Metabolomic Profiling Unveils the Impact of Non-Doped and Heteroatom-Doped Carbon Nanodots on Zebrafish (Danio rerio) Embryos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumental Conditions
2.3. CND Synthesis and Characterization
2.4. Exposure of Zebrafish to CNDs
2.5. Metabolome Extraction and Analysis
2.6. Metabolome Data Processing
3. Results
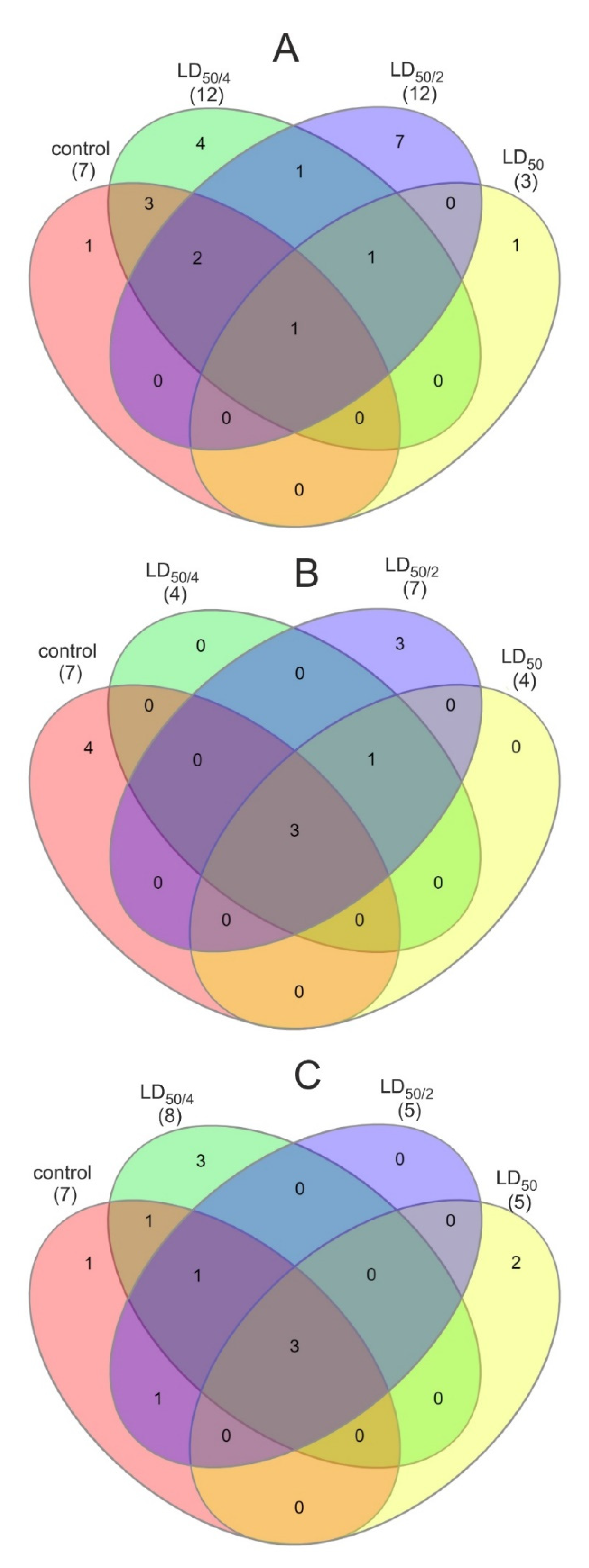
3.1. Metabolomic Study of Non-doped CNDs Effect
3.2. Metabolomic Study of N-doped CNDs Effect
3.3. Metabolomic Study of N,S-codoped CND Effect
4. Discussion
4.1. Glutathione Metabolism
4.2. Glycerolipid and Glycerophospholipid Metabolism
4.3. Riboflavin Metabolism
4.4. Biotin Metabolism
4.5. Nitrogen Metabolism
4.6. Glucose Alterations—Energy Metabolism
4.7. Amino Acid Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Du, F.; Zeng, F.; Ming, Y.; Wu, S. Carbon dots-based fluorescent probes for sensitive and selective detection of iodide. Microchim. Acta 2013, 180, 453–460. [Google Scholar] [CrossRef]
- Yang, K.; Liu, M.; Wang, Y.; Wang, S.; Miao, H.; Yang, L.; Yang, X. Carbon dots derived from fungus for sensing hyaluronic acid and hyaluronidase. Sens. Actuators B Chem. 2017, 251, 503–508. [Google Scholar] [CrossRef]
- Huang, H.; Li, C.; Zhu, S.; Wang, H.; Chen, C.; Wang, Z.; Bai, T.; Shi, Z.; Feng, S. Histidine-derived nontoxic nitrogen-doped carbon dots for sensing and bioimaging applications. Langmuir 2014, 30, 13542–13548. [Google Scholar] [CrossRef]
- Roy, A.K.; Kim, S.M.; Paoprasert, P.; Park, S.Y.; In, I. Preparation of biocompatible and antibacterial carbon quantum dots derived from resorcinol and formaldehyde spheres. RSC Adv. 2015, 5, 31677–31682. [Google Scholar] [CrossRef]
- Su, X.; Xu, Y.; Che, Y.; Liao, X.; Jiang, Y. A type of novel fluorescent magnetic carbon quantum dots for cells imaging and detection. J. Biomed. Mater. Res. Part A 2015, 103, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.F.; Wu, H.C.; Kuan, C.H.; Lin, C.J.; Wang, L.W.; Chang, C.W.; Wang, T.W. Multi-functionalized carbon dots as theranostic nanoagent for gene delivery in lung cancer therapy. Sci. Rep. 2016, 6, 21170. [Google Scholar] [CrossRef]
- Hassan, M.; Gomes, V.G.; Dehghani, A.; Ardekani, S.M. Engineering carbon quantum dots for photomediated theranostics. Nano Res. 2018, 11, 1–41. [Google Scholar] [CrossRef]
- Ghosal, K.; Ghosh, A. Carbon dots: The next generation platform for biomedical applications. Mater. Sci. Eng. C 2019, 96, 887–903. [Google Scholar] [CrossRef]
- Miao, S.; Liang, K.; Zhu, J.; Yang, B.; Zhao, D.; Kong, B. Hetero-atom-doped carbon dots: Doping strategies, properties and applications. Nano Today 2020, 33, 100879. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Y.; Tsai, P.; Wang, J.; Chen, X. Metal ions doped carbon quantum dots: Synthesis, physicochemical properties, and their applications. TrAC-Trends Anal. Chem. 2018, 103, 87–101. [Google Scholar] [CrossRef]
- Gwinn, M.R.; Vallyathan, V. Nanoparticles: Health effects-Pros and cons. Environ. Health Perspect. 2006, 114, 1818–1825. [Google Scholar] [CrossRef] [Green Version]
