Room-Temperature Reduction of Graphene Oxide in Water by Metal Chloride Hydrates: A Cleaner Approach for the Preparation of Graphene@Metal Hybrids
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Graphene Oxide
2.2. Preparation of Aqueous Suspension of GO
2.3. Preparation of 2 M Aqueous Solution of Metal Chloride
2.4. Synthesis of GO@MO Hybrids
2.5. Characterization Methods
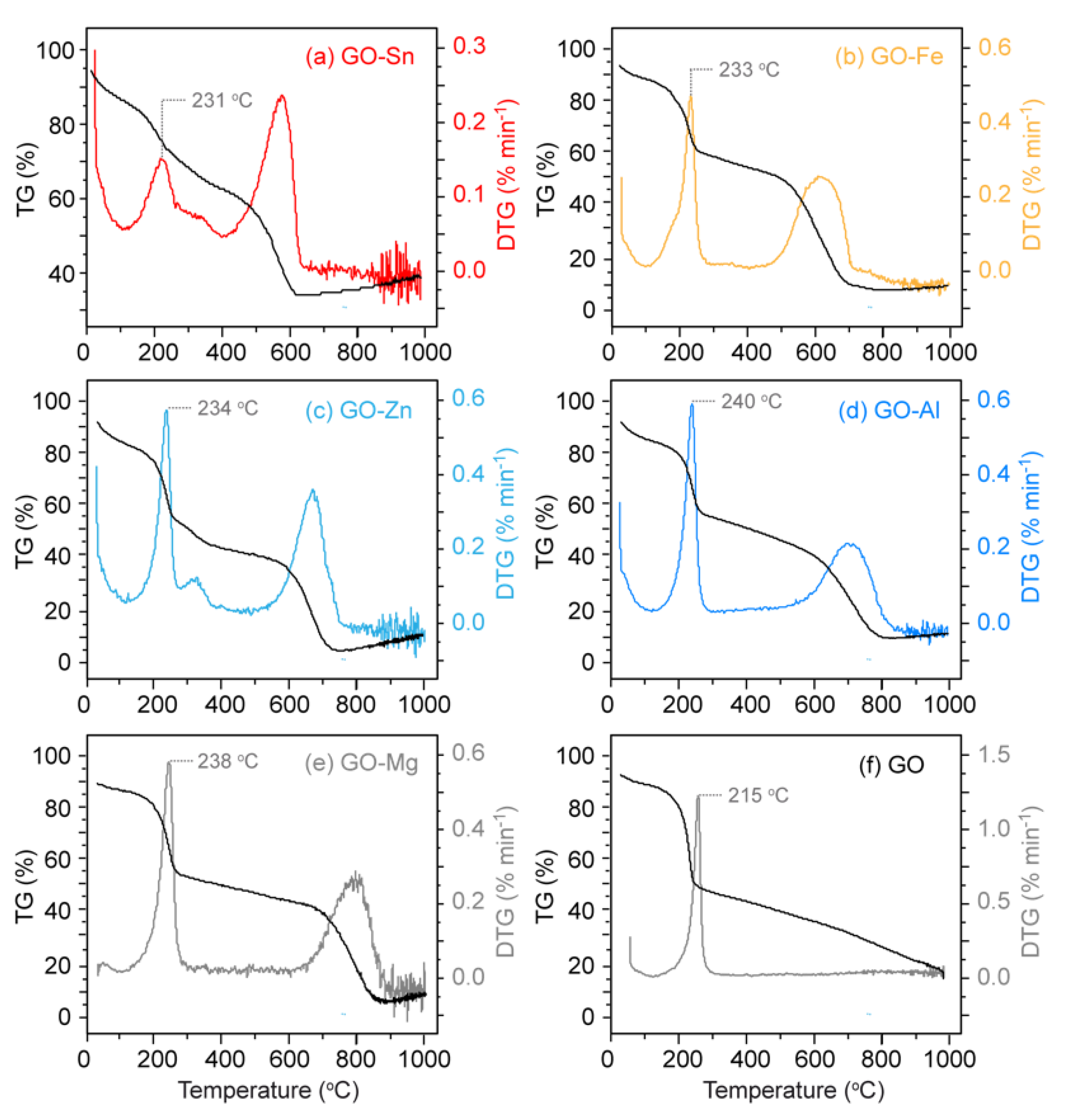
3. Results and Discussion

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Lu, T.; Chen, F.; Zhang, R. A brief review of graphene–metal oxide composites synthesis and applications in photocatalysis. J. Chin. Adv. Mater. Soc. 2013, 1, 21–39. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J. Graphene-Metal Particle Nanocomposites. J. Phys. Chem. C 2008, 112, 19841–19845. [Google Scholar] [CrossRef]
- Ogata, C.; Koinuma, M.; Hatakeyama, K.; Tateishi, H.; Asrori, M.Z.; Taniguchi, T.; Funatsu, A.; Matsumoto, Y. Metal permeation into multi-layered graphene oxide. Sci. Rep. 2014, 4, 3647. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Ma, Y.; Huang, Y.; Wang, Y.; Chen, Y. Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J. Mater. Chem. 2009, 19, 2710–2714. [Google Scholar] [CrossRef]
- Ma, X.; Tao, H.; Yang, K.; Feng, L.; Cheng, L.; Shi, X.; Li, Y.; Guo, L.; Liu, Z. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
- Cong, H.P.; He, J.J.; Lu, Y.; Yu, S.H. Water-soluble magnetic-functionalized reduced graphene oxide sheets: In situ synthesis and magnetic resonance imaging applications. Small 2010, 6, 169–173. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, T.; Zhou, X.; Zhang, Y.; Lou, X.W.; Chen, X.; Zhang, H.; Hng, H.H.; Yan, Q. Facile synthesis of metal oxide/reduced graphene oxide hybrids with high lithium storage capacity and stable cyclability. Nanoscale 2011, 3, 1084–1089. [Google Scholar] [CrossRef]
- Kim, H.-K.; Park, S.-H.; Yoon, S.-B.; Lee, C.-W.; Jeong, J.H.; Roh, K.C.; Kim, K.-B. In Situ Synthesis of Three-Dimensional Self-Assembled Metal Oxide–Reduced Graphene Oxide Architecture. Chem. Mater. 2014, 26, 4838–4843. [Google Scholar] [CrossRef]
- Pendashteh, A.; Mousavi, M.F.; Rahmanifar, M.S. Fabrication of anchored copper oxide nanoparticles on graphene oxide nanosheets via an electrostatic coprecipitation and its application as supercapacitor. Electrochim. Acta 2013, 88, 347–357. [Google Scholar] [CrossRef]
- Xiang, C.; Li, M.; Zhi, M.; Manivannan, A.; Wu, N. Reduced graphene oxide/titanium dioxide composites for supercapacitor electrodes: Shape and coupling effects. J. Mater. Chem. 2012, 22, 19161–19167. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Wu, X.; Han, Q.; Wang, X. Graphene Oxide−MnO2 Nanocomposites for Supercapacitors. ACS Nano 2010, 4, 2822–2830. [Google Scholar] [CrossRef] [PubMed]
