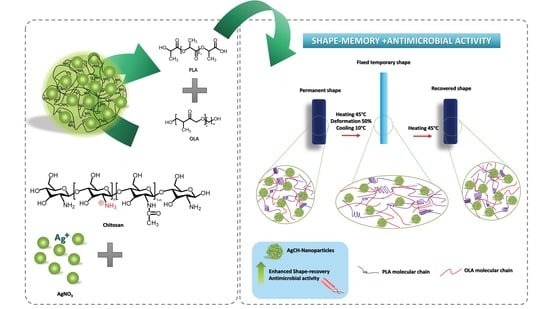
Biodegradable and Antimicrobial PLA–OLA Blends Containing Chitosan-Mediated Silver Nanoparticles with Shape Memory Properties for Potential Medical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing of Shape Memory Plasticized PLA/OLA Nanocomposites
2.3. Characterization Techniques
3. Results
3.1. Glass Transition Temperature, Activation Energy and Crystallinity of the Systems
3.2. Shape Memory Properties; Thermal Activation
3.3. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yakacki, C.M.; Gall, K. Shape-Memory Polymers for Biomedical Applications. In Shape-Memory Polymers. Advances in Polymer Science Series; Lendlein, A., Ed.; Springer: Berlín/Heidelberg, Germany, 2009; Volume 226, pp. 147–175. [Google Scholar]
- Lendlein, A.; Behl, M.; Hiebl, B.; Wischke, C. Shape-memory polymers as a technology platform for biomedical applications. Expert Rev. Med. Devices 2010, 7, 357–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, M.C.; Ameer, G.A. Recent insights into the biomedical applications of shape-memory polymers. Macromol. Biosci. 2012, 12, 1156–1171. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, J. Biodegradable polydepsipeptides. Int. J. Mol. Sci. 2009, 10, 589–615. [Google Scholar] [CrossRef]
- Grijpma, D.W.; Pennings, A.J. (Co)polymers of L-lactide, 2. Mechanical properties. Macromol. Chem. Phys. 1994, 195, 1649–1663. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Toughening modification of poly(lactic acid) via melt blending. ACS Symp. Ser. 2012, 1105, 27–46. [Google Scholar]
- Choi, N.-Y.; Kelch, S.; Lendlein, A. Synthesis, Shape-Memory Functionality and Hydrolytical Degradation Studies on Polymer Networks from Poly(rac-lactide)-b-poly(propylene oxide)-b-poly(rac-lactide) dimethacrylates. Adv. Eng. Mater. 2006, 8, 439–445. [Google Scholar] [CrossRef]
- Kelch, S.; Choi, N.Y.; Wang, Z.; Lendlein, A. Amorphous, elastic AB copolymer networks from acrylates and poly[(L-lactide)-ran-glycolide]dimetfiacrylates. Adv. Eng. Mater. 2008, 10, 494–502. [Google Scholar] [CrossRef]
- Min, C.; Cui, W.; Bei, J.; Wang, S. Effect of comonomer on thermal/mechanical and shape memory property of L-lactide-based shape-memory copolymers. Polym. Adv. Technol. 2007, 18, 299–305. [Google Scholar] [CrossRef]
- Yang, J.; Liu, F.; Yang, L.; Li, S.M. Hydrolytic and enzymatic degradation of poly(trimethylene carbonate-co-D,L-lactide) random copolymers with shape memory behavior. Eur. Polym. J. 2010, 46, 783–791. [Google Scholar] [CrossRef]
- Liu, C.; Mather, P.T. Thermomechanical characterization of blends of poly(vinyl acetate) with semicrystalline polymers for shape memory applications. In Proceedings of the 61st Annual Technical Conference ANTEC 2003, Nashville, TN, USA, 4–8 May 2003; pp. 1962–1966. [Google Scholar]
- Wang, L.S.; Chen, H.C.; Xiong, Z.C.; Pang, X.B.; Xiong, C.D. Novel degradable compound shape-memory-polymer blend: Mechanical and shape-memory properties. Mater. Lett. 2010, 64, 284–286. [Google Scholar] [CrossRef]
- Sessini, V.; Navarro-Baena, I.; Arrieta, M.P.; Dominici, F.; López, D.; Torre, L.; Kenny, J.M.; Dubois, P.; Raquez, J.M.; Peponi, L. Effect of the addition of polyester-grafted-cellulose nanocrystals on the shape memory properties of biodegradable PLA/PCL nanocomposites. Polym. Degrad. Stab. 2018, 152, 126–138. [Google Scholar] [CrossRef]
- Leonés, A.; Sonseca, A.; López, D.; Fiori, S.; Peponi, L. Shape memory effect on electrospun PLA-based fibers tailoring their thermal response. Eur. Polym. J. 2019, 117, 217–226. [Google Scholar] [CrossRef]
- Choi, A.H.; Ben-Nissan, B.; Matinlinna, J.P.; Conway, R.C. Current perspectives: Calcium phosphate nanocoatings and nanocomposite coatings in dentistry. J. Dent. Res. 2013, 92, 853–859. [Google Scholar] [CrossRef]
