Self-Assembled Polyaniline/Ti3C2Tx Nanocomposites for High-Performance Electrochromic Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
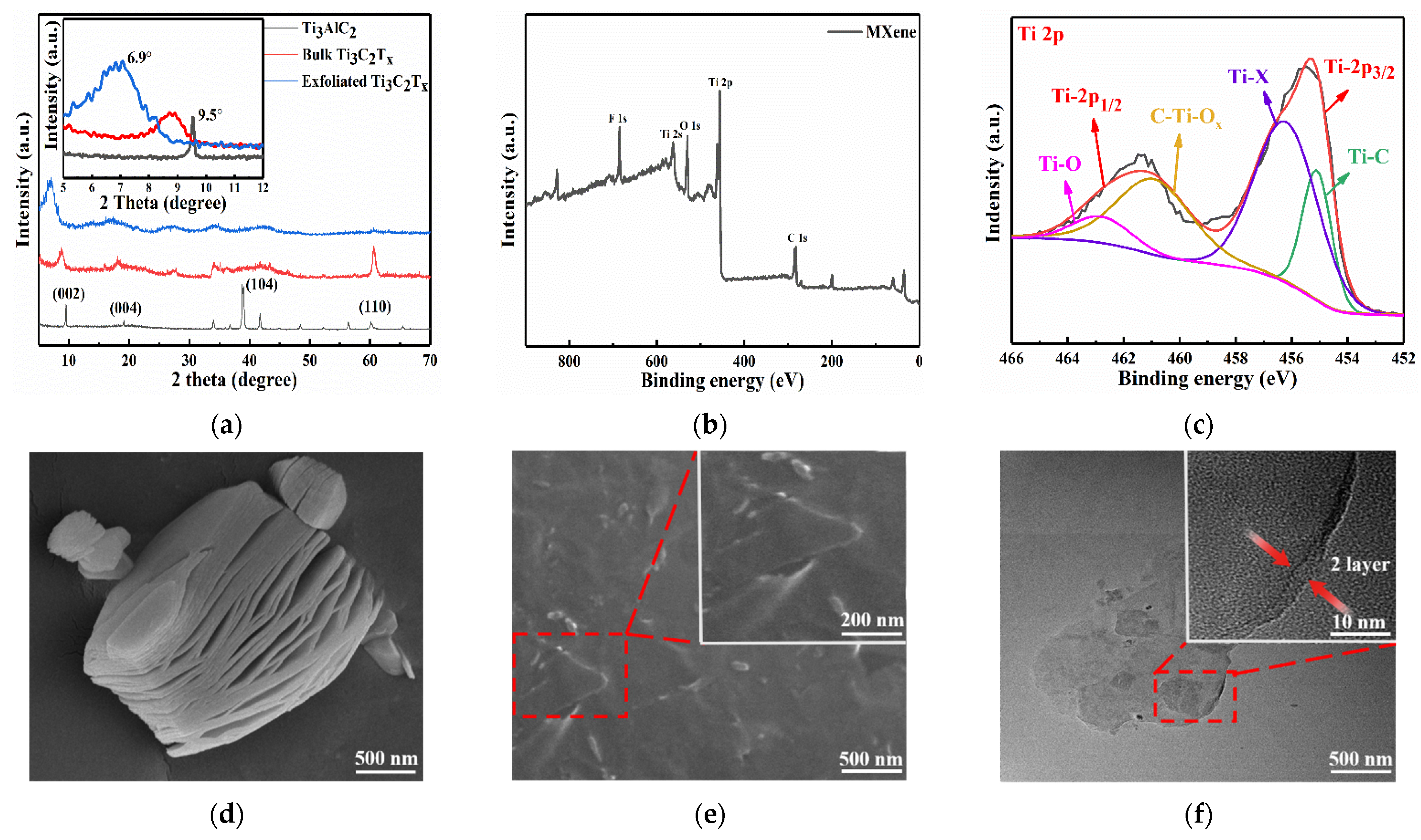
2.2.1. Preparation of Ti3C2Tx MXene
2.2.2. Synthesis of PANI
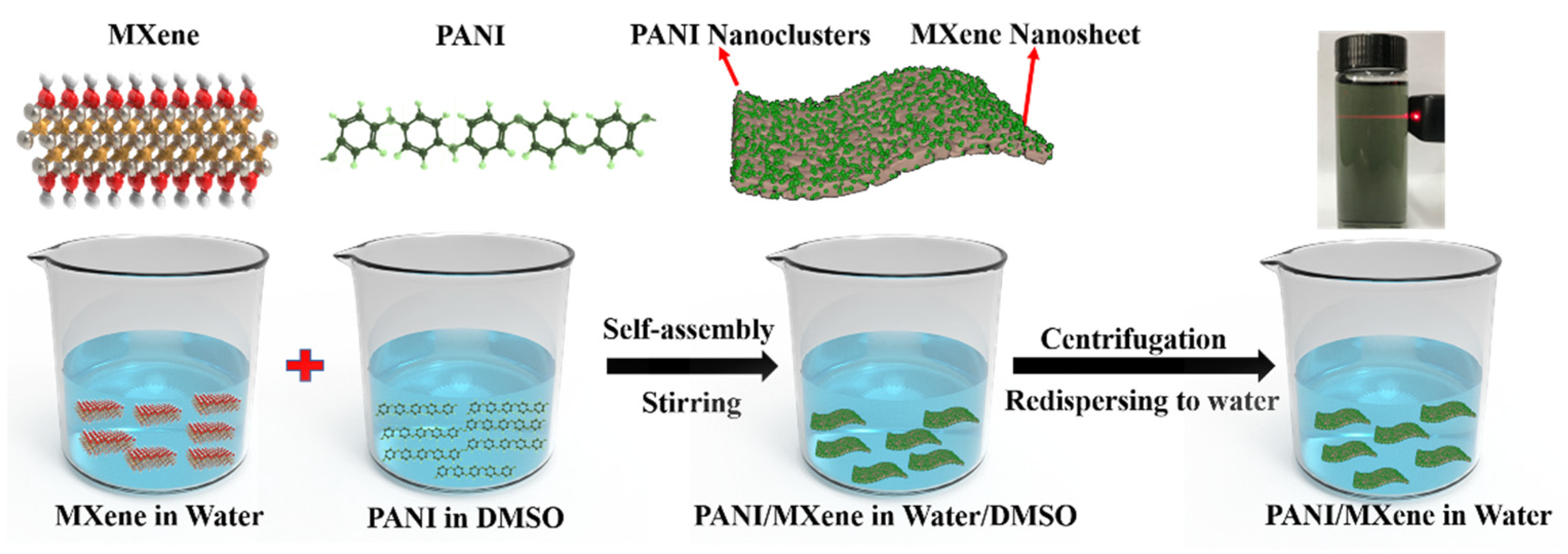
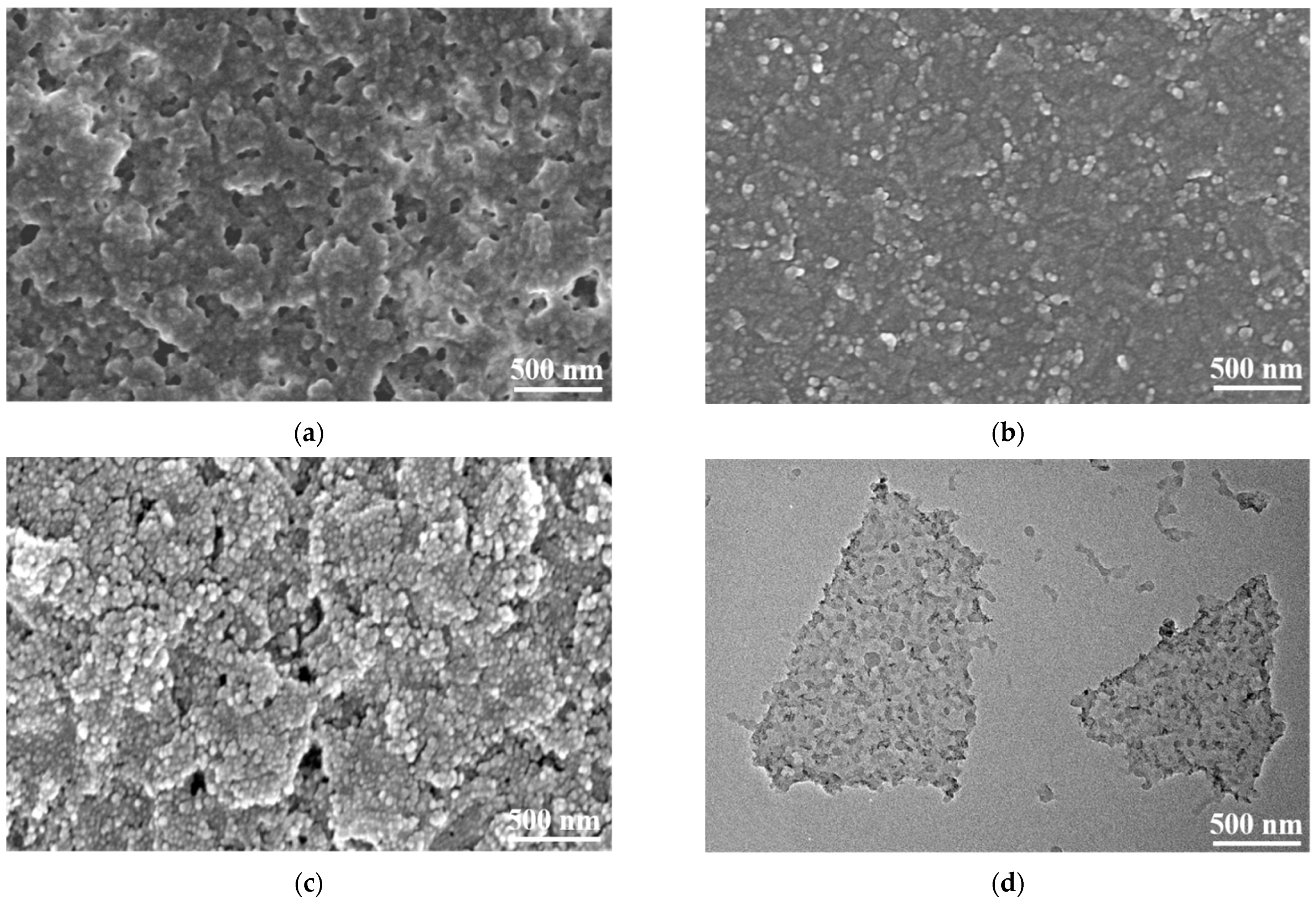
2.2.3. Preparation of PANI/MXene Nanocomposite and Electrochromic Films
2.3. Characterization
2.4. Electrochemical Measurements
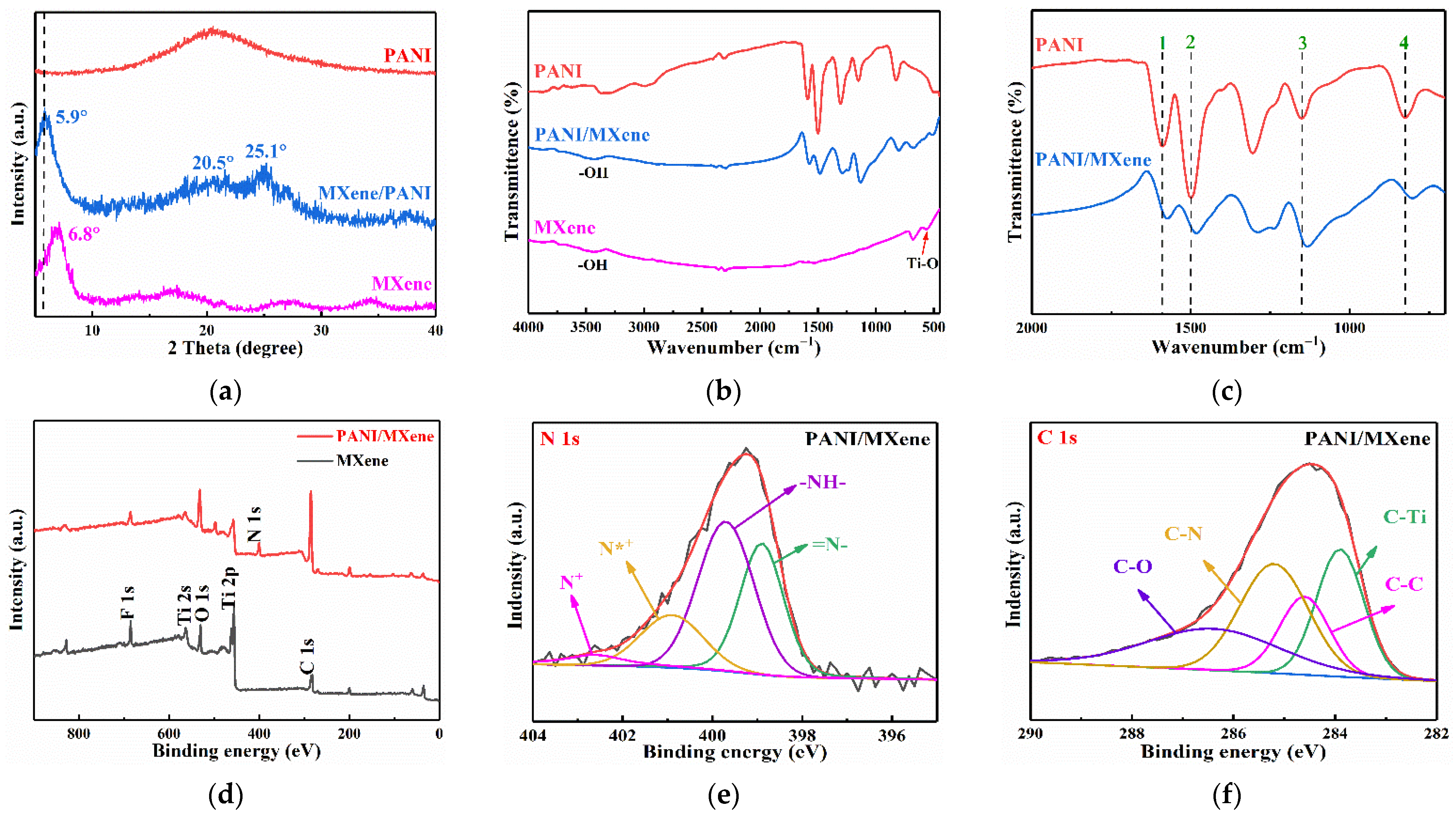
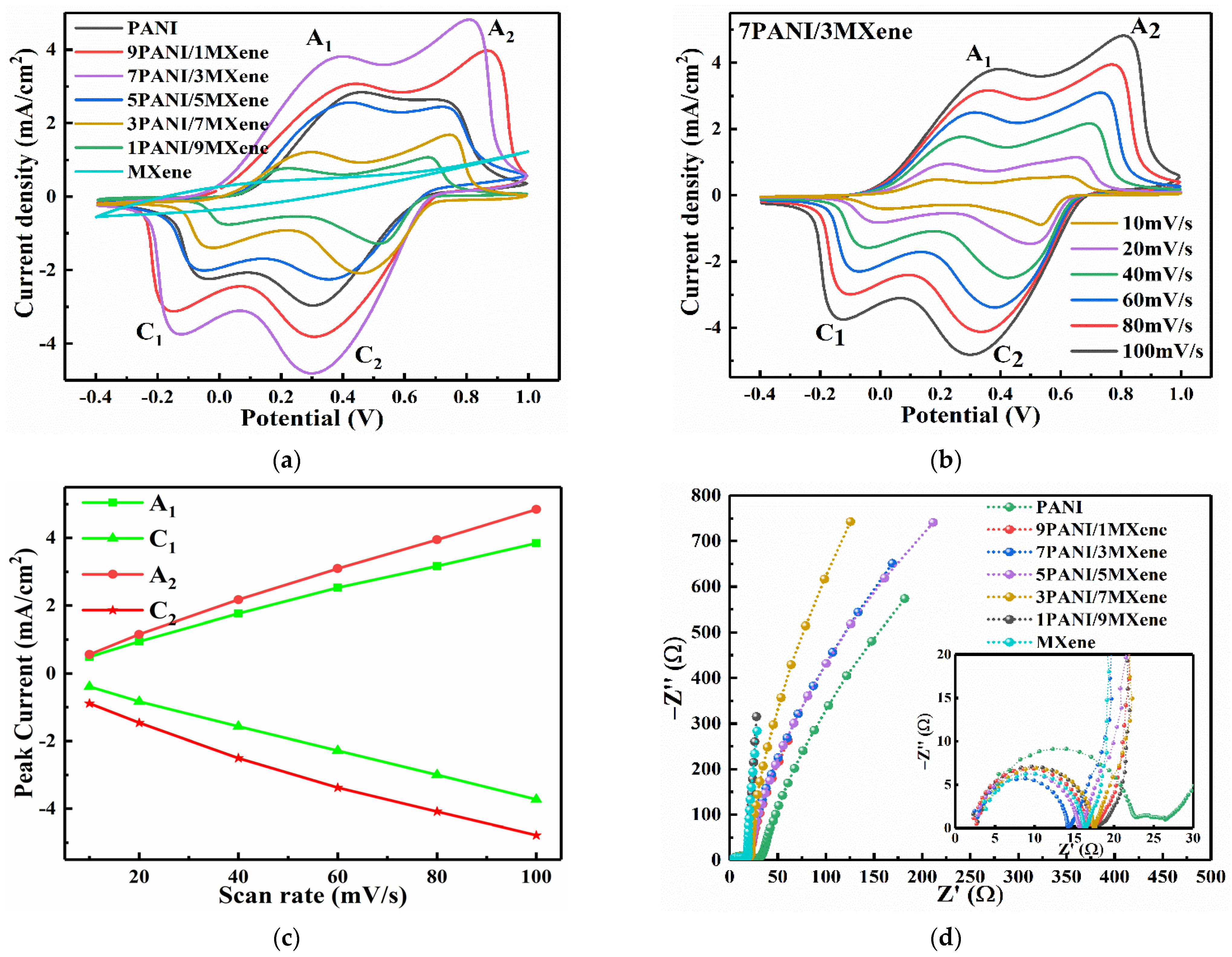
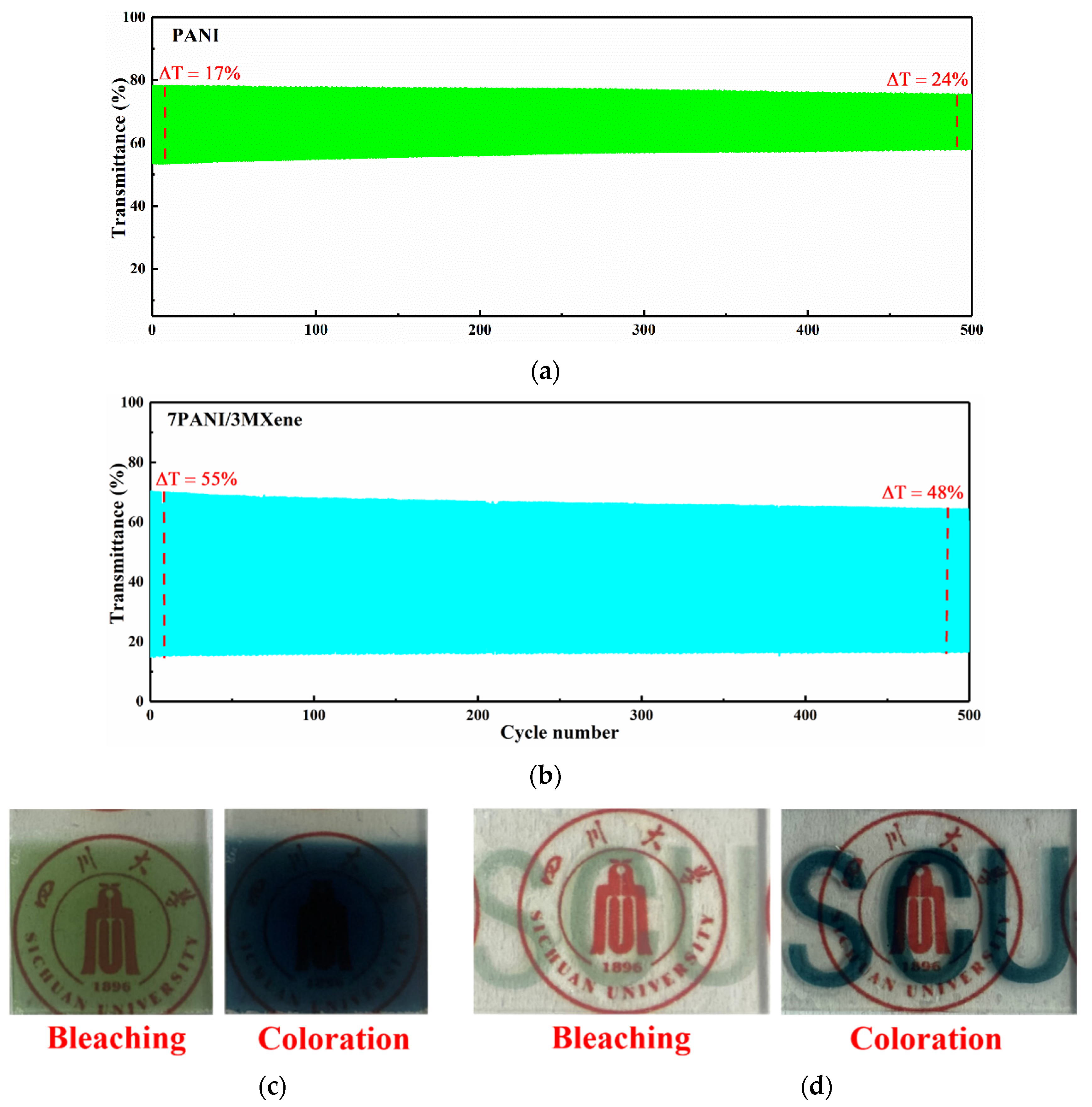
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 4, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Granqvist, C.G.; Arvizu, M.A.; Pehlivan, İ.B.; Qu, H.Y.; Wen, R.T. Electrochromic materials and devices for energy efficiency and human comfort in buildings: A critical revie. Electrochim. Acta 2018, 259, 1170–1182. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Runnerstrom, E.L.; Milliron, D.J. Switchable materials for smart windows. Annu. Rev. Chem. Biomol. 2016, 7, 283–304. [Google Scholar] [CrossRef]
- Pathak, D.K.; Chaudhary, A.; Tanwar, M.; Goutam, U.K.; Kumar, R. Nano-cobalt oxide/viologen hybrid solid state device: Electrochromism beyond chemical cell. Appl. Phys. Lett. 2020, 116, 141901. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhang, P.; Fu, J.J.; Wei, C.Y.; Cai, G.F. A mini-review: Pyridyl-based coordination polymers for energy efficient electrochromic application. Front. Energy Res. 2021, 9, 620203. [Google Scholar] [CrossRef]
- Kumar, R.; Pathak, D.K.; Chaudhary, A. Current status of some electrochromic materials and devices: A brief revie. J. Phys. D 2021, 54, 503002. [Google Scholar] [CrossRef]