- Sawangphruk, M.; Srimuk, P.; Chiochan, P.; Krittayavathananon, A.; Luanwuthi, S.; Limtrakul, J. High-performance supercapacitor of manganese oxide/reduced graphene oxide nanocomposite coated on flexible carbon fiber paper. Carbon 2013, 60, 109–116. [Google Scholar] [CrossRef]
- Stengl, V.; Bakardjieva, S.; Grygar, T.M.; Bludská, J.; Kormunda, M. TiO2-graphene oxide nanocomposite as advanced photocatalytic materials. Chem. Cent. J. 2013, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xiong, Z.; Zhao, X.S. Graphene–metal–oxide composites for the degradation of dyes under visible light irradiation. J. Mater. Chem. 2011, 21, 3634–3640. [Google Scholar] [CrossRef]
- Akhavan, O.; Choobtashani, M.; Ghaderi, E. Protein Degradation and RNA Efflux of Viruses Photocatalyzed by Graphene–Tungsten Oxide Composite Under Visible Light Irradiation. J. Phys. Chem. C 2012, 116, 9653–9659. [Google Scholar] [CrossRef]
- Chen, C.; Cai, W.; Long, M.; Zhou, B.; Wu, Y.; Wu, D.; Feng, Y. Synthesis of Visible-Light Responsive Graphene Oxide/TiO2 Composites with p/n Heterojunction. ACS Nano 2010, 4, 6425–6432. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, T. Fabrication and characterization of graphene oxide/zinc oxide nanorods hybrid. Appl. Surf. Sci. 2011, 257, 8950–8954. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Sun, J.; He, P.; Xu, X.; Ding, G. Tungsten oxide nanowire-reduced graphene oxide aerogel for high-efficiency visible light photocatalysis. Carbon 2014, 78, 38–48. [Google Scholar] [CrossRef]
- Xu, S.; Yong, L.; Wu, P. One-Pot, Green, Rapid Synthesis of Flowerlike Gold Nanoparticles/Reduced Graphene Oxide Composite with Regenerated Silk Fibroin As Efficient Oxygen Reduction Electrocatalysts. ACS Appl. Mater. Interfaces 2013, 5, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, G.; Chen, J.; Chen, X.; Xie, Z.; Wang, X. Synthesis of “Clean” and Well-Dispersive Pd Nanoparticles with Excellent Electrocatalytic Property on Graphene Oxide. J. Am. Chem. Soc. 2011, 133, 3693–3695. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ganguly, A.; Papakonstantinou, P.; Miao, X.; Li, M.; Hutchison, J.L.; Delichatsios, M.; Ukleja, S. Rapid Microwave Synthesis of CO Tolerant Reduced Graphene Oxide-Supported Platinum Electrocatalysts for Oxidation of Methanol. J. Phys. Chem. C 2010, 114, 19459–19466. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Bae, H.S.; Seo, E.; Jang, S.; Park, K.H.; Kim, B.-S. Hybrid gold nanoparticle-reduced graphene oxide nanosheets as active catalysts for highly efficient reduction of nitroarenes. J. Mater. Chem. 2011, 21, 15431–15436. [Google Scholar] [CrossRef]
- Nie, R.; Wang, J.; Wang, L.; Qin, Y.; Chen, P.; Hou, Z. Platinum supported on reduced graphene oxide as a catalyst for hydrogenation of nitroarenes. Carbon 2012, 50, 586–596. [Google Scholar] [CrossRef]
