Graphene Oxide Protected Copper Benzene-1,3,5-Tricarboxylate for Clean Energy Gas Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Sample Characterization
2.4. Hydrogen, Methane and Carbon Dioxide Adsorption
2.5. Effect of Humidity
3. Results and Discussion
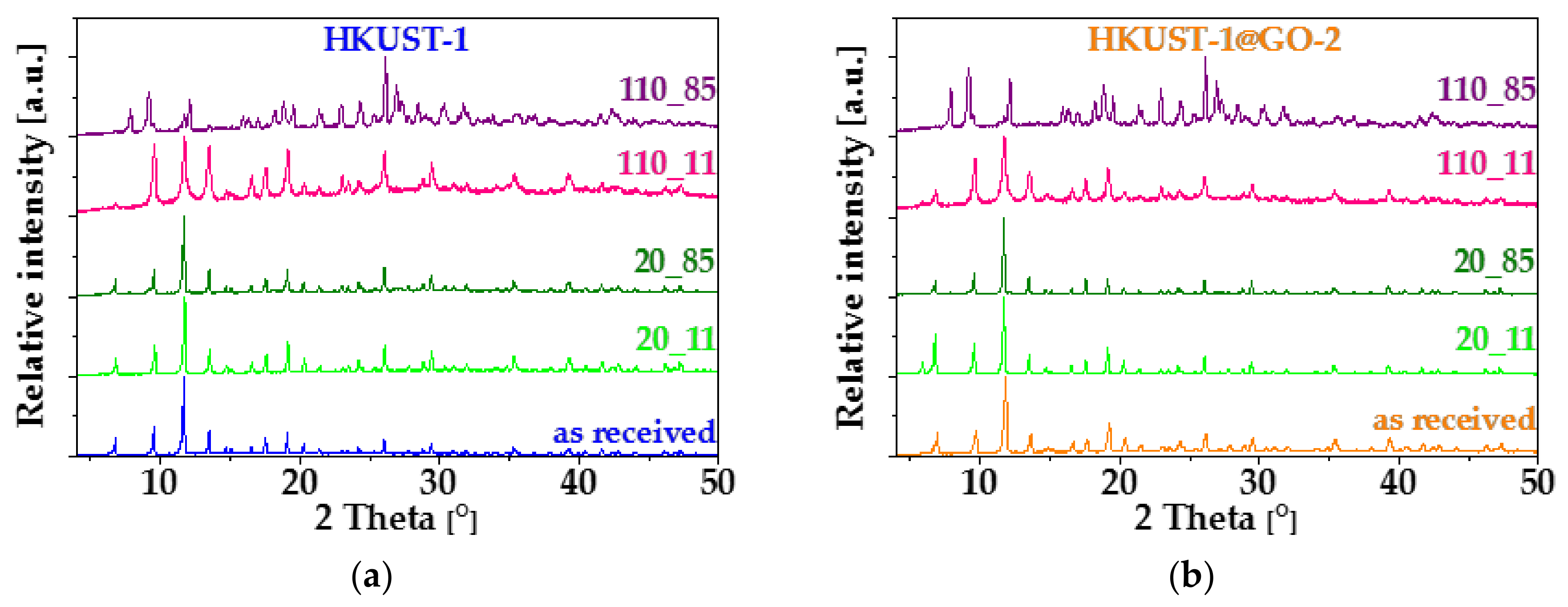
3.1. Characterization of the Composite Materials
3.2. Adsorption Capacity for Clean Energy Gases and CO2
3.3. Effect of Water Vapor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moellmer, J.; Moeller, A.; Dreisbach, F.; Glaeser, R.; Staudt, R. High pressure adsorption of hydrogen. nitrogen. carbon dioxide and methane on the metal–organic framework HKUST-1. Micropor. Mesopor. Mater. 2011, 138, 140–148. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Wang, B.; Xie, L.-H.; Wang, X.; Liu, X.-M.; Li, J.; Li, J.-R. Applications of metal–organic frameworks for green energy and environment: New advance in adsorptive gas separation, storage and removal. Green Energy Environ. 2018, 3, 191–228. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to metal−organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal–organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef] [Green Version]
- Domán, A.; Czakkel, O.; Porcar, L.; Madarász, J.; Geissler, E.; László, K. Role of water molecules in the decomposition of HKUST-1: Evidence from adsorption, thermoanalytical, X-ray and neutron scattering measurements. Appl. Surf. Sci. 2019, 480, 138–147. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Z.; Wang, B.; Chen, Y.; Wang, H.; Chen, X.; Zhang, H.; Sun, N.; Wei, W.; Sun, Y. Synthesis of HKUST-1#MCF compositing materials for CO2 adsorption. Micropor. Mesopor. Mater. 2016, 226, 476–481. [Google Scholar]
- Mu, X.; Chen, Y.; Lester, E.; Wu, T. Optimized synthesis of nano-scale high quality HKUST-1 under mild conditions and its application in CO2 capture. Micropor. Mesopor. Mater. 2018, 270, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Tsivadze, A.Y.; Aksyutin, O.E.; Ishkov, A.G.; Knyazeva, M.K.; Solovtsova, O.V.; Men’shchikov, I.E.; Fomkin, A.A.; Shkolin, A.V.; Khozina, E.V.; Grachev, V.A. Metal-organic framework structures: Adsorbents for natural gas storage. Russ. Chem. Rev. 2019, 88, 925–978. [Google Scholar] [CrossRef]
- Langmia, H.W.; Rena, J.; Northa, B.; Mathea, M.; Bessarabov, D. Hydrogen storage in metal-organic frameworks: A review. Electrochim. Acta 2014, 128, 368–392. [Google Scholar] [CrossRef]