- Yu, H.T.; Shao, S.; Yan, L.J.; Meng, H.; He, Y.W.; Yao, C.; Xu, P.P.; Zhang, X.T.; Hu, W.P. Side-chain engineering of green color electrochromic polymer materials: Toward adaptive camouflage application. J. Mater. Chem. C 2016, 4, 2269–2273. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.M.; Hwang, J.; Kim, E. Polyoxometalates as promising materials for electrochromic devices. J. Mater. Chem. C 2019, 7, 7828–7850. [Google Scholar] [CrossRef]
- Rai, V.; Singh, R.S.; Blackwood, D.J.; Zhili, D. A Review on Recent Advances in Electrochromic Devices: A Material Approach. Adv. Eng. Mater. 2020, 22, 2000082. [Google Scholar] [CrossRef]
- Celiesiute, R.; Ramanaviciene, A.; Gicevicius, M.; Ramanavicius, A. Electrochromic sensors based on conducting polymers, metal oxides, and coordination complexes. Nanomaterials 2019, 49, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.D.; Sun, M.J.; Zhang, Y.; Cui, J.W.; Shu, X.; Wang, Y.; Qin, Y.Q.; Liu, J.Q.; Tan, H.H.; Wu, Y.C. Rational design of oxygen deficiency-controlled tungsten oxide electrochromic films with an exceptional memory effect. ACS Appl. Mater. Int. 2020, 12, 32658–32665. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.X.; Lan, J.P.; Yin, S.Y.; Wang, Y.Y.; Kong, Z.Z.; Gong, M.; Wu, B.H.; Chu, J.; Wang, X.Q.; Zhang, R.L.; et al. Enhancing the electrochromic properties of polyaniline via coordinate bond tethering the polyaniline with gold colloids. Sol. Energy Mat. Sol. C 2018, 177, 134–141. [Google Scholar] [CrossRef]
- Mei, Y.T.; Logan, S.; Harish, D. The: In situ synthesis of conductive polyaniline patterns using micro-reactive inkjet printing. J. Mater. Chem. C 2019, 7, 2219–2224. [Google Scholar]
- An, J.B.; Li, Y.L.; Chen, W.; Li, G.Q.; He, J.H.; Feng, H.X. Electrochemically-deposited PANI on iron mesh-based metal-organic framework with enhanced visible-light response towards elimination of thiamphenicol and E. coli. Environ. Res. 2020, 191, 110067. [Google Scholar] [CrossRef] [PubMed]
