The Heating Efficiency and Imaging Performance of Magnesium Iron Oxide@tetramethyl Ammonium Hydroxide Nanoparticles for Biomedical Applications
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
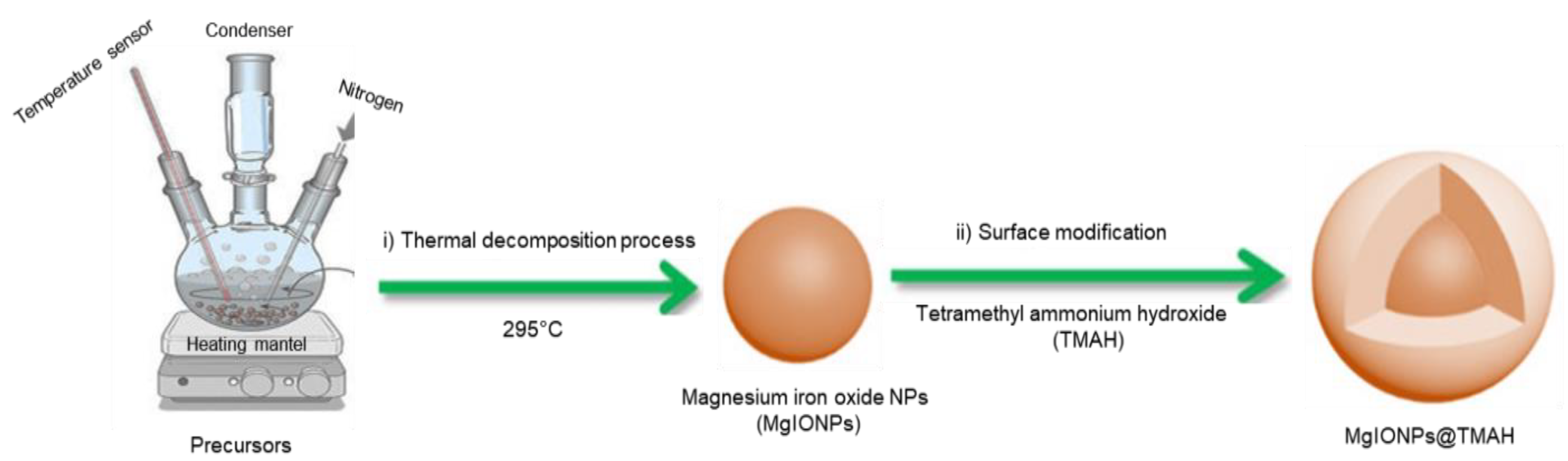
2.2. Synthesis of Magnesium Iron Oxide@Tetramethyl Ammonium Hydroxide Nanoparticles (MgIONPs@TMAH)
2.2.1. Preparation of Hydrophobic Nanoparticles
2.2.2. Surface Modification of MgIONPs with Tetramethyl Ammonium Hydroxide
2.3. Characterization
2.4. In Vitro Cytocompatibility Test
3. Results and Discussion
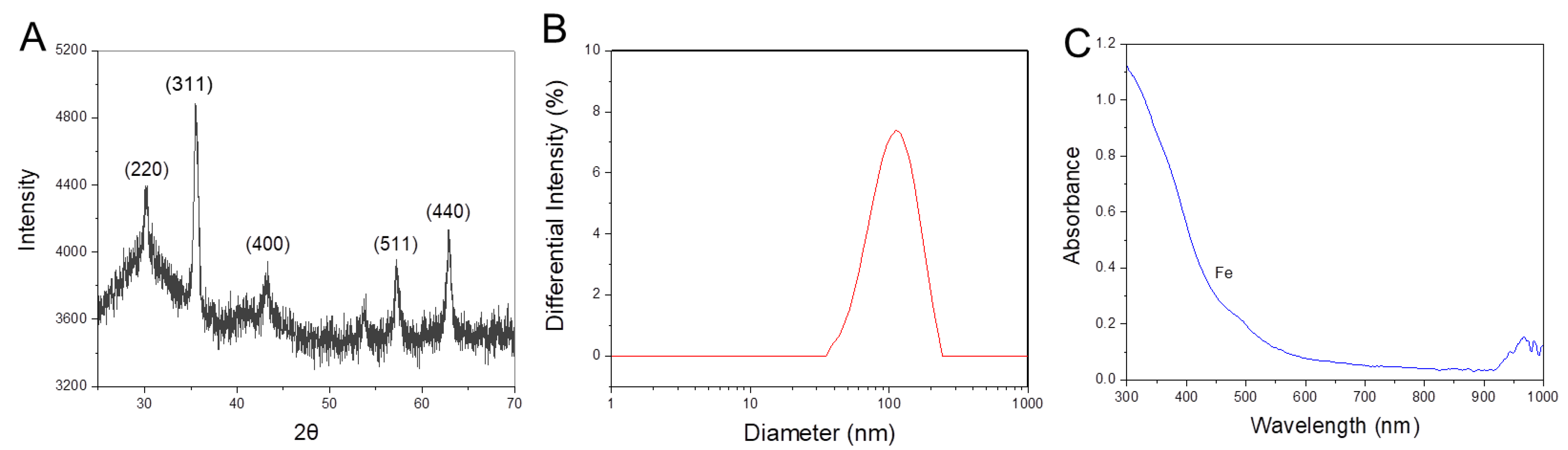
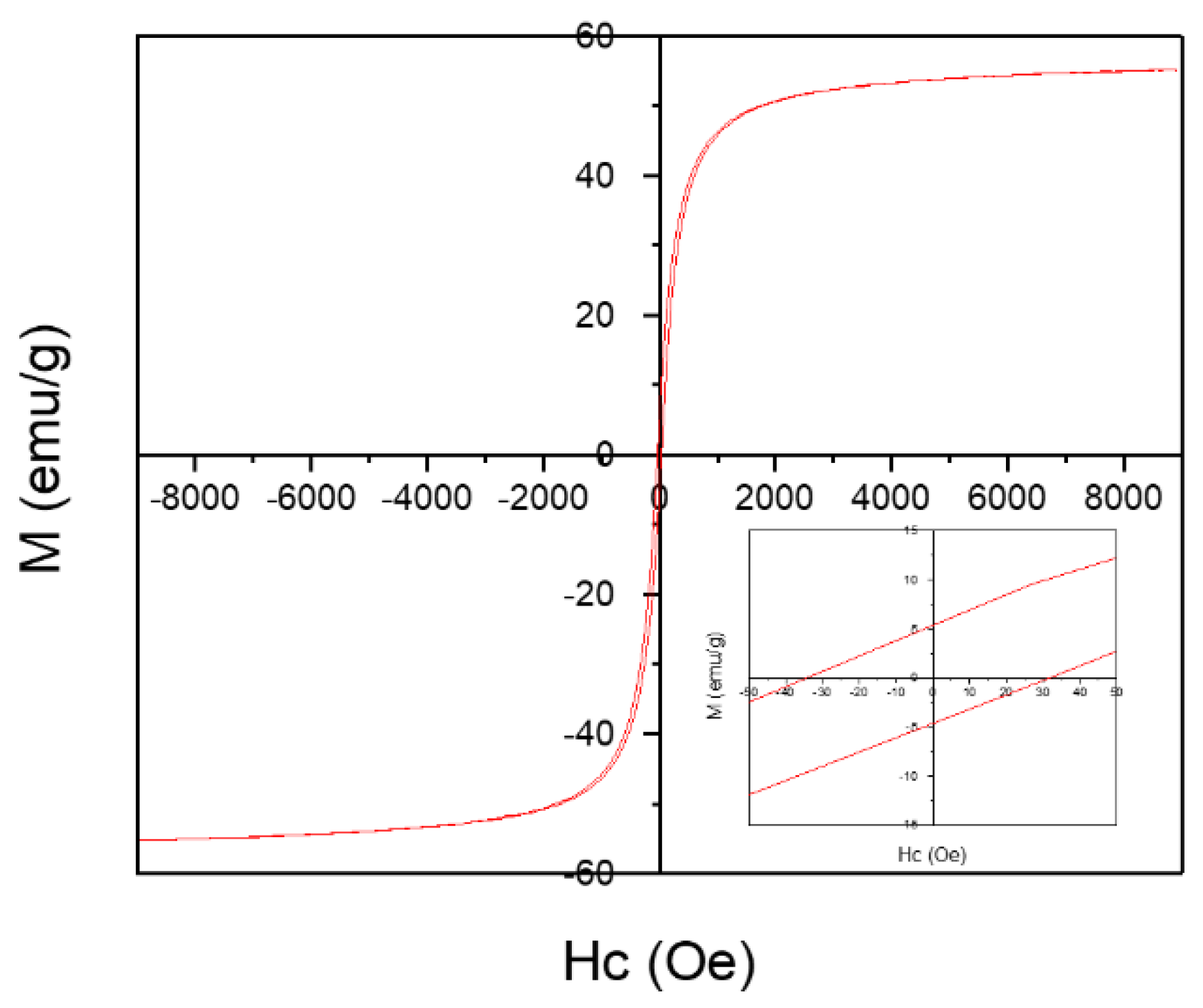
3.1. Synthesis and Characterization of MgIONPs@TMAH
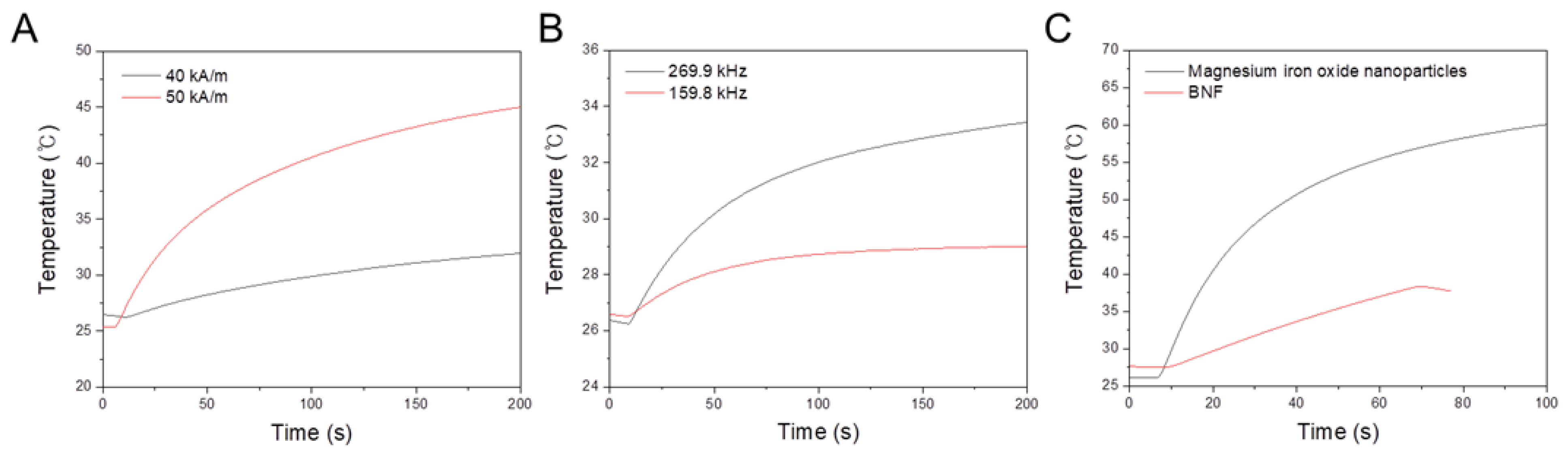
3.2. Hyperthermia Performance
3.2.1. Effects of Magnetic-Field Strength Variation with a Constant Frequency
3.2.2. Effects of Field Frequency Variation with a Constant Magnetic Field Strength
3.2.3. Effects of a High-Field Frequency with a Low Magnetic Field Strength
3.3. Magnetic Particle Imaging Performance
3.4. In Vitro Cytotoxicity Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Cabezas, S.; Montes-Robles, R.; Gallo, J.; Sancenón, F.; Martínez-Máñez, R. Combining magnetic hyperthermia and dual T1/T2 MR imaging using highly versatile iron oxide nanoparticles. Dalton Trans. 2019, 48, 3883–3892. [Google Scholar] [CrossRef]
- Carvalho, A.; Gallo, J.; Pereira, D.; Valentão, P.; Andrade, P.; Hilliou, L.; Ferreira, P.; Bañobre-López, M.; Martins, J. Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications. Nanomaterials 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.; Phu Bui, M.; Yoon, J. Theoretical Analysis for Wireless Magnetothermal Deep Brain Stimulation Using Commercial Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2873. [Google Scholar] [CrossRef] [Green Version]
- Appa Rao, P.; Srinivasa Rao, K.; Pydi Raju, T.R.K.; Kapusetti, G.; Choppadandi, M.; Chaitanya Varma, M.; Rao, K.H. A systematic study of cobalt-zinc ferrite nanoparticles for self-regulated magnetic hyperthermia. J. Alloys Compd. 2019, 794, 60–67. [Google Scholar] [CrossRef]