- Tian, T.; Zeng, Z.; Vulpe, D.; Casco, M.E.; Divitini, G.; Midgley, P.A.; Silvestre-Albero, J.; Tan, J.-C.; Moghadam, P.Z.; Fairen-Jimenez, D. A sol–gel monolithic metal–organic framework with enhanced methane uptake. Nat. Mater. 2018, 17, 174–179. [Google Scholar] [CrossRef]
- He, Y.; Chen, F.; Li, B.; Qian, G.; Zhou, W.; Chen, B. Porous metal–organic frameworks for fuel storage. Coordin. Chem. Rev. 2018, 373, 167–198. [Google Scholar] [CrossRef]
- Gomez, D.A.; Toda, J.; Sastre, G. Screening of hypothetical metal–organic frameworks for H2 storage. Phys. Chem. Chem. Phys. 2014, 16, 19001–19010. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Rodríguez-Reinoso, F. Nanoporous Materials for Gas Storage; Springer: Singapore, 2019; pp. 8–12. [Google Scholar]
- Wang, H.; Bai, J.Q.; Qu, Z.G.; Chang, M. Numerical study of heat transfer in process of gas adsorption in a Cu-benzene-1,3,5-tricarboxylic acid particle adsorption bed. Int. J. Hydrog. Energy 2019, 44, 11989–12002. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Li, S.; Huo, F. Metal–organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef]
- Domán, A.; Nagy, B.; Nichele, L.P.; Srankó, D.; Madarász, J.; László, K. Pressure resistance of copper benzene-1,3,5-tricarboxylate-carbon aerogel composites. Appl. Surf. Sci. 2018, 434, 1300–1310. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Porous metal–organic frameworks for fuel storage: Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Muschi, M.; Serre, C. Progress and challenges of graphene oxide/metal-organic composites. Coordin. Chem. Rev. 2019, 387, 262–272. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Kim, H.S. Fast and fully-scalable synthesis of reduced graphene oxide. Sci. Rep. 2015, 5, 10160–10167. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, H.; Zhong, Q. Synthesis and characterization of MOF-aminated graphite oxide composites for CO2 capture. Appl. Surf. Sci. 2013, 284, 138–144. [Google Scholar] [CrossRef]
- Kumar, R.; Jayaramulu, K.; Maji, T.K.; Rao, C.N.R. Hybrid nanocomposites of ZIF-8 with graphene oxide exhibiting tunable morphology, significant CO2 uptake and other novel properties. Chem. Commun. 2013, 49, 4947–4949. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, Y.; Lv, Z.; Song, F.; Zhong, Q. Preparation and enhanced CO2 adsorption capacity of UiO 66/graphene oxide composites. J. Ind. Eng. Chem. 2015, 27, 102–107. [Google Scholar] [CrossRef]
