Influence of Metal Oxide Particles on Bandgap of 1D Photocatalysts Based on SrTiO3/PAN Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrospinning of SrTiO3/PAN-Based Fibers with the Addition of Metal Oxide Particles
2.2. X-ray Diffraction Analysis of Samples
2.3. Scanning Electron Microscope Characterization of the Surface Morphology of Samples
2.4. Measurement of the Transmission and Reflection of SrTiO3/PAN Fibers with the Addition of Metal Oxide Particles in a Wide Spectral Region from Ultraviolet (185 nm) to Near-Infrared Radiation (3600 nm)
2.5. Investigation of the Activity of Photocatalysts Based on SrTiO3/PAN Fibers with the Addition of Metal Oxide Particles
3. Results and Discussion
3.1. The Synthesis of Fibers Based on SrTiO3/PAN with the Addition of Metal Oxide Particles and a Study of Their Physicochemical Properties
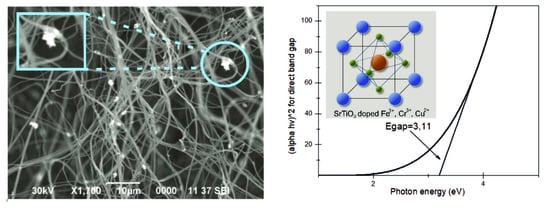
3.2. Investigation of the Transmission and Reflection Spectra of the Obtained Photocatalytic Fibers
3.3. Investigation of the Activity of Photocatalysts Based on SrTiO3/PAN Fibers with the Addition of Metal Oxide Particles by the Output of Hydrogen during the Splitting of Water–Methanol Mixture
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shtarev, D.S.; Shtareva, A.V.; Kevorkyants, R.; Rudakova, A.V.; Molokeev, M.S.; Bakiev, T.V.; Bulanin, K.M.; Ryabchuk, V.K.; Serpone, N. Materials synthesis, characterization and DFT calculations of the visible-light-active perovskite-like barium bismuthate Ba1.264(4) Bi1.971(4)O4 photocatalyst. J. Mater. Chem. C 2020, 8, 3509–3519. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, A.; Chen, X.; Liu, L.; Ren, L.; Tong, L.; Ye, J. Highly efficient Cu induced photocatalysis for visible-light hydrogen evolution. Catal. Today 2019, 335, 166–172. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Sultanov, F.R.; Daulbayev, C.; Bakbolat, B.; Mansurov, Z.A.; Urazgaliyeva, A.A.; Ebrahim, R.; Pei, S.S.; Huang, K.-P. Microwave-enhanced chemical vapor deposition graphene nanoplatelets-derived 3D porous materials for oil/water separation. Carbon Lett. 2020, 30, 81–92. [Google Scholar] [CrossRef]
- Phoon, B.L.; Lai, C.W.; Juan, J.C.; Show, P.-L.; Pan, G.-T. Recent developments of strontium titanate for photocatalytic water splitting application. Int. J. Hydrogen Energy 2019, 44, 14316–14340. [Google Scholar] [CrossRef]
- Collignon, C.; Lin, X.; Rischau, C.W.; Fauqué, B.; Behnia, K. Metallicity and superconductivity in doped strontium titanate. Annu. Rev. Condens. Matter Phys. 2019, 10, 25–44. [Google Scholar] [CrossRef]
- Xu, B.; Ahmed, M.B.; Zhou, J.L.; Altaee, A. Visible and UV photocatalysis of aqueous perfluorooctanoic acid by TiO2 and peroxymonosulfate: Process kinetics and mechanistic insights. Chemosphere 2020, 243, 125366. [Google Scholar] [CrossRef]
- Sinhmar, A.; Setia, H.; Kumar, V.; Sobti, A.; Toor, A.P. Enhanced photocatalytic activity of nickel and nitrogen codoped TiO2 under sunlight. Environ. Technol. Innovation 2020, 18, 100658. [Google Scholar] [CrossRef]
- Gonçalves, N.P.F.; Paganini, M.C.; Armillotta, P.; Cerrato, E.; Calza, P. The effect of cobalt doping on the efficiency of semiconductor oxides in the photocatalytic water remediation. J. Environ. Chem. Eng. 2019, 7, 103475. [Google Scholar] [CrossRef]
- Wang, R.; Ni, S.; Liu, G.; Xu, X. Hollow CaTiO3 cubes modified by La/Cr co-doping for efficient photocatalytic hydrogen production. Appl. Catal. B 2018, 225, 139–147. [Google Scholar] [CrossRef]
- Jiang, L.; Ni, S.; Liu, G.; Xu, X. Photocatalytic hydrogen production over Aurivillius compound Bi3TiNbO9 and its modifications by Cr/Nb co-doping. Appl. Catal. B 2017, 217, 342–352. [Google Scholar] [CrossRef]
- Chen, P.-W.; Li, K.; Yu, Y.-X.; Zhang, W.-D. Cobalt-doped graphitic carbon nitride photocatalysts with high activity for hydrogen evolution. Appl. Surf. Sci. 2017, 392, 608–615. [Google Scholar] [CrossRef]