- Shchegolkov, A.V.; Jang, S.H.; Shchegolkov, A.V.; Rodionov, Y.V.; Sukhova, A.O.; Lipkin, M.S. A brief overview of electrochromic materials and related devices: A nanostructured materials perspective. Nanomaterials 2021, 11, 2376. [Google Scholar] [CrossRef]
- Xu, H.; Cao, Q.; Wang, X.; Li, W.; Li, X.; Deng, H. Properties and chemical oxidation polymerization of polyaniline/neutral red/TiO2 composite electrode. Mater. Sci. Eng. B 2010, 1771, 104–108. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Kim, H.S.; Hyunsik, I.M. Mesoporous Ni-PANI composite electrode for electrochromic energy storage applications. Sol. Energy Mat. Sol. C 2019, 201, 110121. [Google Scholar] [CrossRef]
- Zhang, K.; Li, N.; Wang, Y.; Ma, X.X.; Zhao, J.P.; Qiang, L.S.; Hou, S.; Ji, J.J.; Li, Y. Bifunctional urchin-like WO3@PANI electrodes for superior electrochromic behavior and lithium-ion battery. J. Mater. Sci. Mater. Electron. 2018, 29, 14803–14812. [Google Scholar] [CrossRef]
- Dong, Y.; Shi, H.; Wu, Z.S. Recent advances and promise of MXene-based nanostructures for high-performance metal ion batteries. Adv. Funct. Mater. 2020, 30, 2000706. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.G.; Liu, S.C.; Li, G.Y.; Zhang, G.D.; Cheng, G.H. Nonlinear optical response of reflective MXene molybdenum carbide films as saturable absorbers. Nanomaterials 2020, 10, 2391. [Google Scholar] [CrossRef]
- Du, C.F.; Zhao, X.Y.; Wang, Z.J.; Yu, H.; Ye, Q. Recent advanced on the MXene–organic hybrids: Design, synthesis, and their applications. Nanomaterials 2021, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Wang, L.; Zhang, J.L.; Song, P.; Xiao, Z.R.; Liang, C.B.; Qiu, H.; Kong, J. Fabrication and investigation on the ultra-thin and flexible Ti3C2Tx/Co-doped polyaniline electromagnetic interference shielding composite films. Compos Sci. Technol. 2019, 183, 107833. [Google Scholar] [CrossRef]