- Yang, T.B.; Sun, L.X.; Xu, F.; Wang, Z.Q.; Zou, Y.J.; Chu, H.L. Synthesis of MIL-125/graphene oxide composites and hydrogen storage properties. Key Eng. Mater. 2017, 727, 683–687. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, W.; Liu, J.; Wang, H.; Li, Z. Quenched breathing effect, enhanced CO2 uptake and improved CO2/CH4 selectivity of MIL-53(Cr)/graphene oxide composites. Chem. Eng. Sci. 2017, 167, 98–104. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, W.; Miao, J.; Xia, Q.; Zhang, Z.; Wang, H.; Li, Z. Enhanced separation performance of a novel composite material GrO@MIL-101 for CO2/CH4 binary mixture. Chem. Eng. J. 2015, 266, 339–344. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, D.; Wub, J.; Xiao, J.; Xi, H.; Xia, Q.; Li, Z. A new MOF-505@GO composite with high selectivity for CO2/CH4 and CO2/N2 separation. Chem. Eng. J. 2017, 308, 1065–1072. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Development of activated graphene-MOF composites for H2 and CH4 adsorption. Adsorption 2019, 25, 1–12. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M.; Bessarabov, D. Synthesis of rGO/Zr-MOF composite for hydrogen storage application. J. Alloys Compd. 2017, 724, 450–455. [Google Scholar] [CrossRef]
- Asgharnejad, L.; Abbasi, A.; Shakeri, A. Ni-based metal-organic framework/GO nanocomposites as selective adsorbent for CO2 over N2. Micropor. Mesopor. Mater. 2018, 262, 227–234. [Google Scholar] [CrossRef]
- Li, W.; Chuah, C.Y.; Yang, Y.; Bae, T.-H. Nanocomposites formed by in situ growth of NiDOBDC nanoparticles on graphene oxide sheets for enhanced CO2 and H2 storage. Micropor. Mesopor. Mater. 2018, 265, 35–42. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xue, H.; Pang, H. Metal-organic frameworks/graphene-based materials: Preparations and applications. Adv. Funct. Mater. 2018, 28, 1804950. [Google Scholar] [CrossRef]
- Arslanov, V.V.; Kalinina, M.A.; Ermakova, E.V.; Raitman, O.A.; Gorbunova, Y.G.; Aksyutin, O.E.; Ishkov, A.G.; Grachev, V.A.; Tsivadze, A.Y. Hybrid materials based on graphene derivatives and porphyrin metal-organic frameworks. Russ. Chem. Rev. 2019, 88, 775–799. [Google Scholar] [CrossRef]