- Bauer, L.; Situ, S.; Griswold, M.; Samia, A. High-performance iron oxide nanoparticles for magnetic particle imaging-guided hyperthermia (hMPI). Nanoscale 2016, 8, 12162–12169. [Google Scholar] [CrossRef]
- Dadfar, S.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvara, P.; Metselaar, J.; Banala, S.; Straub, M.; Guvener, N.; Engelmann, U.; et al. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J Nanobiotechnol. 2020, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Kenney, M.; Chen, Y.; Zheng, X.; Deng, Y.; Chen, Z.; Wang, S.; Gambhir, S.; Dai, H.; Rao, J. Carbon-coated FeCo nanoparticles as sensitive magnetic-particle-imaging tracers with photothermal and magnetothermal properties. Nat. Biomed. Eng. 2020, 4, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.P.; Wang, Y.; Li, F.S. Magnetic properties of nanocrystalline Co–Ni ferrite. J. Mater. Sci. 2006, 41, 5726–5730. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef]
- Sorescu, M.; Grabias, A.; Tarabasanu-Mihaila, D.; Diamandescu, L. Influence of cobalt and nickel substitutions on populations, hyperfine fields, and hysteresis phenomenon in magnetite. J. Appl. Phys. 2002, 91, 8135–8137. [Google Scholar] [CrossRef]
- Rani, S.; Varma, G.D. Superparamagnetism and metamagnetic transition in Fe3O4 nanoparticles synthesized via co-precipitation method at different pH. Phys. B Condens. Matter 2015, 472, 66–77. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Chen, Y.; Peng, X. Size- and shape-controlled magnetic (Cr, Mn, Fe, Co, Ni) oxide nanocrystals via a simple and general approach. Chem. Mater. 2004, 16, 3931–3935. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Petersen, E.J.; Nelson, B.C. Mechanisms and measurements of nanomaterial-induced oxidative damage to DNA. Anal. Bioanal. Chem. 2010, 398, 613–650. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Hyeon, T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small 2013, 9, 1450. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6.4, 3080–3091. [Google Scholar] [CrossRef]