- Li, J.M.; Levitt, A.; Kurra, N.; Juan, K.; Noriega, N.; Xiao, X.; Wang, X.H.; Wang, H.Z.; Alshareef, H.N.; Gototsi, Y. MXene-conducting polymer electrochromic microsupercapacitors. Energy Stor. Mater. 2019, 20, 455–461. [Google Scholar] [CrossRef]
- Wu, W.T.; Fang, H.J.; Ma, H.L.; Wu, L.L.; Zhang, W.Q.; Wang, H. Boosting transport kinetics of ions and electrons simultaneously by Ti3C2Tx (MXene) addition for enhanced electrochromic performance. Nanomicro Lett. 2021, 13, 20. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Lipatov, A.; Alhabeb, M.; Lukatskaya, M.R.; Boson, A.; Gogotsi, Y.; Sinitskii, A. Effect of synthesis on quality, electronic properties and environmental stability of individual monolayer Ti3C2 MXene flakes. Adv. Electron. Mater. 2016, 2, 1600255. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.; Joseph, H.; Lar-Åke, N. Chemical bonding in carbide MXene nanosheets. J. Electron. Spectrosc. 2018, 224, 27–32. [Google Scholar]
- Joseph, H.; Kevin, C.; Michael, N.; Gogotsi, Y.; Barsoum, M.W. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXene). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar]
- Zhao, R.Z.; Di, H.X.; Hui, X.B.; Zhao, D.Y.; Wang, R.T.; Wang, C.X.; Yin, L.W. Self-assembled Ti3C2 MXene and N-rich porous carbon hybrids as superior anodes for high-performance potassium-ion batteries. Energy Environ. Sci. 2020, 13, 246–257. [Google Scholar] [CrossRef]