- Liu, X.-W.; Sun, T.-J.; Hu, J.-L.; Wang, S.-D. Composites of metal–organic frameworks and carbon-based materials: Preparations, functionalities and applications. J. Mater. Chem. A 2016, 4, 3584–3616. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Gao, F.; Ni, J.; Zhang, Y.; Lin, Z. Graphene oxide directed one-step synthesis of flowerlike graphene@HKUST-1 for enzyme-free detection of hydrogen peroxide in biological samples. ACS Appl. Mater. Interfaces 2016, 8, 32477–32487. [Google Scholar] [CrossRef]
- Travlou, N.A.; Singh, K.; Rodríguez-Castellón, E.; Bandosz, T.J. Cu–BTC MOF–graphene-based hybrid materials as low concentration ammonia sensors. J. Mater. Chem. A 2015, 3, 11417–11429. [Google Scholar] [CrossRef]
- Ma, B.; Guo, H.; Wang, M.; Li, L.; Jia, X.; Chen, H.; Xue, R.; Yang, W. Electrocatalysis of Cu MOF/graphene composite and its sensing application for electrochemical simultaneous determination of dopamine and paracetamol. Electroanalysis 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Sun, K.K.; Li, L.; He, Y.Q.; Fan, L.; Wu, Y.Q.; Liu, L. Preparation and drug-delivery properties of HKUST-1/GO hybrid. J. Nanosci. Nanotechnol. 2016, 16, 242–245. [Google Scholar] [CrossRef]
- Li, Y.; Miao, J.; Sun, X.; Xiao, J.; Li, Y.; Wang, H.; Xia, Q.; Li, Z. Mechanochemical synthesis of Cu-BTC@GO with enhanced water stability and toluene adsorption capacity. Chem. Eng. J. 2016, 298, 191–197. [Google Scholar] [CrossRef]
- Srimuk, P.; Luanwuthi, S.; Krittayavathananon, A.; Sawangphruk, M. Solid-type supercapacitor of reduced graphene oxide-metalorganic framework composite coated on carbon fiber paper. Electrochim. Acta 2015, 157, 69–77. [Google Scholar] [CrossRef]
- Yu, D.; Ge, L.; Wei, X.; Wu, B.; Ran, J.; Wang, H.; Xu, T. A general route to the synthesis of layer-by-layer structured metal organic framework/graphene oxide hybrid films for high-performance supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 16865–16872. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Gas adsorption properties of hybrid graphene-MOF materials. J. Colloid Interface Sci. 2018, 514, 801–813. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Gas adsorption properties of graphene-based materials. Adv. Colloid Interface Sci. 2017, 243, 46–59. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Chowdhury, S. Recent advances and progress in the development of graphene-based adsorbents for CO2 capture. J. Mater. Chem. A 2015, 3, 21968–21989. [Google Scholar] [CrossRef]