- Thirunavukkarasu, G.K.; Cherukula, K.; Lee, H.; Yeon Jeong, Y.; Park, I.; Young Lee, J. Magnetic field-inducible drug-eluting nanoparticles for image-guided thermo-chemotherapy. Biomaterials 2018, 180, 240–252. [Google Scholar] [CrossRef]
- Ayyappan, S.; Mahadevan, S.; Chandramohan, P.; Srinivasan, M.P.; Philip, J.; Raj, B. Influence of Co2+ Ion Concentration on the Size, Magnetic Properties, and Purity of CoFe2O4 Spinel Ferrite Nanoparticles. J. Phys. Chem. C 2010, 114, 6334–6341. [Google Scholar] [CrossRef]
- Chinnasamy, C.N.; Senoue, M.; Jeyadevan, B.; Perales-Perez, O.; Shinoda, K.; Tohji, K. Synthesis of size-controlled cobalt ferrite particles with high coercivity and squareness ratio. J. Colloid Interface Sci. 2003, 263, 80–83. [Google Scholar] [CrossRef]
- Maensiri, S.; Sangmanee, M.; Wiengmoon, A. Magnesium Ferrite (MgFe2O4) Nanostructures Fabricated by Electrospinning. Nanoscale Res. Lett. 2009, 4, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Noh, S.H.; Na, W.; Jang, J.T.; Lee, J.H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.S.; Cheon, J. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, K.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating Magnetic Fluid with Alternating Magnetic Field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, Y.; Palchoudhury, S.; Bao, Y. Water-Soluble Iron Oxide Nanoparticles with High Stability and Selective Surface Functionality. Langmuir 2011, 27, 8990–8997. [Google Scholar] [CrossRef]
- Kmita, A.; Lachowicz, D.; Żukrowski, J.; Gajewska, M.; Szczerba, W.; Kuciakowski, J.; Zapotoczny, S.; Sikora, M. One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water. Materials 2019, 12, 1048. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, S.K.; Lim, P.K. On determining the optical gap associated with an amorphous semiconductor: A generalization of the Tauc model. Solid State Commun. 1997, 104, 17–21. [Google Scholar] [CrossRef]
- Mallick, P.; Dash, B.N. X-ray diffraction and UV–Visible characterizations of γ–Fe2O3 nanoparticles annealed at different temperature. Nanosci. Nanotechnol. 2013, 3, 130–134. [Google Scholar]