- Thien, G.S.H.; Omar, F.S.; Blya, N.I.S.A.; Chiu, W.S.; Lim, H.N.; Yousefi, R.; Sheini, F.-J.; Huang, N.M. Improved Synthesis of Reduced Graphene Oxide-Titanium Dioxide Composite with Highly Exposed 001 Facets and Its Photoelectrochemical Response. Int. J. Photoenergy 2014, 2014, 650583. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Hu, Z.-A.; Chang, Y.-Q.; Wang, H.-W.; Zhang, Z.-Y.; Yang, Y.-Y.; Wu, H.-Y. Zinc Oxide/Reduced Graphene Oxide Composites and Electrochemical Capacitance Enhanced by Homogeneous Incorporation of Reduced Graphene Oxide Sheets in Zinc Oxide Matrix. J. Phys. Chem. C 2011, 115, 2563–2571. [Google Scholar] [CrossRef]
- Kholmanov, I.N.; Domingues, S.H.; Chou, H.; Wang, X.; Tan, C.; Kim, J.-Y.; Li, H.; Piner, R.; Zarbin, A.J.G.; Ruoff, R.S. Reduced Graphene Oxide/Copper Nanowire Hybrid Films as High-Performance Transparent Electrodes. ACS Nano 2013, 7, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Parnianchi, F.; Nazari, M.; Maleki, J.; Mohebi, M. Combination of graphene and graphene oxide with metal and metal oxide nanoparticles in fabrication of electrochemical enzymatic biosensors. Int. Nano Lett. 2018, 8, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Li, R.; Chen, R.; Wang, J.; Xiang, L. 3D Architectured Graphene/Metal Oxide Hybrids for Gas Sensors: A Review. Sensors 2018, 18, 1456. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Ji, L.; Wu, K.; Yang, N. Electrochemistry of ZnO@reduced graphene oxides. Carbon 2018, 130, 480–486. [Google Scholar] [CrossRef]
- Guo, D.; Cai, P.; Sun, J.; He, W.; Wu, X.; Zhang, T.; Wang, X.; Zhang, X. Reduced-graphene-oxide/metal-oxide p-n heterojunction aerogels as efficient 3D sensing frameworks for phenol detection. Carbon 2016, 99, 571–578. [Google Scholar] [CrossRef]
- Mao, S.; Cui, S.; Lu, G.; Yu, K.; Wen, Z.; Chen, J. Tuning gas-sensing properties of reduced graphene oxide using tin oxide nanocrystals. J. Mater. Chem. 2012, 22, 11009–11013. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, X.; Chen, J.; Zheng, X.; Liu, C.; Xue, T.; Wang, H.; Jin, Z.; Qiao, L.; Zheng, W. Well-dispersed palladium nanoparticles on graphene oxide as a non-enzymatic glucose sensor. RSC Adv. 2012, 2, 6245–6249. [Google Scholar] [CrossRef]
- Marlinda, A.R.; Huang, N.M.; Muhamad, M.R.; An’amt, M.N.; Chang, B.Y.S.; Yusoff, N.; Harrison, I.; Lim, H.N.; Chia, C.H.; Kumar, S.V. Highly efficient preparation of ZnO nanorods decorated reduced graphene oxide nanocomposites. Mater. Lett. 2012, 80, 9–12. [Google Scholar] [CrossRef]
- Mura, S.; Jiang, Y.; Vassalini, I.; Gianoncelli, A.; Alessandri, I.; Granozzi, G.; Calvillo, L.; Senes, N.; Enzo, S.; Innocenzi, P.; et al. Graphene Oxide/Iron Oxide Nanocomposites for Water Remediation. ACS Appl. Nano Mater. 2018, 1, 6724–6732. [Google Scholar] [CrossRef]