- Jain, V.; Kandasubramanian, B. Functionalized graphene materials for hydrogen storage. J. Mater. Sci. 2020, 55, 1865–1903. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, X.; Xia, Q.; Peng, J.; Wang, H.; Li, Z. Preparation and adsorption performance of GrO@Cu-BTC for separation of CO2/CH4. Ind. Eng. Chem. Res. 2014, 53, 11176–11184. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Y.; Zhong, Q. CO2 capture on metal-organic framework and graphene oxide composite using a high-pressure static adsorption apparatus. J. Clean Energy Technol. 2014, 2, 34–37. [Google Scholar] [CrossRef] [Green Version]
- Al-Naddaf, Q.; Al-Mansour, M.; Thakkar, H.; Rezaei, F. MOF-GO hybrid nanocomposite adsorbents for methane storage. Ind. Eng. Chem. Res. 2018, 57, 17470–17479. [Google Scholar] [CrossRef]
- Liu, S.; Sun, L.; Xu, F.; Zhang, J.; Jiao, C.; Li, F.; Li, Z.; Wang, S.; Wang, Z.; Jiang, X.; et al. Nanosized Cu-MOFs induced by graphene oxide and enhanced gas storage capacity. Energy Environ. Sci. 2013, 6, 818–823. [Google Scholar] [CrossRef]
- Xu, F.; Yu, Y.; Yan, J.; Xia, Q.; Wang, H.; Li, J.; Li, Z. Ultrafast room temperature synthesis of GrO@HKUST-1 composites with high CO2 adsorption capacity and CO2/N2 adsorption selectivity. Chem. Eng. J. 2016, 303, 231–237. [Google Scholar] [CrossRef]
- Bian, Z.; Zhu, X.; Jin, T.; Gao, J.; Hu, J.; Liu, H. Ionic liquid-assisted growth of Cu3(BTC)2 nanocrystals on graphene oxide sheets: Towards both high capacity and high rate for CO2 adsorption. Micropor. Mesopor. Mater. 2014, 200, 159–164. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, X.; Zhang, J.; Yan, X.; Liu, Y.; Yuan, A. Enhanced room-temperature hydrogen storage capacity in Pt-loaded graphene oxide/HKUST-1 composites. Int. J. Hydrog. Energy 2014, 39, 2160–2167. [Google Scholar] [CrossRef]
- Shang, S.; Tao, Z.; Yang, C.; Hanif, A.; Lid, L.; Tsange, D.C.W.; Guf, Q.; Shang, J. Facile synthesis of CuBTC and its graphene oxide composites as efficient adsorbents for CO2 capture. Chem. Eng. J. 2020, 393, 124666. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Engineering the surface of a new class of adsorbents: Metal–organic framework/graphite oxide composites. J. Colloid Interface Sci. 2015, 447, 139–151. [Google Scholar] [CrossRef]