- Viswanath, B.; Kim, S. Influence of nanotoxicity on human health and environment: The alternative strategies. Rev. Environ. Contam. Toxicol. 2017, 242, 61–104. [Google Scholar] [CrossRef]
- Wang, Z.G.; Zhou, R.; Jiang, D.; Song, J.E.; Xu, Q.; Si, J.; Chen, Y.P.; Zhou, X.; Gan, L.; Li, J.Z.; et al. Toxicity of graphene quantum dots in zebrafish embryo. Biomed. Environ. Sci. 2015, 28, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Y.; Liu, L.; Chen, Y.; Zeng, Y.L.; Liu, M.Z.; Jin, L. Developmental toxicity of carbon quantum dots to the embryos/larvae of rare minnow (Gobiocypris rarus). Biomed Res. Int. 2016, 2016, 4016402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, C.; Vasimalai, N.; Sárria, M.P.; Pinheiro, I.; Vilas-Boas, V.; Peixoto, J.; Espiña, B. Biocompatibility and bioimaging potential of fruit-based carbon dots. Nanomaterials 2019, 9, 199. [Google Scholar] [CrossRef] [Green Version]
- Chousidis, I.; Stalikas, C.D.; Leonardos, I.D. Induced toxicity in early-life stage zebrafish (Danio rerio) and its behavioral analysis after exposure to non-doped, nitrogen-doped and nitrogen, sulfur-co doped carbon quantum dots. Environ. Toxicol. Pharmacol. 2020, 79, 103426. [Google Scholar] [CrossRef]
- Berghoff, B.A.; Konzer, A.; Mank, N.N.; Looso, M.; Rische, T.; Förstner, K.U.; Krüger, M.; Klug, G. Integrative “Omics”-Approach Discovers Dynamic and Regulatory Features of Bacterial Stress Responses. PLoS Genet. 2013, 9, e1003576. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Kasouni, A.I.; Troganis, A.N.; Stalikas, C.D. Exploring the antibacterial potential and unraveling the mechanism of action of non-doped and heteroatom-doped carbon nanodots. J. Nanoparticle Res. 2020, 22, 36. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. In A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th ed.; University Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Wilhelm, K.-P.; Zhai, H.; Maibach, H.I.; Wilhelm, K.-P.; Maibach, H.I. OECD guidelines for testing of chemicals. Dermatotoxicology 2012, 1, 497–499. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Chousidis, I.; Chatzimitakos, T.; Leonardos, D.; Filiou, M.D.; Stalikas, C.D.; Leonardos, I.D. Cannabinol in the spotlight: Toxicometabolomic study and behavioral analysis of zebrafish embryos exposed to the unknown cannabinoid. Chemosphere 2020, 252, 126417. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.; Chen, Z.F.; Zhang, H.; Chen, Y.; Qi, Z.; Liu, G.; Cai, Z. Evaluation and optimization of sample pretreatment for GC/MS-based metabolomics in embryonic zebrafish. Talanta 2020, 207, 120260. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Stalikas, C.D. Qualitative Alterations of Bacterial Metabolome after Exposure to Metal Nanoparticles with Bactericidal Properties: A Comprehensive Workflow Based on 1H NMR, UHPLC-HRMS, and Metabolic Databases. J. Proteome Res. 2016, 15, 3322–3330. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Stalikas, C.D. Metabolic fingerprinting of bacteria exposed to nanomaterials, using online databases, nmr, and high-resolution mass spectrometry. Methods Mol. Biol. 2019, 1894, 271–280. [Google Scholar] [PubMed]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Havrdova, M.; Hola, K.; Skopalik, J.; Tomankova, K.; Petr, M.; Cepe, K.; Polakova, K.; Tucek, J.; Bourlinos, A.B.; Zboril, R. Toxicity of carbon dots-Effect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle. Carbon N. Y. 2016, 99, 238–248. [Google Scholar] [CrossRef]