- El Ghandoor, H.; Zidan, H.M.; Khalil, M.M.; Ismail, M.I.M. Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 5734–5745. [Google Scholar]
- Prabhakaran, T.; Hemalatha, J. Combustion synthesis and characterization of cobalt ferrite nanoparticles. Ceram. Int. 2016, 42, 14113–14120. [Google Scholar] [CrossRef]
- Engelmann, U.; Buhl, E.; Draack, S.; Viereck, T.; Ludwig, F.; Schmitz-Rode, T.; Slabu, I. Magnetic Relaxation of Agglomerated and Immobilized Iron Oxide Nanoparticles for Hyperthermia and Imaging Applications. IEEE Magn. Lett. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Myrovali, E.; Maniotis, N.; Samaras, T.; Angelakeris, M. Spatial focusing of magnetic particle hyperthermia. Nanoscale Adv. 2020, 2, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Brero, F.; Albino, M.; Antoccia, A.; Arosio, P.; Avolio, M.; Berardinelli, F.; Bettega, D.; Calzolari, P.; Ciocca, M.; Corti, M.; et al. Hadron Therapy, Magnetic Nanoparticles and Hyperthermia: A Promising Combined Tool for Pancreatic Cancer Treatment. Nanomaterials 2020, 10, 1919. [Google Scholar] [CrossRef] [PubMed]
- Al-Musawi, S.; Albukhaty, S.; Al-Karagoly, H.; Almalki, F. Design and Synthesis of Multi-Functional Superparamagnetic Core-Gold Shell Nanoparticles Coated with Chitosan and Folate for Targeted Antitumor Therapy. Nanomaterials 2021, 11, 32. [Google Scholar] [CrossRef]
- Salimi, M.; Sarkar, S.; Hashemi, M.; Saber, R. Treatment of Breast Cancer-Bearing BALB/c Mice with Magnetic Hyperthermia using Dendrimer Functionalized Iron-Oxide Nanoparticles. Nanomaterials 2020, 10, 2310. [Google Scholar] [CrossRef]
- Ma, M.; Wu, Y.; Zhou, J.; Sun, Y.; Zhang, Y.; Gu, N. Size dependence of specific power absorption of Fe3O4 particles in AC magnetic field. J. Magn. Magn. Mater. 2004, 268, 33–39. [Google Scholar] [CrossRef]