- Policicchio, A.; Zhao, Y.; Zhong, Q.; Agostino, R.G.; Bandosz, T.J. Cu-BTC/aminated graphite oxide composites as high-efficiency CO2 capture media. ACS Appl. Mater. Interfaces 2014, 6, 101–108. [Google Scholar] [CrossRef]
- Bian, Z.; Xu, J.; Zhang, S.; Zhu, X.; Liu, H.; Hu, J. Interfacial growth of metal organic framework/graphite oxide composites through Pickering emulsion and their CO2 capture performance in the presence of humidity. Langmuir 2015, 31, 7410–7417. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Burress, J.; Bandosz, T.J. The synthesis and characterization of copper-based metal–organic framework/graphite oxide composites. Carbon 2011, 49, 563–572. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, J.; Yan, X.-F.; Shen, X.-P.; Yuan, A.-H. Spillover enhanced hydrogen storage in Pt-doped MOF/graphene oxide composite produced via an impregnation method. Inorg. Chem. Commun. 2015, 54, 54–56. [Google Scholar] [CrossRef]
- Zhao, Y.; Seredych, M.; Zhong, Q.; Bandosz, T.J. Superior performance of copper based MOF and aminated graphite oxide composites as CO2 adsorbents at room temperature. Appl. Mater. Interfaces 2013, 5, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Seredych, M.; Jagiello, J.; Zhong, Q.; Bandosz, T.J. Insight into the mechanism of CO2 adsorption on Cu–BTC and its composites with graphite oxide or aminated graphite oxide. Chem. Eng. J. 2014, 239, 399–407. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, M.; Tang, B.; Li, S.; Jiang, L.; Sun, X.; Que, M.; Tao, C.; Wu, Z. Graphene modified Cu-BTC with high stability in water and controllable selective adsorption of various gases. J. Alloys Compd. 2019, 808, 151721. [Google Scholar] [CrossRef]
- Lamagni, P.; Pedersen, B.L.; Godiksen, A.; Mossin, S.; Hu, X.-M.; Pedersen, S.U.; Daasbjerg, K.; Lock, N. Graphene inclusion controlling conductivity and gas sorption of metal–organic framework. RSC Adv. 2018, 8, 13921–13932. [Google Scholar] [CrossRef] [Green Version]
- Zu, D.-D.; Lu, L.; Liu, X.-Q.; Zhang, D.-Y.; Sun, L.-B. Improving hydrothermal stability and catalytic activity of metal—organic frameworks by graphite oxide incorporation. J. Phys. Chem. C 2014, 118, 19910–19917. [Google Scholar] [CrossRef]