- Yang, X.; Chen, C.; Li, J.; Zhao, G.; Ren, X.; Wang, X. Graphene oxide-iron oxide and reduced graphene oxide-iron oxide hybrid materials for the removal of organic and inorganic pollutants. RSC Adv. 2012, 2, 8821–8826. [Google Scholar] [CrossRef]
- Thebo, K.H.; Qian, X.; Wei, Q.; Zhang, Q.; Cheng, H.-M.; Ren, W. Reduced graphene oxide/metal oxide nanoparticles composite membranes for highly efficient molecular separation. J. Mater. Sci. Technol. 2018, 34, 1481–1486. [Google Scholar] [CrossRef]
- Chook, S.W.; Chia, C.H.; Zakaria, S.; Ayob, M.K.; Chee, K.L.; Huang, N.M.; Neoh, H.M.; Lim, H.N.; Jamal, R.; Rahman, R. Antibacterial performance of Ag nanoparticles and AgGO nanocomposites prepared via rapid microwave-assisted synthesis method. Nanoscale Res. Lett. 2012, 7, 541. [Google Scholar] [CrossRef] [Green Version]
- Richtera, L.; Chudobova, D.; Cihalova, K.; Kremplova, M.; Milosavljevic, V.; Kopel, P.; Blazkova, I.; Hynek, D.; Adam, V.; Kizek, R. The Composites of Graphene Oxide with Metal or Semimetal Nanoparticles and Their Effect on Pathogenic Microorganisms. Materials 2015, 8, 2994–3011. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Papers Modified by Divalent Ions—Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Scheer, E.; Polarz, S. Synthesis of graphene-transition metal oxide hybrid nanoparticles and their application in various fields. Beilstein J. Nanotechnol. 2017, 8, 688–714. [Google Scholar] [CrossRef] [Green Version]
- Nethravathi, C.; Rajamathi, M.; Ravishankar, N.; Basit, L.; Felser, C. Synthesis of graphene oxide-intercalated α-hydroxides by metathesis and their decomposition to graphene/metal oxide composites. Carbon 2010, 48, 4343–4350. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; He, J. Facile Synthesis of Graphene-Wrapped Honeycomb MnO2 Nanospheres and Their Application in Supercapacitors. ACS Appl. Mater. Interfaces 2012, 4, 1770–1776. [Google Scholar] [CrossRef]
- Sohn, M.; Park, E.; Yoo, B.M.; Han, T.H.; Park, H.B.; Kim, H. Metal-assisted mechanochemical reduction of graphene oxide. Carbon 2016, 110, 79–86. [Google Scholar] [CrossRef]
- Annamalai, K.P.; Zheng, X.; Gao, J.; Chen, T.; Tao, Y. Nanoporous ruthenium and manganese oxide nanoparticles/reduced graphene oxide for high-energy symmetric supercapacitors. Carbon 2019, 144, 185–192. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; El-Khouly, M.E.; El-Kemary, M.; Ramadan, M.S.; Masoud, M.S. Graphene oxide–metal oxide nanocomposites: Fabrication, characterization and removal of cationic rhodamine B dye. RSC Adv. 2018, 8, 13323–13332. [Google Scholar] [CrossRef] [Green Version]