- Ruiz, V.; Yate, L.; García, I.; Cabanero, G.; Grande, H.J. Tuning the antioxidant activity of graphene quantum dots: Protective nanomaterials against dye decoloration. Carbon N. Y. 2017, 116, 366–374. [Google Scholar] [CrossRef]
- Morello, J.; Derks, R.J.E.; Lopes, S.S.; Steenvoorden, E.; Monteiro, E.C.; Mayboroda, O.A.; Pereira, S.A. Zebrafish larvae are a suitable model to investigate the metabolic phenotype of drug-induced renal tubular injury. Front. Pharmacol. 2018, 9, 1193. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.; Yin, Z.; Jia, Z.; Wei, J. Carbon Nanodots Derived from Urea and Citric Acid in Living Cells: Cellular Uptake and Antioxidation Effect. Langmuir 2020, 36, 8632–8640. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, Z.; Eeza, M.N.H.; Matysik, J.; Berry, J.P.; Alia, A. NMR-based metabolic profiles of intact zebrafish embryos exposed to aflatoxin b1 recapitulates hepatotoxicity and supports possible neurotoxicity. Toxins (Basel) 2019, 11, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.J.; Olsen, R.K.; Bross, P.; Gomes, C.M. Emerging Roles for Riboflavin in Functional Rescue of Mitochondrial β-Oxidation Flavoenzymes. Curr. Med. Chem. 2010, 17, 3842–3854. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, N.; Corydon, T.J.; Gregersen, N.; Olsen, R.K.J. Cellular consequences of oxidative stress in riboflavin responsive multiple acyl-CoA dehydrogenation deficiency patient fibroblasts. Hum. Mol. Genet. 2014, 23, 4285–4301. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez Meléndez, R. Importancia del metabolismo de la biotina. Rev. Investig. Clin. 2000, 52, 194–199. [Google Scholar]
- Fernandes, H.; Peres, H.; Carvalho, A.P. Dietary protein requirement during Juvenile Growth of Zebrafish (Danio rerio). Zebrafish 2016, 13, 548–555. [Google Scholar] [CrossRef]
- Yossa, R.; Sarker, P.K.; Karanth, S.; Ekker, M.; Vandenberg, G.W. Effects of dietary biotin and avidin on growth, survival, feed conversion, biotin status and gene expression of zebrafish Danio rerio. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 160, 150–158. [Google Scholar] [CrossRef]
- Fu, J.; Gong, Z.; Kelly, B.C. Metabolomic profiling of zebrafish (Danio rerio) embryos exposed to the antibacterial agent triclosan. Environ. Toxicol. Chem. 2019, 38, 240–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K.; Ward, S.A.; Kalantar-Zadeh, K.; El-Omar, E.M. Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles. ACS Nano 2020, 14, 5179–5182. [Google Scholar] [CrossRef] [PubMed]

| Metabolic Pathway | Control | 138 μg mL−1 (LC50/4) | 275 μg mL−1 (LC50/2) | 550 μg mL−1 (LC50) |
|---|---|---|---|---|
| Starch and sucrose metabolism | + | + | + | + |
| Galactose metabolism | + | + | + | - |
| Nitrogen metabolism | + | + | + | - |
| Amino sugar and nucleotide sugar metabolism | + | + | - | - |
| Fructose and mannose metabolism | + | + | - | - |