- Hergt, R.; Hiergeist, R.; Zeisberger, M.; Glockl, G.; Weitschies, W.; Ramirez, L.P.; Hilger, I.; Kaiser, W.A. Enhancement of AC-losses of magnetic nanoparticles for heating applications. J. Magn. Magn. Mater. 2004, 280, 358–368. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic Particle Hyperthermia—Biophysical Limitations of a Visionary Tumour Therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Muller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919. [Google Scholar] [CrossRef]
- Nigam, S.; Barick, K.; Bahadur, D. Development of citrate-stabilized Fe3O4 nanoparticles: Conjugation and release of doxorubicin for therapeutic applications. J. Magn. Magn. Mater. 2011, 323, 237–243. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Delgado, Á.V.; Schneider, E.K.; Checa Fernández, B.L.; Iglesias, G.R. Magnetic Nanoparticles Coated with a Thermosensitive Polymer with Hyperthermia Properties. Polymers 2018, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Shete, P.B.; Patil, R.M.; Thorat, N.D.; Prasad, A.; Ningthoujam, R.S.; Ghosh, S.J.; Pawar, S.H. Magnetic chitosan nanocomposite for hyperthermia therapy application: Preparation, characterization and in vitro experiments. Appl. Surf. Sci. 2014, 288, 149. [Google Scholar] [CrossRef]
- Chauhan, A.; Midha, S.; Kumar, R.; Meena, R.; Singh, P.; Jhab, S.; Kuanr, K. Rapid tumor inhibition via magnetic hyperthermia regulated by caspase 3 with time-dependent clearance of iron oxide nanoparticles. Biomater. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Fan, H.M.; Yi, J.B.; Yang, Y.; Choo, E.S.G.; Xue, J.M.; Ding, J. Optimization of surface coating on Fe3O4 nanoparticles for high performance magnetic hyperthermia agents. J. Mater. Chem. 2012, 22, 8235–8244. [Google Scholar] [CrossRef]
- Fuentes-García, J.A.; Carvalho Alavarse, A.; Moreno Maldonado, A.C.; Toro-Córdova, A.; Ibarra, M.R.; Goya, G.F. Simple Sonochemical Method to Optimize the Heating Efficiency of Magnetic Nanoparticles for Magnetic Fluid Hyperthermia. ACS Omega 2020, 5, 26357–26364. [Google Scholar] [CrossRef]
- Gupta, J.; Hassan, P.A.; Barick, K.C. Core-shell Fe3O4@ ZnO nanoparticles for magnetic hyperthermia and bio-imaging applications. AIP Adv. 2021, 11, 025207. [Google Scholar] [CrossRef]



| Conditions | f = 614.4 kHz; H = 9.5 kA/m | f = 97 kHz; H = 40 kA/m |