- Regmi, C.; Kshetri, Y.K.; Pandey, R.P.; Kim, T.-H.; Gyawali, G.; Lee, S.W. Understanding the multifunctionality in Cu-doped BiVO4 semiconductor photocatalyst. J. Environ. Sci. 2019, 75, 84–97. [Google Scholar] [CrossRef]
- Habba, Y.; Capochichi-Gnambodoe, M.; Leprince-Wang, Y. Enhanced Photocatalytic Activity of Iron-Doped ZnO Nanowires for Water Purification. Appl. Sci. 2017, 7, 1185. [Google Scholar] [CrossRef] [Green Version]
- Sultanov, F.R.; Daulbayev, C.; Bakbolat, B.; Zhurintayeva, A.; Daulbayev, O.; Mansurov, Z.A. Comparison of oil/water separating efficiency of oleophobic membranes based on fluorine containing and fluorine non-containing coatings. RJC 2019, 12, 1091–1097. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, X.; Song, X.; Chen, X.; Dai, W.; Yuan, R.; Fu, X. Visible-light-driven deep oxidation of NO over Fe doped TiO2 catalyst: Synergic effect of Fe and oxygen vacancies. Appl. Catal. B 2020, 277, 119196. [Google Scholar] [CrossRef]
- Ismael, M. Enhanced photocatalytic hydrogen production and degradation of organic pollutants from Fe(III) doped TiO2 nanoparticles. J. Environ. Chem. Eng. 2020, 8, 103676. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Li, X. Synthesis and visible light photocatalysis water splitting property of chromium-doped Bi4Ti3O12. Solid State Ionics 2009, 180, 1599–1603. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X.; Zhu, C.; Shi, C. Chromium-modified Bi4Ti3O12 photocatalyst: Application for hydrogen evolution and pollutant degradation. Appl. Catal. B 2016, 199, 241–251. [Google Scholar] [CrossRef]
- Zhu, H.; Fang, M.; Huang, Z.; Liu, Y.; Chen, K.; Guan, M.; Tang, C.; Zhang, L.; Wang, M. Novel chromium doped perovskites A2ZnTiO6 (A = Pr, Gd): Synthesis, crystal structure and photocatalytic activity under simulated solar light irradiation. Appl. Surf. Sci. 2017, 393, 348–356. [Google Scholar] [CrossRef]
- Liu, J.; Hodes, G.; Yan, J.; Liu, S.F. Metal-doped Mo2C (metal = Fe, Co, Ni, Cu) as catalysts on TiO2 for photocatalytic hydrogen evolution in neutral solution. Chin. J. Catal. 2021, 42, 205–216. [Google Scholar] [CrossRef]
- Nikhila, M.P.; John, D.; Pai, M.R.; Renuka, N.K. Cu and Ag modified mesoporous TiO2 nanocuboids for visible light driven photocatalysis. Nanostruct. Nano-Objects. 2020, 21, 100420. [Google Scholar] [CrossRef]
- Onwubiko, I.; Khan, W.S.; Subeshan, B.; Asmatulu, R. Investigating the effects of carbon-based counter electrode layers on the efficiency of hole-transporter-free perovskite solar cells. Energ. Ecol. Environ. 2020, 5, 141–152. [Google Scholar] [CrossRef]
- Alharbi, A.R.; Alarifi, I.M.; Khan, W.S.; Swindle, A.; Asmatulu, R. Synthesis and characterization of electrospun polyacrylonitrile/graphene nanofibers embedded with SrTiO3/NiO nanoparticles for water splitting. J. Nanosci. Nanotechnol. 2017, 17, 5294–5302. [Google Scholar] [CrossRef]
- Asmatulu, R.; Shinde, M.A.; Alharbi, A.R.; Alarifi, I.M. Integrating graphene and C60 into TiO2 nanofibers via electrospinning process for enhanced conversion efficiencies of DSSCs. Macromol. Symp. 2016, 365, 128–139. [Google Scholar] [CrossRef]
- Sultanov, F.; Daulbayev, C.; Bakbolat, B.; Daulbayev, O.; Bigaj, M.; Mansurov, Z.; Kuterbekov, K.; Bekmyrza, K. Aligned composite SrTiO3/PAN fibers as 1D photocatalyst obtained by electrospinning method. Chem. Phys. Lett. 2019, 737, 136821. [Google Scholar] [CrossRef]
- Roy, P.K.; Bera, J. Formation of SrTiO3 from Sr-oxalate and TiO2. Mater. Res. Bull. 2005, 40, 599–604. [Google Scholar] [CrossRef]
- Havlíček, K.; Svobodová, L.; Bakalova, T.; Lederer, T. Influence of electrospinning methods on characteristics of polyvinyl butyral and polyurethane nanofibres essential for biological applications. Mater. Des. 2020, 194, 108898. [Google Scholar] [CrossRef]