- Wang, F.; Guo, H.; Chai, Y.; Li, Y.; Liu, C. The controlled regulation of morphology and size of HKUST-1 by “coordination modulation method”. Micropor. Mesopor. Mater. 2013, 173, 181–188. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Justh, N.; Berke, B.; László, K.; Szilágyi, I.M. Thermal analysis of the improved Hummers synthesis of graphene oxide. J. Therm. Anal. Calorim. 2018, 131, 2267–2272. [Google Scholar] [CrossRef] [Green Version]
- Molnár, N. Spectroscopic properties of GO nanostructures. Master’s Thesis, Budapest University of Technology and Economics, Budapest, Hungary, 2019. [Google Scholar]
- Mohai, M.; László, K.; Bertóti, I. Reduction and covalent modification of graphene-oxide by nitrogen in glow discharge plasma. Surf. Interface Anal. 2018, 50, 1207–1212. [Google Scholar] [CrossRef]
- Farah, S.; Farkas, A.; Madarász, J.; László, K. Comparison of thermally and chemically reduced graphene oxides by thermal analysis and Raman spectroscopy. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge structural database: A quarter of a million crystal structures and rising. Acta Cryst. B 2002, 58, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Jacco van de Streek, W.P.A. Mercury CSD 20-new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1983, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristic curve of activated charcoal. Chem. Zent. 1947, 1, 875–890. [Google Scholar]
- Barret, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Liang, H.; Xing, Y.; Yan, L.; Dai, P.; Gu, X.; Zhao, G.; Zhao, X. Superstructure of a metal−organic framework derived from microdroplet flow reaction: An intermediate state of crystallization by particle attachment. ACS Nano 2019, 13, 2901–2912. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Wahiduzzaman, A.K.; Stone, J.; Harp, S.; Mujibur, K. Synthesis and electrospraying of nanoscale MOF (metal organic framework) for high-performance CO2 adsorption membrane. Nanoscale Res. Lett. 2017, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domán, A.; Madarász, J.; László, K. In situ evolved gas analysis assisted thermogravimetric (TG-FTIR andTG/DTA–MS) studies on non-activated copperbenzene-1.3.5-tricarboxylate. Thermochim. Acta 2017, 647, 62–69. [Google Scholar]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfao, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]

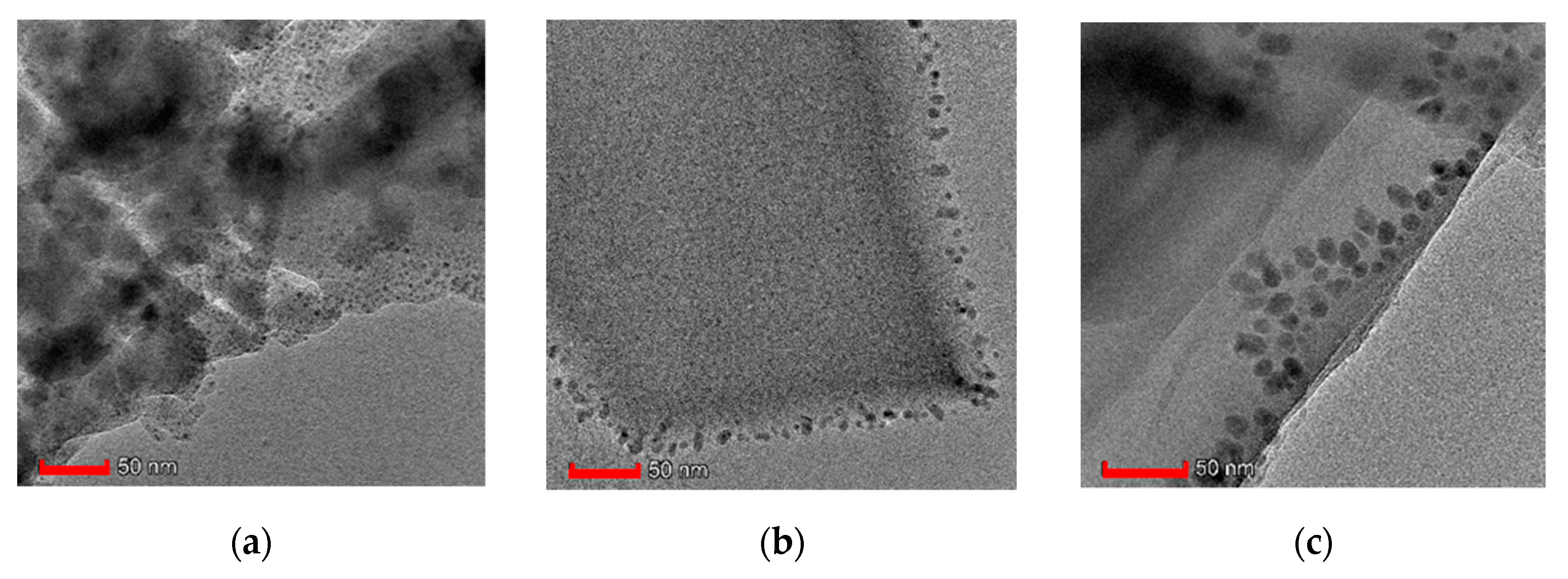