- Beyth, N.; Farah, S.; Domb, A.J.; Weiss, E.I. Antibacterial dental resin composites. React. Funct. Polym. 2014, 75, 81–88. [Google Scholar] [CrossRef]
- Hook, E.R.; Owen, O.J.; Bellis, C.A.; Holder, J.A.; O´Sullivan, D.J.; Barbour, M.E. Development of a novel antimicrobial-releasing glass ionomer cement functionalized with chlorhexidine hexametaphosphate nanoparticles. J. Nanobiotechnol. 2014, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chien, C.Y.; Liu, T.Y.; Kuo, W.H.; Wang, M.J.; Tsai, W.B. Dopamine-assisted immobilization of hydroxyapatite nanoparticles and RGD peptides to improve the osteoconductivity of titanium. J. Biomed. Mater. Res. Part A 2013, 101 A, 740–747. [Google Scholar] [CrossRef]
- Sonseca, A.; Madani, S.; Rodríguez, G.; Hevilla, V.; Echeverría, C.; Fernández-García, M.; Muñoz-Bonilla, A.; Charef, N.; López, D. Multifunctional PLA blends containing chitosan mediated silver nanoparticles: Thermal, mechanical, antibacterial, and degradation properties. Nanomaterials 2020, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef]
- Kalaivani, R.; Maruthupandy, M.; Muneeswaran, T.; Hameedha Beevi, A.; Anand, M.; Ramakritinan, C.M.; Kumaraguru, A.K. Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front. Lab. Med. 2018, 2, 30–35. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Wagermaier, W.; Kratz, K.; Heuchel, M.; Lendlein, A. Characterization Methods for Shape-Memory Polymers. Adv. Polym. Sci. 2010, 226, 97–145. [Google Scholar]
- Ratna, D.; Karger-Kocsis, J. Recent advances in shape memory polymers and composites: A review. J. Mater. Sci. 2008, 43, 254–269. [Google Scholar] [CrossRef]
- ASTM E2149-13a. Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents under Dynamic Contact Conditions; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Shmool, T.A.; Zeitler, J.A. Insights into the structural dynamics of poly lactic-: Co-glycolic acid at terahertz frequencies. Polym. Chem. 2019, 10, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Frequency Dependence of Glass Transition Temperatures. Applications Notes Library, TA Instruments, Thermal Analysis, Rheology, TA423. Available online: https://www.tainstruments.com/applications-library-search/ (accessed on 10 May 2020).
- Kuljanin-Jakovljević, J.; Stojanović, Z.; Nedeljković, J.M. Influence of CdS-filler on the thermal properties of poly(methyl methacrylate). J. Mater. Sci. 2006, 41, 5014–5016. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Chow, L.C.; Yang, M.; Mitchell, J.W. Effects of Inorganic Fillers on the Thermal and Mechanical Properties of Poly(lactic acid). Int. J. Polym. Sci. 2014, 2014, 827028. [Google Scholar] [CrossRef]
- Xu, J.; Shi, W.; Pang, W. Synthesis and shape memory effects of Si–O–Si cross-linked hybrid polyurethanes. Polymer (Guildf) 2006, 47, 457–465. [Google Scholar] [CrossRef]
- Kalichevsky, M.T.; Jaroszkiewicz, E.M.; Ablett, S.; Blanshard, J.M.V.; Lillford, P.J. The glass transition of amylopectin measured by DSC, DMTA and NMR. Carbohydr. Polym. 1992, 18, 77–88. [Google Scholar] [CrossRef]
- Seo, M.K.; Lee, J.R.; Park, S.J. Crystallization kinetics and interfacial behaviors of polypropylene composites reinforced with multi-walled carbon nanotubes. Mater. Sci. Eng. A 2005, 404, 79–84. [Google Scholar] [CrossRef]
- Sheth, M.; Kumar, R.A.; Dave, V. Biodegradable Polymer Blends of Poly (lactic acid) and Poly (ethylene glycol). J. Appl. Polymer Sci. 2008, 66, 1495–1505. [Google Scholar] [CrossRef]