- Ishaq, S.; Moussa, M.; Kanwal, F.; Ehsan, M.; Saleem, M.; Van, T.N.; Losic, D. Facile synthesis of ternary graphene nanocomposites with doped metal oxide and conductive polymers as electrode materials for high performance supercapacitors. Sci. Rep. 2019, 9, 5974. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Kong, B.-S.; Geng, J.; Jung, H.-T. Layer-by-layer assembly of graphene and gold nanoparticles by vacuum filtration and spontaneous reduction of gold ions. Chem. Commun. 2009, 16, 2174–2176. [Google Scholar] [CrossRef]
- Zhuo, Q.; Gao, J.; Peng, M.; Bai, L.; Deng, J.; Xia, Y.; Ma, Y.; Zhong, J.; Sun, X. Large-scale synthesis of graphene by the reduction of graphene oxide at room temperature using metal nanoparticles as catalyst. Carbon 2013, 52, 559–564. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Mei, X.; Ouyang, J. Ultrasonication-assisted ultrafast reduction of graphene oxide by zinc powder at room temperature. Carbon 2011, 49, 5389–5397. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Zhai, Y.; Zhai, J.; Ren, W.; Wang, F.; Dong, S. Controlled synthesis of large-area and patterned electrochemically reduced graphene oxide films. Chemistry 2009, 15, 6116–6120. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, X.; Qi, X.; Wu, S.; Xue, C.; Boey, F.Y.C.; Yan, Q.; Chen, P.; Zhang, H. In Situ Synthesis of Metal Nanoparticles on Single-Layer Graphene Oxide and Reduced Graphene Oxide Surfaces. J. Phys. Chem. C 2009, 113, 10842–10846. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Brisebois, P.P.; Siaj, M. Harvesting graphene oxide—Years 1859 to 2019: A review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- Kaye, G.W.C.; Laby, T.H. Kaye and Labye Tables of Physical Constants & Chemical Constants, 16th ed.; National Physical Laboratory: Middlessex, UK, 1995. [Google Scholar]
- Kumar, N.A.; Gambarelli, S.; Duclairoir, F.; Bidan, G.; Dubois, L. Synthesis of high quality reduced graphene oxide nanosheets free of paramagnetic metallic impurities. J. Mater. Chem. A 2013, 1, 2789–2794. [Google Scholar] [CrossRef]
- Brisebois, P.P.; Kuss, C.; Schougaard, S.B.; Izquierdo, R.; Siaj, M. New Insights into the Diels-Alder Reaction of Graphene Oxide. Chemistry 2016, 22, 5849–5852. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Chen, C.; Yan, L. Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Hashimoto, K.; Nakanishi, S. Instantaneous one-pot synthesis of Fe–N-modified graphene as an efficient electrocatalyst for the oxygen reduction reaction in acidic solutions. Chem. Commun. 2012, 48, 10213–10215. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Wang, X.; Lv, W.; Xu, Y.; Ge, Y.; He, H.; Li, G.; Wu, X.; Li, X.; Li, Q. Facile synthesis of ZnO nanorods grown on graphene sheets and its enhanced photocatalytic efficiency. J. Chem. Technol. Biotechnol. 2015, 90, 550–558. [Google Scholar] [CrossRef]