- Peng, H.C.; Yan, B.; Jiang, M.J.; Liu, B.C.; Gu, Y.C.; Yao, G.; Zhang, Y.; Ye, L.L.; Bai, X.; Chen, S. A coral-like polyaniline/barium titanate nanocomposite electrode with double electric polarization for electrochromic energy storage applications. J. Mater. Chem. A 2021, 9, 1669–1677. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.H.; Duan, Z.H.; Zhao, Q.N.; Zhang, Y.J.; Xie, G.Z.; Jiang, Y.D.; Li, S.R.; Tai, H.L. PANI nanofibers-supported Nb2CTx nanosheets-enabled selective NH3 detection driven by TENG at room temperature. Sens. Actuators B Chem. 2021, 321, 128923. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide “clay” with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, D.K.; Navale, S.T.; Nalage, S.R.; Mane, R.S.; Stadler, F.J.; Aswal, D.K.; Gupta, S.K.; Patil, V.B. Simple and low-temperature polyaniline-based flexible ammonia sensor: A step towards laboratory synthesis to economical device design. J. Mater. Chem. C 2015, 3, 9461–9468. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Duan, Y.P.; Liu, J.; Ma, G.J.; Huang, M.L. Wormlike acid-doped polyaniline: Controllable electrical properties and theoretical investigation. J. Phys. Chem. C 2018, 122, 2032–2040. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, H.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Huang, Y.; Huang, Y.; Deng, Q.; Zhou, J.; et al. Photoluminescent Ti3C2 MXene quantum dots for multicolor cellular imaging. Adv. Mater. 2017, 29, 1604847. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Yan, H.; Li, Y.T.; Li, W.; Li, H.; Fan, X.Q.; Zhu, M.H. Ti3C2Tx/PANI composites with tunable conductivity towards anticorrosion application. Chem. Eng. J. 2010, 410, 128310. [Google Scholar] [CrossRef]