- Patidar, D.; Agrawal, S.; Saxena, N.S. Glass transition activation energy of CdS/PMMA nano-composite and its dependence on composition of CdS nano-particles. J. Therm. Anal. Calorim. 2011, 106, 921–925. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Zhang, Y. Surprising shape-memory effect of polylactide resulted from toughening by polyamide elastomer. Polymer (Guildf) 2009, 50, 1311–1315. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zhong, W.; Du, Q. A novel type of shape memory polymer blend and the shape memory mechanism. Polymer (Guildf) 2009, 50, 1596–1601. [Google Scholar] [CrossRef]
- Lai, S.M.; Lan, Y.C. Shape memory properties of melt-blended polylactic acid (PLA)/thermoplastic polyurethane (TPU) bio-based blends. J. Polym. Res. 2013, 20, 2–9. [Google Scholar] [CrossRef]
- Peponi, L.; Navarro-Baena, I.; Sonseca, A.; Gimenez, E.; Marcos-Fernandez, A.; Kenny, J.M. Synthesis and characterization of PCL–PLLA polyurethane with shape memory behavior. Eur. Polym. J. 2013, 49, 893–903. [Google Scholar] [CrossRef] [Green Version]
- Peponi, L.; Sessini, V.; Arrieta, M.P.; Navarro-Baena, I.; Sonseca, A.; Dominici, F.; Gimenez, E.; Torre, L.; Tercjak, A.; López, D.; et al. Thermally-activated shape memory effect on biodegradable nanocomposites based on PLA/PCL blend reinforced with hydroxyapatite. Polym. Degrad. Stab. 2018, 151, 36–51. [Google Scholar] [CrossRef]








| Sample | Frequency [Hz] | 1 Tg [°C] | 1Ea [kJ] |
|---|---|---|---|
| PLA | 1 | 63 | 435 |
| 0.5 | 60 | ||
| 0.1 | 58 | ||
| PLA/OLA | 1 | 41 | 365 |
| 0.5 | 38 | ||
| 0.1 | 36 | ||
| PLA/OLA-AgCH-0.5% | 1 | 37 | 270 |
| 0.5 | 34 | ||
| 0.1 | 31 | ||
| PLA/OLA-AgCH-1% | 1 | 35 | 215 |
| 0.5 | 34 | ||
| 0.1 | 27 | ||
| PLA/OLA-AgCH-3% | 1 | 37 | 410 |
| 0.5 | 37 | ||
| 0.1 | 33 |
| Sample | 2 TgDMTA [°C] | 1,2 TgDSC [°C] | 1,2 Tcc [°C] | ΔHcc [J/g] | 1,2 Tm [°C] | ΔHm [J/g] | ΔHTotal [J/g] | 1 Xc-DSC [%] |
|---|---|---|---|---|---|---|---|---|
| PLA | 63 | 62 | 123 | 2 | 149 | 2 | 0 | -- |
| PLA/OLA | 41 | 32 | 88 | 25 | 143 | 27 | 2 | 2.8 |
| PLA/OLA AgCH0.5% | 37 | 25 | 83 | 27 | 142 | 27 | 0 | 0.0 |
| PLA/OLA AgCH1% | 35 | 24 | 76 | 23 | 142 | 29 | 6 | 9.2 |
| PLA/OLA AgCH3% | 37 | 50 | 66 | 2 | 142 | 30 | 28 | 38.0 |
| Sample | Cycle [N°] | Rr (N) [%] | Rf (N) [%] | Stress at Max. Strain [MPa] |
|---|---|---|---|---|
| PLA/OLA | 1 | 36 | 99 | 4.9 |
| 2 | 61 | 99 | 5.6 | |
| 3 | 67 | 99 | 6.0 | |
| PLA/OLA-AgCH-0.5% | 1 | 98 | 100 | 0.8 |
| 2 | 98 | 100 | 0.8 | |
| 3 | 98 | 100 | 0.8 | |
| PLA/OLA-AgCH-1% | 1 | 86 | 100 | 1.9 |
| 2 | 99 | 100 | 1.9 | |
| 3 | 100 | 100 | 1.7 | |
| PLA/OLA-AgCH-3% | 1 | 77 | 99 | 2.3 |
| 2 | 84 | 99 | 2.7 | |
| 3 | 100 | 99 | 3.0 |
| Sample | Killing Percentage [%] |
|---|---|
| PLA | - |
| PLA/OLA | - |
| PLA/OLA CH | 90 |
| PLA/OLA-AgCH-0.5% | 99 |
| PLA/OLA-AgCH-1% | 99 |
| PLA/OLA-AgCH-3% | 99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonseca, A.; Madani, S.; Muñoz-Bonilla, A.; Fernández-García, M.; Peponi, L.; Leonés, A.; Rodríguez, G.; Echeverría, C.; López, D. Biodegradable and Antimicrobial PLA–OLA Blends Containing Chitosan-Mediated Silver Nanoparticles with Shape Memory Properties for Potential Medical Applications. Nanomaterials 2020, 10, 1065. https://doi.org/10.3390/nano10061065
Sonseca A, Madani S, Muñoz-Bonilla A, Fernández-García M, Peponi L, Leonés A, Rodríguez G, Echeverría C, López D. Biodegradable and Antimicrobial PLA–OLA Blends Containing Chitosan-Mediated Silver Nanoparticles with Shape Memory Properties for Potential Medical Applications. Nanomaterials. 2020; 10(6):1065. https://doi.org/10.3390/nano10061065
Chicago/Turabian StyleSonseca, Agueda, Salim Madani, Alexandra Muñoz-Bonilla, Marta Fernández-García, Laura Peponi, Adrián Leonés, Gema Rodríguez, Coro Echeverría, and Daniel López. 2020. "Biodegradable and Antimicrobial PLA–OLA Blends Containing Chitosan-Mediated Silver Nanoparticles with Shape Memory Properties for Potential Medical Applications" Nanomaterials 10, no. 6: 1065. https://doi.org/10.3390/nano10061065