- Huang, W.; Tong, Z.; Wang, R.; Liao, Z.; Bi, Y.; Chen, Y.; Ma, M.; Lyu, P.; Ma, Y. A review on electrospinning nanofibers in the field of microwave absorption. Ceram. Int. 2020, in press. [Google Scholar] [CrossRef]
- Guo, L.; Xie, N.; Wang, C.; Kou, X.; Ding, M.; Zhang, H.; Sun, Y.; Song, H.; Wang, Y.; Lu, G. Enhanced hydrogen sulfide sensing properties of Pt-functionalized α-Fe2O3 nanowires prepared by one-step electrospinning. Sens. Actuators B 2018, 255, 1015–1023. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Q.; Wei, M.; Wang, C. Synthesis of one-dimensional α-Fe2O3/Bi2MoO6 heterostructures by electrospinning process with enhanced photocatalytic activity. J. Alloys Compd. 2015, 646, 417–424. [Google Scholar] [CrossRef]
- Koo, B.-R.; Park, I.-K.; Ahn, H.-J. Fe-doped In2O3/α-Fe2O3 core/shell nanofibers fabricated by using a co-electrospinning method and its magnetic properties. J. Alloys Compd. 2014, 603, 52–56. [Google Scholar] [CrossRef]
- Wu, L.; Shi, S.; Li, Q.; Zhang, X.; Cui, X. TiO2 nanoparticles modified with 2D MoSe2 for enhanced photocatalytic activity on hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 720–728. [Google Scholar] [CrossRef]
- Yu, H.; Yan, S.; Li, Z.; Yu, T.; Zou, Z. Efficient visible-light-driven photocatalytic H2 production over Cr/N-codoped SrTiO3. Int. J. Hydrogen Energy 2012, 37, 12120–12127. [Google Scholar] [CrossRef]
- Li, F.; Gu, Q.; Niu, Y.; Wang, R.; Tong, Y.; Zhu, S.; Zhang, H.; Zhang, Z.; Wang, X. Hydrogen evolution from aqueous-phase photocatalytic reforming of ethylene glycol over Pt/TiO2 catalysts: Role of Pt and product distribution. Appl. Surf. Sci. 2017, 391, 251–258. [Google Scholar] [CrossRef]
- López-Tenllado, F.J.; Hidalgo-Carrillo, J.; Montes, V.; Marinas, A.; Urbano, F.J.; Marinas, J.M.; Ilieva, L.; Tabakova, T.; Reid, F. A comparative study of hydrogen photocatalytic production from glycerol and propan-2-ol on M/TiO2 systems (M = Au, Pt, Pd). Catal. Today 2017, 280, 58–64. [Google Scholar] [CrossRef]



| Type of Photocatalyst | Parameters of the Process | Composition of the Water Mixture | The Output of Hydrogen, µmol h−1 g−1 | Reference |
|---|---|---|---|---|
| SrTiO3/PAN-based fibers | 40 W UV lamp, quartz reactor | 80% of water and 20% of CH3OH | 305.96 | [26] |
| SrTiO3/PAN/Fe2O3 fibers | 40 W UV lamp, quartz reactor | 80% of water and 20% of CH3OH | 344.67 | This work |
| SrTiO3/PAN/Cr2O3 fibers | 40 W UV lamp, quartz reactor | 80% of water and 20% of CH3OH | 398.93 | This work |
| SrTiO3/PAN/CuO fibers | 40 W UV lamp, quartz reactor | 80% of water and 20% of CH3OH | 420.82 | This work |
| MoSe2/TiO2 | Xe arc lamp | 90% of water and 10% of CH3OH | 4.9 | [33] |
| SrTiO3 doped with Cr and N | 300 W xenon lamp, quartz reactor | 81.5% of water and 18.5% of CH3OH | 106.7 | [34] |
| Pt/TiO2 | 125 W xenon lamp, quartz reactor | 70% of water and 30% of CH3OH | 523.71 | [35] |
| Pt/ZrO2/TaOn | 300 W mercury lamp, quartz reactor | 85% of water and 15% of CH3OH | 9 | [36] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultanov, F.; Daulbayev, C.; Azat, S.; Kuterbekov, K.; Bekmyrza, K.; Bakbolat, B.; Bigaj, M.; Mansurov, Z. Influence of Metal Oxide Particles on Bandgap of 1D Photocatalysts Based on SrTiO3/PAN Fibers. Nanomaterials 2020, 10, 1734. https://doi.org/10.3390/nano10091734
Sultanov F, Daulbayev C, Azat S, Kuterbekov K, Bekmyrza K, Bakbolat B, Bigaj M, Mansurov Z. Influence of Metal Oxide Particles on Bandgap of 1D Photocatalysts Based on SrTiO3/PAN Fibers. Nanomaterials. 2020; 10(9):1734. https://doi.org/10.3390/nano10091734
Chicago/Turabian StyleSultanov, Fail, Chingis Daulbayev, Seitkhan Azat, Kairat Kuterbekov, Kenzhebatyr Bekmyrza, Baglan Bakbolat, Magdalena Bigaj, and Zulkhair Mansurov. 2020. "Influence of Metal Oxide Particles on Bandgap of 1D Photocatalysts Based on SrTiO3/PAN Fibers" Nanomaterials 10, no. 9: 1734. https://doi.org/10.3390/nano10091734