- George, M.; Pandey, A.K.; Rahim, N.A.; Tyagi, V.V.; Shahabuddin, S.; Saidur, R. A novel polyaniline (PANI)/paraffin wax nano composite phase change material: Superior transition heat storage capacity, thermal conductivity and thermal reliability. Sol. Energy 2020, 204, 448–458. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Heum Park, S.; Heeger, A.J.; Lee, C.W.; Lee, S.H. Metallic transport in polyaniline. Nature 2016, 441, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.M.; Li, R.M.; Ma, Y.W.; Chen, R.F.; Shi, N.E.; Fan, Q.L.; Huang, W. One-step electrochemical synthesis of graphene/polyaniline composite film and its applications. Adv. Funct. Mater. 2011, 21, 2989–2996. [Google Scholar] [CrossRef]
- Wu, G.; Li, L.; Li, J.H.; Xu, B.Q. Methanol electrooxidation on Pt particles dispersed into PANI/SWNT composite films. J. Power Sources 2006, 155, 118–127. [Google Scholar] [CrossRef]
- Golczak, S.; Kanciurzewska, A.; Fahlman, M.; Langer, K.; Langer, J. Comparative XPS surface study of polyaniline thin films. Solid State Ionics 2008, 179, 2234–2239. [Google Scholar] [CrossRef]
- Wu, W.L.; Wang, C.W.; Zhao, C.H.; Wei, D.; Zhu, J.F.; Xu, Y.L. Facile strategy of hollow polyaniline nanotubes supported on Ti3C2-MXene nanosheets for high-performance symmetric supercapacitors. J. Colloid Interface Sci. 2020, 580, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Chen, S.; Zhao, Y.H.; Kang, J.; Chen, J.Y.; Yan, B.; Gu, Y.C.; Yang, F.; Cao, Y. All-solid-state electrochromic device based on nanocellulose/PANI/PEDOT ternary hybrid system for high optical contrast and excellent cycling stability. J. Electrochem. Soc. 2019, 166, 77–86. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited gold nanostructures for the enhancement of electrochromic properties of PANI–PEDOT film deposited on transparent electrode. Polymer 2020, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; Liu, Y.Y.; Do, J.S. Room temperature amperometric ammonia sensor based on Pt and Pt-Ir porous ceramic electrodes. IEEE Sens J. 2016, 16, 1872–1879. [Google Scholar] [CrossRef]