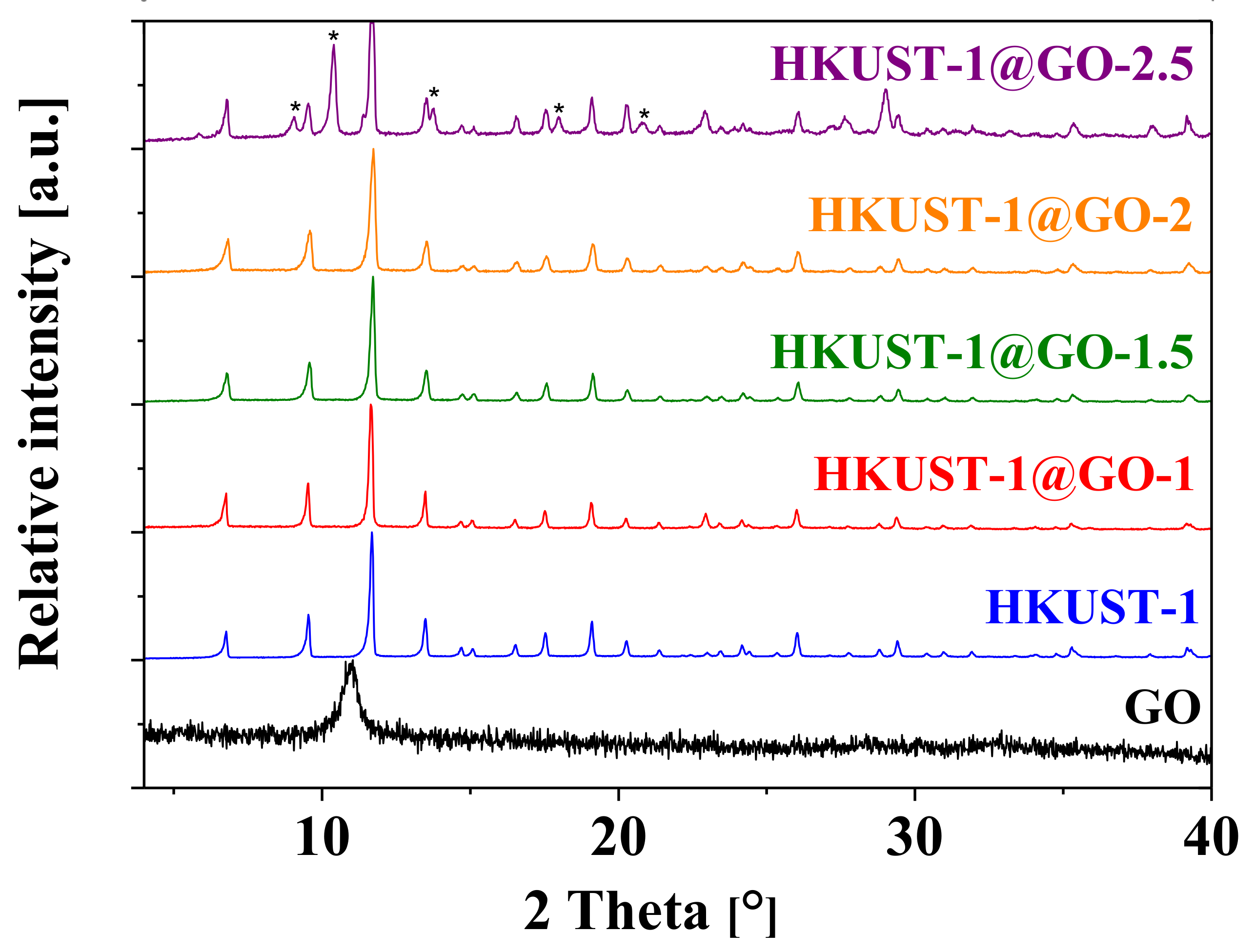


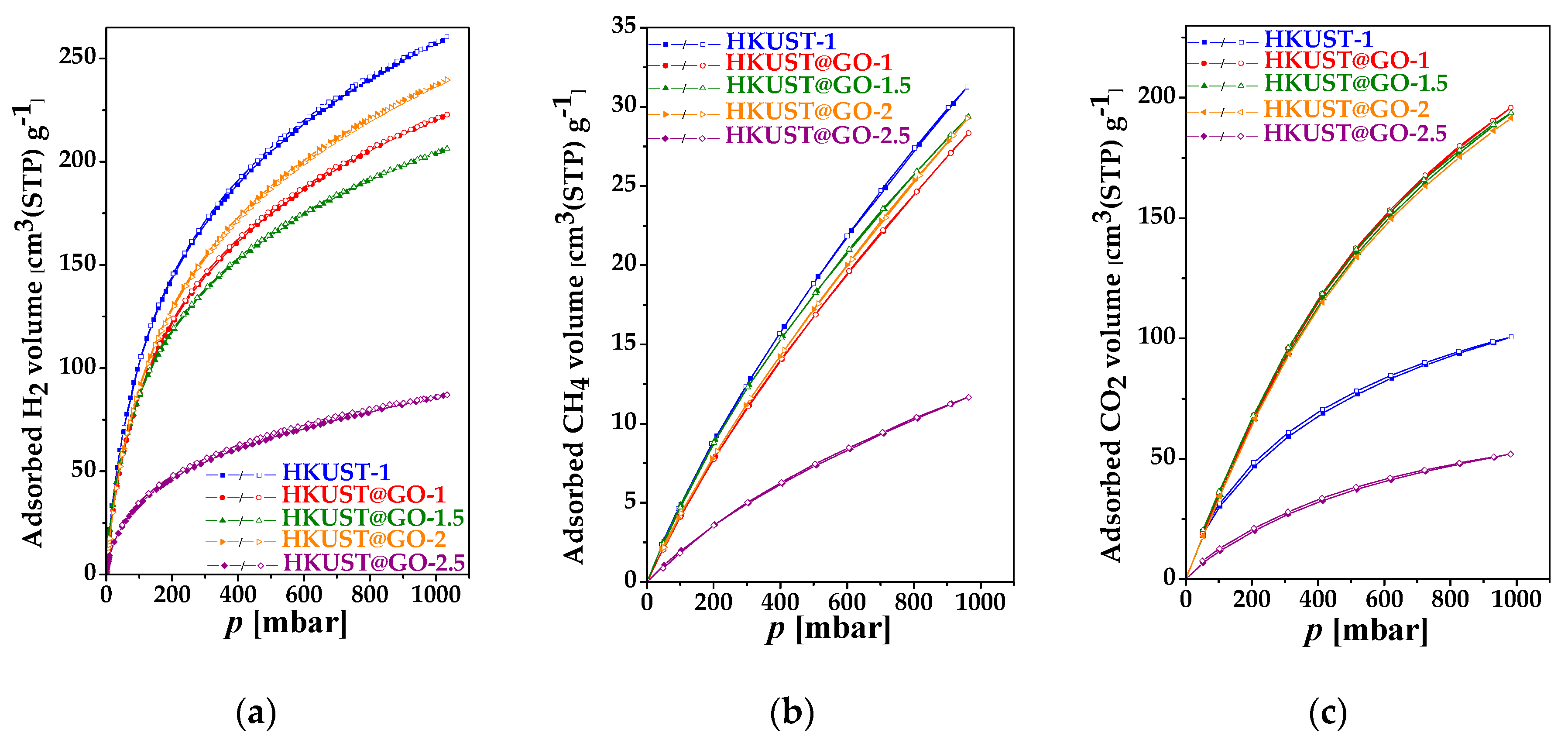
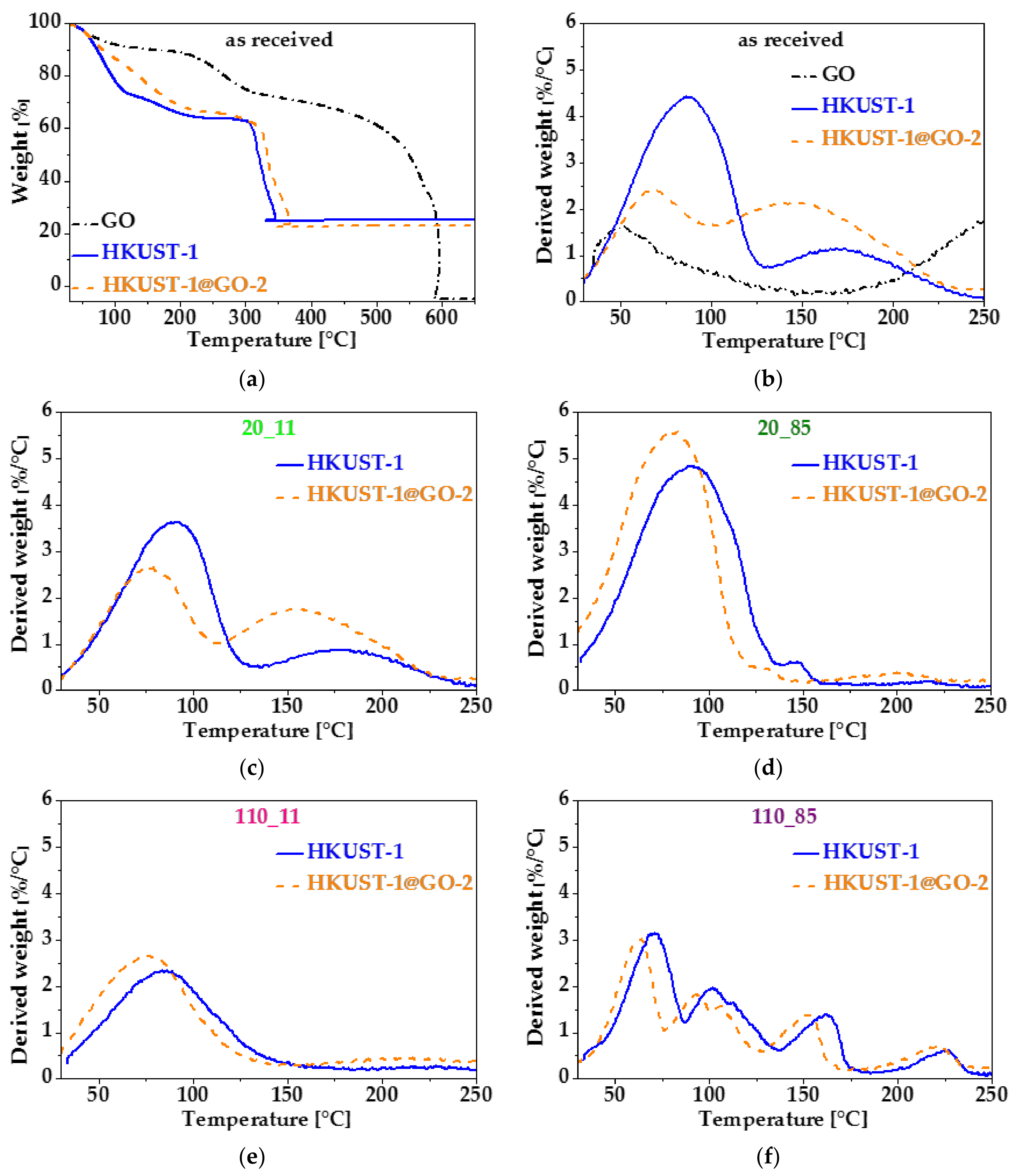
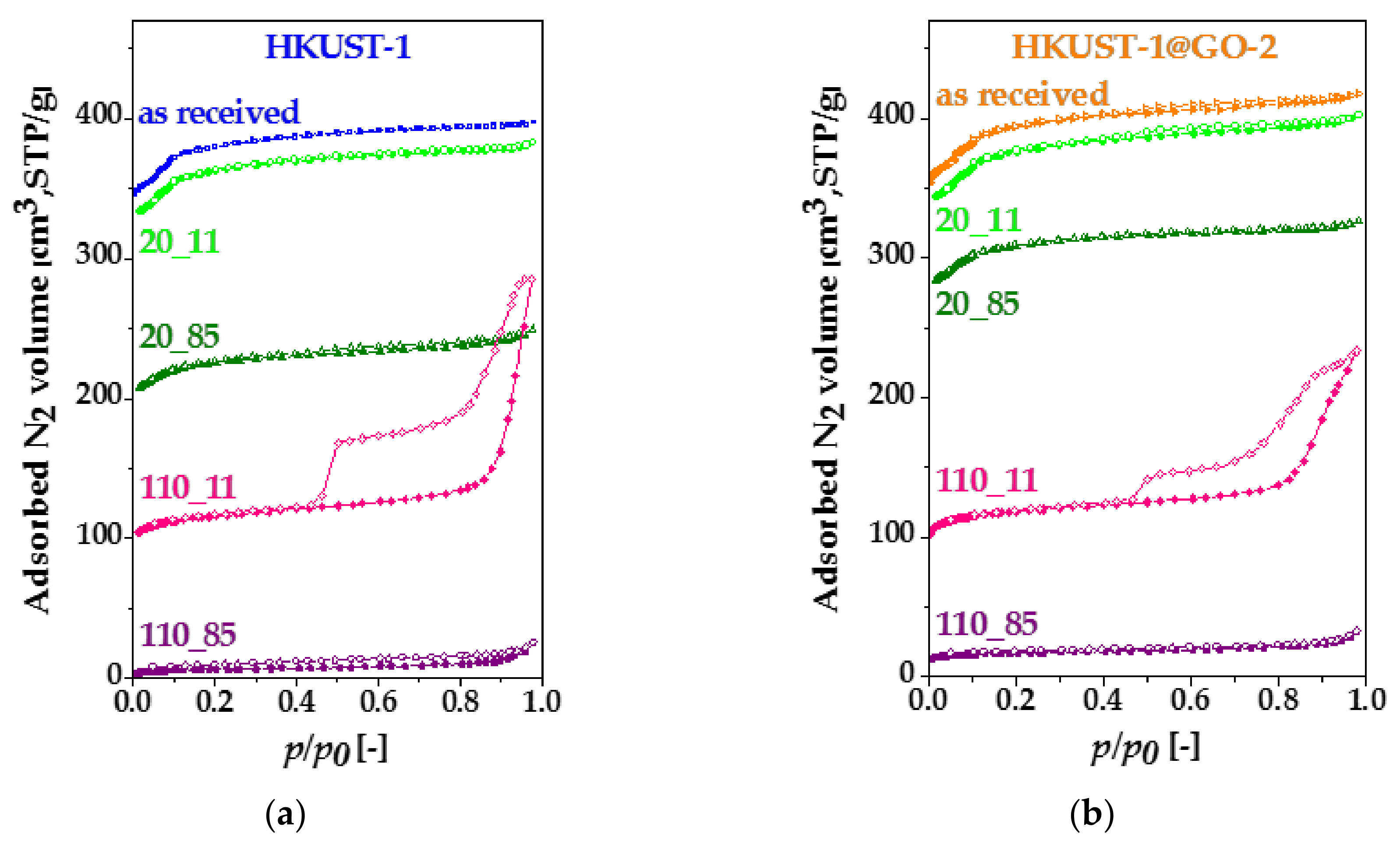

| Sample. | mGO | mdry | Yield of HKUST-1 | GO in Composite | SBET | Vmicro | Vtot | Vmeso |
|---|---|---|---|---|---|---|---|---|
| mg | % | % | m2 g−1 | cm3 g−1 | ||||
| GO | - | - | - | - | 20 | 0.01 | 0.07 | 0.06 |
| HKUST-1 | 0 | 213 | 84 | 0 | 1500 | 0.55 | 0.62 | 0.07 |
| HKUST-1@GO-1 | 20 | 215 | 77 | 9 | 1470 | 0.54 | 0.61 | 0.07 |
| [email protected] | 30 | 227 | 78 | 13 | 1500 | 0.56 | 0.63 | 0.07 |
| HKUST-1@GO-2 | 40 | 249 | 82 | 16 | 1550 | 0.57 | 0.65 | 0.08 |
| [email protected] | 50 | 212 | 64 | 24 | 560 | 0.21 | 0.27 | 0.06 |
| Sample | Uptake | |||
|---|---|---|---|---|
| cm3·(STP)·g−1 | ||||
| N2 | H2 | CH4 | CO2 | |
| HKUST-1 | 402 | 261 | 31 | 91 |
| HKUST-1@GO-1 | 394 | 223 | 28 | 196 |
| [email protected] | 406 | 206 | 29 | 194 |
| HKUST-1@GO-2 | 418 | 247 | 31 | 192 |
| [email protected] | 174 | 87 | 12 | 52 |
| Sample | SBET | Vmicro | Vtot | Vmeso |
|---|---|---|---|---|
| m2·g−1 | cm3·(STP)·g−1 | |||
| HKUST-1 | 1500 | 0.56 | 0.61 | 0.05 |
| HKUST-1_20_11 | 1410 | 0.53 | 0.59 | 0.06 |
| HKUST-1_20_85 | 880 | 0.34 | 0.39 | 0.05 |
| HKUST-1_110_11 | 450 | 0.18 | 0.44 | 0.26 |
| HKUST-1_110_85 | 25 | 0.01 | 0.04 | 0.03 |
| HKUST-1@GO-2 | 1550 | 0.57 | 0.65 | 0.08 |
| HKUST-1@GO-2_20_11 | 1460 | 0.56 | 0.62 | 0.06 |
| HKUST-1@GO-2_20_85 | 1200 | 0.46 | 0.50 | 0.04 |
| HKUST-1@GO-2_110_11 | 470 | 0.18 | 0.36 | 0.18 |
| HKUST-1@GO-2_110_85 | 65 | 0.03 | 0.05 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domán, A.; Klébert, S.; Madarász, J.; Sáfrán, G.; Wang, Y.; László, K. Graphene Oxide Protected Copper Benzene-1,3,5-Tricarboxylate for Clean Energy Gas Adsorption. Nanomaterials 2020, 10, 1182. https://doi.org/10.3390/nano10061182
Domán A, Klébert S, Madarász J, Sáfrán G, Wang Y, László K. Graphene Oxide Protected Copper Benzene-1,3,5-Tricarboxylate for Clean Energy Gas Adsorption. Nanomaterials. 2020; 10(6):1182. https://doi.org/10.3390/nano10061182
Chicago/Turabian StyleDomán, Andrea, Szilvia Klébert, János Madarász, György Sáfrán, Ying Wang, and Krisztina László. 2020. "Graphene Oxide Protected Copper Benzene-1,3,5-Tricarboxylate for Clean Energy Gas Adsorption" Nanomaterials 10, no. 6: 1182. https://doi.org/10.3390/nano10061182





