenko, V.; Rodríguez-Fernández, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzán, L.M.; Abajo, F.J.G. de Modelling the Optical Response of Gold Nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1805. [Google Scholar] [CrossRef] [Green Version]
- Movsesyan, A.; Marguet, S.; Muravitskaya, A.; Béal, J.; Adam, P.-M.; Baudrion, A.-L. Influence of the CTAB Surfactant Layer on Optical Properties of Single Metallic Nanospheres. J. Opt. Soc. Am. A JOSAA 2019, 36, C78–C84. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Karayil, J.; Kumar, S.; Hassan, P.A.; Talmon, Y.; Sreejith, L. Microstructural Transition of Aqueous CTAB Micelles in the Presence of Long Chain Alcohols. RSC Adv. 2015, 5, 12434–12441. [Google Scholar] [CrossRef]
- Koppen, J.V.; Hapka, M.; Szczęśniak, M.M.; Chałasiński, G. Optical Absorption Spectra of Gold Clusters Au(n) (n = 4, 6, 8,12, 20) from Long-Range Corrected Functionals with Optimal Tuning. J. Chem. Phys. 2012, 137, 114302. [Google Scholar] [CrossRef] [PubMed]


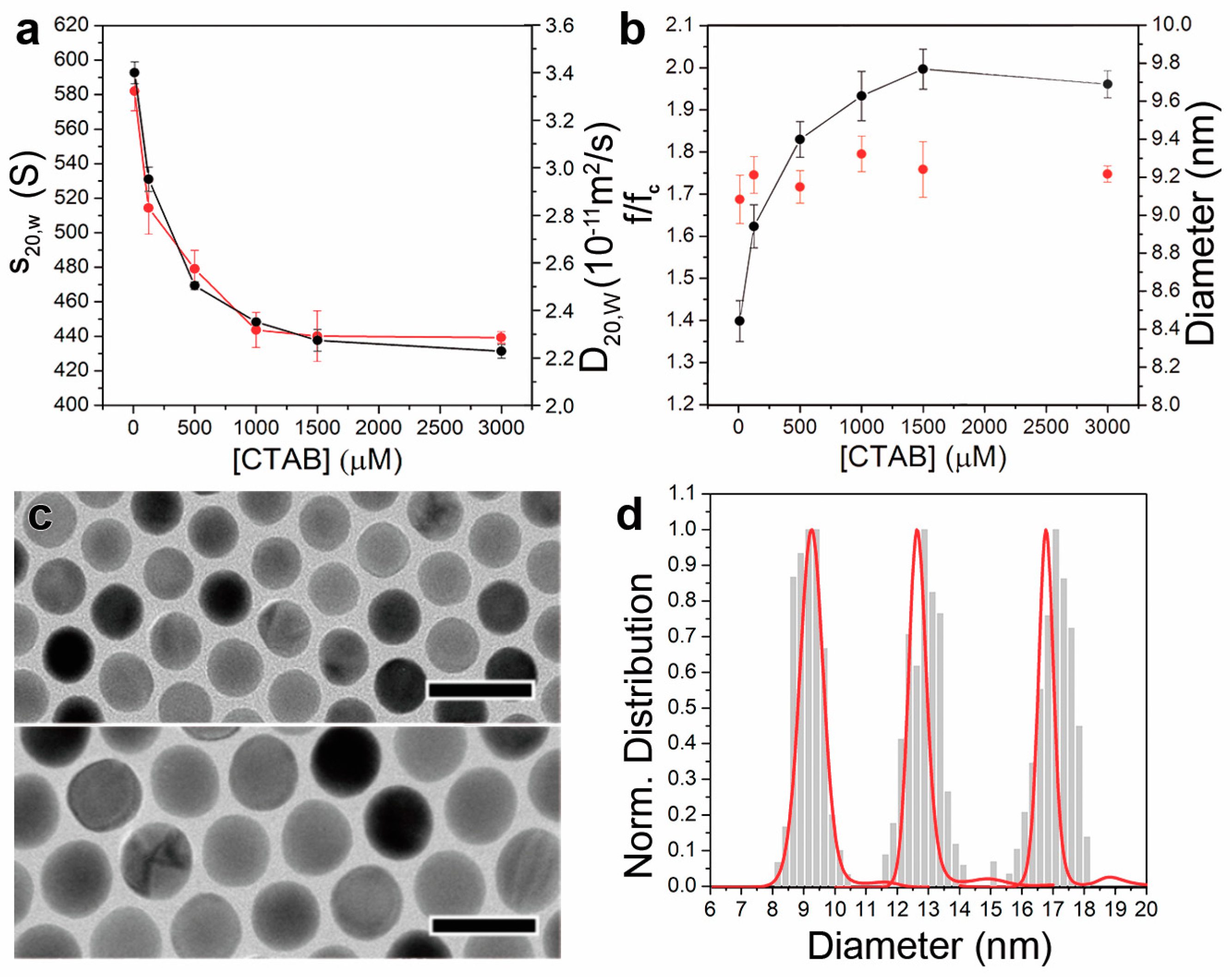

| [CTAB] µM | s20,w,AuNC@CTAB@W (S, 10−13 s) | D20,w,AuNC@CTAB@W (10−11 m2/s) | (f/fc)AuNC@CTAB@W | MAuNC (MDa) |
|---|---|---|---|---|
| 10 | 590 ± 7 | 3.32 ± 0.08 | 1.40 ± 0.05 | 4.5 ± 0.2 |
| 125 | 526 ± 4 | 2.8 ± 0.1 | 1.62 ± 0.05 | 4.8 ± 0.2 |
| 500 | 469 ± 4 | 2.58 ± 0.07 | 1.83 ± 0.05 | 4.8 ± 0.2 |
| 1000 | 447 ± 2 | 2.32 ± 0.07 | 1.93 ± 0.06 | 4.8 ± 0.2 |
| 1500 | 430 ± 1 | 2.3 ± 0.1 | 2.00 ± 0.05 | 4.8 ± 0.2 |
| 3000 | 426 ± 6 | 2.29 ± 0.02 | 1.96 ± 0.03 | 4.9 ± 0.1 |
| [CTAB] µM | dAuNC@CTAB@W by AUC (nm) | dAuNC@CTAB@W by DLS (nm) | dAuNC by AUC (nm) | dAuNC by TEM (nm) | tCTAB@W by AUC (nm) |
|---|---|---|---|---|---|
| 10 | 12.6 ± 0.3 | 13 ± 4 | 9.1 ± 0.1 | 9.1 ± 0.4 | 1.9 ± 0.2 |