| Biotin metabolism | + | + | - | - |
| Riboflavin metabolism | + | - | - | - |
| Aminoacyl-tRNA biosynthesis | - | + | - | - |
| Arginine and proline metabolism | - | + | - | - |
| Glutathione metabolism | - | + | + | - |
| Histidine metabolism | - | + | - | - |
| Selenoamino acid metabolism | - | + | - | - |
| Pantothenate and CoA biosynthesis | - | - | + | - |
| Pentose and glucuronate interconversions | - | - | + | - |
| Purine metabolism | - | - | + | - |
| Valine, leucine, and isoleucine biosynthesis | - | - | + | - |
| Valine, leucine, and isoleucine degradation | - | - | + | - |
| Glycerolipid metabolism | - | - | + | - |
| Glycerophospholipid metabolism | - | - | + | - |
| Glycine, serine, and threonine metabolism | - | - | - | + |
| Cysteine and methionine metabolism | - | + | + | + |
| Metabolic Pathway | Control | 100 μg mL−1 (LC50/4) | 200 μg mL−1 (LC50/2) | 400 μg mL−1 (LC50) |
|---|---|---|---|---|
| Starch and sucrose metabolism | + | + | + | + |
| Galactose metabolism | + | + | + | + |
| Amino sugar and nucleotide sugar metabolism | + | + | + | + |
| Nitrogen metabolism | + | - | - | - |
| Fructose and mannose metabolism | + | - | - | - |
| Biotin metabolism | + | - | - | - |
| Riboflavin metabolism | + | - | - | - |
| Arginine and proline metabolism | - | - | + | - |
| Pentose and glucuronate interconversions | - | - | + | - |
| Cysteine and methionine metabolism | - | + | + | + |
| Valine, leucine, and isoleucine biosynthesis | - | - | + | - |
| Metabolic Pathway | Control | 38 μg mL−1 (LC50/4) | 75 μg mL−1 (LC50/2) | 150 μg mL−1 (LC50) |
|---|---|---|---|---|
| Starch and sucrose metabolism | + | + | + | + |
| Galactose metabolism | + | + | + | + |
| Amino sugar and nucleotide sugar metabolism | + | + | + | + |
| Nitrogen metabolism | + | + | + | - |
| Fructose and mannose metabolism | + | + | - | - |
| Biotin metabolism | + | - | + | - |
| Riboflavin metabolism | + | - | - | - |
| Pentose and glucuronate interconversions | - | + | - | - |
| Valine, leucine, and isoleucine biosynthesis | - | + | - | - |
| Aminoacyl-tRNA biosynthesis | - | + | - | - |
| Cysteine and methionine metabolism | - | - | - | + |
| Alanine, aspartate and glutamate metabolism | - | - | - | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
G. Chatzimitakos, T.; Pliatsika, C.; Chousidis, I.; D. Leonardos, I.; Stalikas, C.D. Metabolomic Profiling Unveils the Impact of Non-Doped and Heteroatom-Doped Carbon Nanodots on Zebrafish (Danio rerio) Embryos. Nanomaterials 2021, 11, 483. https://doi.org/10.3390/nano11020483
G. Chatzimitakos T, Pliatsika C, Chousidis I, D. Leonardos I, Stalikas CD. Metabolomic Profiling Unveils the Impact of Non-Doped and Heteroatom-Doped Carbon Nanodots on Zebrafish (Danio rerio) Embryos. Nanomaterials. 2021; 11(2):483. https://doi.org/10.3390/nano11020483
Chicago/Turabian StyleG. Chatzimitakos, Theodoros, Claire Pliatsika, Ieremias Chousidis, Ioannis D. Leonardos, and Constantine D. Stalikas. 2021. "Metabolomic Profiling Unveils the Impact of Non-Doped and Heteroatom-Doped Carbon Nanodots on Zebrafish (Danio rerio) Embryos" Nanomaterials 11, no. 2: 483. https://doi.org/10.3390/nano11020483