|---|---|---|
| MgIONPs@TMAH | SLP: 216.18 W/g ILP:3.8 nHm2/kg | SLP:10.84 W/g ILP:0.069 nHm2/kg |
| BNF | SLP:14 W/g ILP:0.25 nHm2/kg | SLP:195.2 W/g ILP:1.25 nHm2/kg |
| Sample | Size (nm) | Ms (emu/g) | SLP | Alternating Current (AC) Field Condition | ILP | MPI Good Performance |
|---|---|---|---|---|---|---|
| Chitosan-coated Fe3O4 [44] | 37 | 71.5 | 595 | H: 14, f: 335 | 9 | - |
| PEG-coated Fe3O4 [45] | 31 | 54 | 355 | H: 27, f: 400 | 1.22 | - |
| Fe3O4 [46] | 37 | 67 | 213 | H: 23.9, f: 571 | 0.72 | - |
| Fe3O4,Sph [5] | 19.2 ± 1.3 | 101.5 | 189.6 | H: 16, f: 380 | 1.9 | + |
| Fe3O4,Cube | 15.5 ± 1.1 | 107.3 | 356.2 | 3.6 | ||
| Zn0.4Fe2.6O4,Zn-Sph | 19.1 ± 1.0 | 125.7 | 438.6 | 4.5 | ||
| Zn0.4Fe2.6O4,Zn-Cube | 15.4 ± 1.1 | 130.4 | 1019.2 | 10.47 | ||
| FeCo@C [7] | 40 nm | 192 | 406 | H: 100, f: 30 | 1.3 | + |
| Citrate-coated IONPs [6] | 10.6 ± 1.8 | 73.8 | 230 | H: 46, f: 186 | 0.58 | + |
| 13.1 ± 2.2 | 82.5 | 350 | 0.88 | |||
| Fe3O4@ZnO [47] | 10 | 31.2 | 80 | H: 25.12, f: 250 | 0.49 | - |
| MgIONPs@TMAH | 15.0 ± 5.0 | 55.1 | 216.18 | H: 9.5, f: 614.4 | 3.8 | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, M.S.A.; Kim, H.; Bui, M.P.; Le, T.-A.; Lee, H.; Ryu, C.; Lee, J.Y.; Yoon, J. The Heating Efficiency and Imaging Performance of Magnesium Iron Oxide@tetramethyl Ammonium Hydroxide Nanoparticles for Biomedical Applications. Nanomaterials 2021, 11, 1096. https://doi.org/10.3390/nano11051096
Darwish MSA, Kim H, Bui MP, Le T-A, Lee H, Ryu C, Lee JY, Yoon J. The Heating Efficiency and Imaging Performance of Magnesium Iron Oxide@tetramethyl Ammonium Hydroxide Nanoparticles for Biomedical Applications. Nanomaterials. 2021; 11(5):1096. https://doi.org/10.3390/nano11051096
Chicago/Turabian StyleDarwish, Mohamed S. A., Hohyeon Kim, Minh Phu Bui, Tuan-Anh Le, Hwangjae Lee, Chiseon Ryu, Jae Young Lee, and Jungwon Yoon. 2021. "The Heating Efficiency and Imaging Performance of Magnesium Iron Oxide@tetramethyl Ammonium Hydroxide Nanoparticles for Biomedical Applications" Nanomaterials 11, no. 5: 1096. https://doi.org/10.3390/nano11051096