| 125 | 14.8 ± 0.6 | 14 ± 5 | 9.21 ± 0.09 | 2.4 ± 0.3 | |
| 500 | 16.3 ± 0.5 | 15 ± 5 | 9.16 ± 0.09 | 2.9 ± 0.2 | |
| 1000 | 18.1 ± 0.6 | 16 ± 4 | 9.32 ± 0.09 | 3.3 ± 0.2 | |
| 1500 | 18.3 ± 0.8 | 16 ± 5 | 9.2 ± 0.1 | 3.4 ± 0.2 | |
| 3000 | 18.3 ± 0.2 | 16 ± 3 | 9.22 ± 0.05 | 3.4 ± 0.2 |
| s20,w,AuNC@CTAB@W (S, 10−13 s) | D20,w,AuNC@CTAB@W (10−11 m2/s) | dAuNC@CTAB@W (nm) | (f/fc)AuNC@CTAB@W | MAuNC (kDa) | dAuNC (nm) | Rel. Conc. % |
|---|---|---|---|---|---|---|
| 1.8 ± 0.1 | 3.8 ± 0.2 | 9.9 ±0.5 | 9.23 ± 0.05 | 11 ± 2 | 1.2 ± 0.2 | 25.6 |
| 2.6 ± 0.2 | 3.3 ± 0.1 | 11.5 ± 0.4 | 9.06 ± 0.05 | 19 ± 2 | 1.5 ± 0.2 | 18.1 |
| 3.6 ± 0.3 | 2.9 ± 0.1 | 12.8 ± 0.4 | 8.9 ± 0.1 | 28 ± 4 | 1.6 ± 0.2 | 11.0 |
| 5.2 ± 0.3 | 2.5 ± 0.6 | 15.1 ± 0.3 | 8.5 ± 0.1 | 47 ± 6 | 2.0 ± 0.2 | 18.2 |
| 14.8 ± 0.7 | 2.32 ± 0.1 | 16.3 ± 0.2 | 6.4 ± 0.1 | 145 ± 7 | 2.9 ± 0.2 | 21.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rubio, G.; Hilbert, H.; Rosenberg, R.; Ni, B.; Fuhrer, L.; Cölfen, H. Simple Determination of Gold Nanocrystal Dimensions by Analytical Ultracentrifugation via Surface Ligand-Solvent Density Matching. Nanomaterials 2021, 11, 1427. https://doi.org/10.3390/nano11061427
González-Rubio G, Hilbert H, Rosenberg R, Ni B, Fuhrer L, Cölfen H. Simple Determination of Gold Nanocrystal Dimensions by Analytical Ultracentrifugation via Surface Ligand-Solvent Density Matching. Nanomaterials. 2021; 11(6):1427. https://doi.org/10.3390/nano11061427
Chicago/Turabian StyleGonzález-Rubio, Guillermo, Holger Hilbert, Rose Rosenberg, Bing Ni, Lisa Fuhrer, and Helmut Cölfen. 2021. "Simple Determination of Gold Nanocrystal Dimensions by Analytical Ultracentrifugation via Surface Ligand-Solvent Density Matching" Nanomaterials 11, no. 6: 1427. https://doi.org/10.3390/nano11061427