- Chen, W.K.; Hu, C.W.; Hsu, C.Y.; Ho, K.C. A study on the electrochromic properties of polyaniline/silica composite films with an enhanced optical contrast. Electrochim. Acta. 2009, 54, 4408–4415. [Google Scholar] [CrossRef]
- Ratautaite, V.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. An application of conducting polymer polypyrrole for the design of electrochromic pH and CO2 sensors. J. Electrochem. Soc. 2019, 166, 297–303. [Google Scholar] [CrossRef]
- Pathak, D.K.; Chaudhary, A.; Tanwar, M.; Goutam, U.K.; Mondal, P.; Kumar, R. Nickel cobalt oxide nanoneedles for electrochromic glucose sensors. ACS Appl. Nano Mater. 2021, 4, 2143–2152. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Finkelsteinas, A.; Ramanaviciene, A. Electrochemical impedance spectroscopy of polypyrrole based electrochemical immunosensor. Bioelectrochemistry 2010, 79, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Bi, Z.; Chen, Y.; He, X.; Guo, X.; Gao, X.; Li, X. Electrodeposited Mo-doped WO3 film with large optical modulation and high areal capacitance toward electrochromic energy-storage applications. Appl. Surf. Sci. 2018, 459, 774–781. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Gu, C.; Ma, Y. Electropolymerized conjugated microporous Poly(zinc-porphyrin) films as potential electrode materials in supercapacitors. Adv. Energy Mater. 2015, 5, 1402175–1402180. [Google Scholar] [CrossRef]
- Ma, C.; Fan, Q.C.; Dirican, M.; Subjalearndeee, N.; Cheng, H.; Li, J.J.; Song, Y.; Shi, J.L.; Zhang, X.W. Rational design of meso-/micro-pores for enhancing ion transportation in highly-porous carbon nanofibers used as electrode for supercapacitors. Appl. Surf. Sci. 2021, 545, 148933. [Google Scholar] [CrossRef]