- Maruyama, B.; Ohuchi, F.S.; Rabenberg, L. Catalytic carbide formation at aluminium-carbon interfaces. J. Mater. Sci. Lett. 1990, 9, 864–866. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Fadoni, M.; Vercelli, B. Magnesium salts and oxide: An XPS overview. Appl. Surf. Sci. 1997, 119, 253–259. [Google Scholar] [CrossRef]
- Paparazzo, E. On the interpretation of XPS spectra of metal (Pt, Pt–Sn) nanoparticle/graphene systems. Carbon 2013, 63, 578–581. [Google Scholar] [CrossRef]
- Liu, C.-J.; Huang, H.; Cao, G.-Z.; Xue, F.-H.; Paredes Camacho, R.A.; Dong, X.-L. Enhanced Electrochemical Stability of Sn-Carbon Nanotube Nanocapsules as Lithium-Ion Battery Anode. Electrochim. Acta 2014, 144, 376–382. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Jordan, J. International Union of Pure and Applied Chemistry. Standard Potentials in Aqueous Solution, 1st ed.; Marcel Dekker: New York, NY, USA, 1985. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Al Shboul, A.M.; Siaj, M.; Claverie, J.P. Selective Process To Extract High-Quality Reduced Graphene Oxide Leaflets. ACS Appl. Nano Mater. 2018, 1, 5920–5926. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Salas, E.C.; Sun, Z.; Lüttge, A.; Tour, J.M. Reduction of Graphene Oxide via Bacterial Respiration. ACS Nano 2010, 4, 4852–4856. [Google Scholar] [CrossRef] [PubMed]


| Compound | % C | % O | % M | % Cl | C/O Ratio |
|---|---|---|---|---|---|
| GO-Sn | 55.4 | 34.8 | 6.8 | 3.1 | 1.6 |
| GO-Fe | 64.2 | 34.0 | 1.0 | 0.8 | 1.9 |
| GO-Zn | 65.1 | 34.6 | 0.2 | 0.1 | 1.9 |
| GO-Al | 65.6 | 33.9 | 0.2 | 0.1 | 1.9 |
| GO-Mg | 64.9 | 34.4 | 0.3 | 0.2 | 1.9 |
| GO | 6 | 35.7 | 0.0 | 0.0 | 1.8 |
| Entries | Compound | FRC (%) | Weight Loss (%) |
|---|---|---|---|
| a | GO-Sn | 75 | 16 |
| b | GO-Fe | 38 | 35 |
| c | GO-Zn | 31 | 31 |
| d | GO-Al | 28 | 39 |
| e | GO-Mg | 26 | 39 |
| f | GO | 24 | 45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brisebois, P.P.; Izquierdo, R.; Siaj, M. Room-Temperature Reduction of Graphene Oxide in Water by Metal Chloride Hydrates: A Cleaner Approach for the Preparation of Graphene@Metal Hybrids. Nanomaterials 2020, 10, 1255. https://doi.org/10.3390/nano10071255
Brisebois PP, Izquierdo R, Siaj M. Room-Temperature Reduction of Graphene Oxide in Water by Metal Chloride Hydrates: A Cleaner Approach for the Preparation of Graphene@Metal Hybrids. Nanomaterials. 2020; 10(7):1255. https://doi.org/10.3390/nano10071255
Chicago/Turabian StyleBrisebois, Patrick. P., Ricardo Izquierdo, and Mohamed Siaj. 2020. "Room-Temperature Reduction of Graphene Oxide in Water by Metal Chloride Hydrates: A Cleaner Approach for the Preparation of Graphene@Metal Hybrids" Nanomaterials 10, no. 7: 1255. https://doi.org/10.3390/nano10071255