- Ghenaatian, H.R.; Mousavi, M.F.; Rahmanifar, M.S. High performance hybrid supercapacitor based on two nanostructured conducting polymers: Self-doped polyaniline and polypyrrole nanofibers. Electrochim. Acta. 2012, 78, 212–222. [Google Scholar] [CrossRef]
- Wang, B.; He, X.; Li, H.; Liu, Q.; Wang, J.; Yu, L.; Yan, H.; Li, Z.; Wang, P. Optimizing the charge transfer process by designing Co3O4@PPy@MnO2 ternary core-shell composite. J. Mater. Chem. A 2014, 2, 12968–12973. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Do, H.H.; Trung, T.Q.; Le, Q.V.; Nguyen, T.P.; Hong, S.H.; Jang, H.W.; Ahn, S.H.; Kim, S.Y. Stable and multicolored electrochromic device based on polyaniline-tungsten oxide hybrid thin film. J. Alloys Compound. 2021, 882, 160818. [Google Scholar] [CrossRef]
- Xia, M.T.; Chen, B.J.; Gu, F.; Zu, L.H.; Xu, M.Z.; Feng, Y.T.; Wang, Z.J.; Zhang, H.J.; Zhang, C.; Yang, J.H. Ti3C2Tx MXene nanosheets as a robust and conductive tight on Si anodes significantly enhance electrochemical lithium storage performance. ACS Nano 2020, 14, 5111–5120. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Liu, W.; Yan, B.; Li, J.; Lin, Y.; Zhao, Y.; Shi, Z.; Chen, S. Self-Assembled Polyaniline/Ti3C2Tx Nanocomposites for High-Performance Electrochromic Films. Nanomaterials 2021, 11, 2956. https://doi.org/10.3390/nano11112956
Lin T, Liu W, Yan B, Li J, Lin Y, Zhao Y, Shi Z, Chen S. Self-Assembled Polyaniline/Ti3C2Tx Nanocomposites for High-Performance Electrochromic Films. Nanomaterials. 2021; 11(11):2956. https://doi.org/10.3390/nano11112956
Chicago/Turabian StyleLin, Tao, Wenlong Liu, Bin Yan, Jing Li, Yi Lin, Yinghui Zhao, Zheng Shi, and Sheng Chen. 2021. "Self-Assembled Polyaniline/Ti3C2Tx Nanocomposites for High-Performance Electrochromic Films" Nanomaterials 11, no. 11: 2956. https://doi.org/10.3390/nano11112956





