Attachment Efficiency of Nanomaterials to Algae as an Important Criterion for Ecotoxicity and Grouping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Nanomaterials
2.2. Preparation of Suspensions
2.3. Algal Growth Inhibition Test
2.4. Microscopy
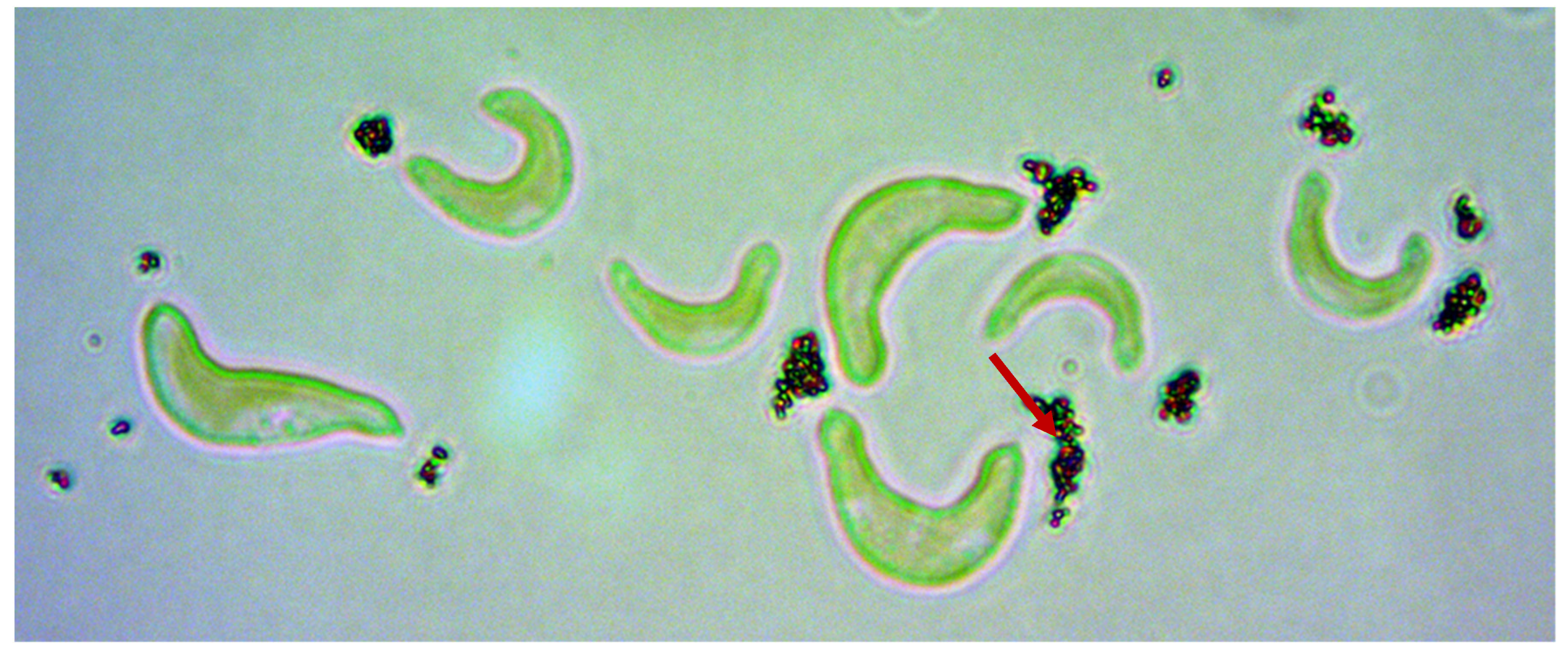
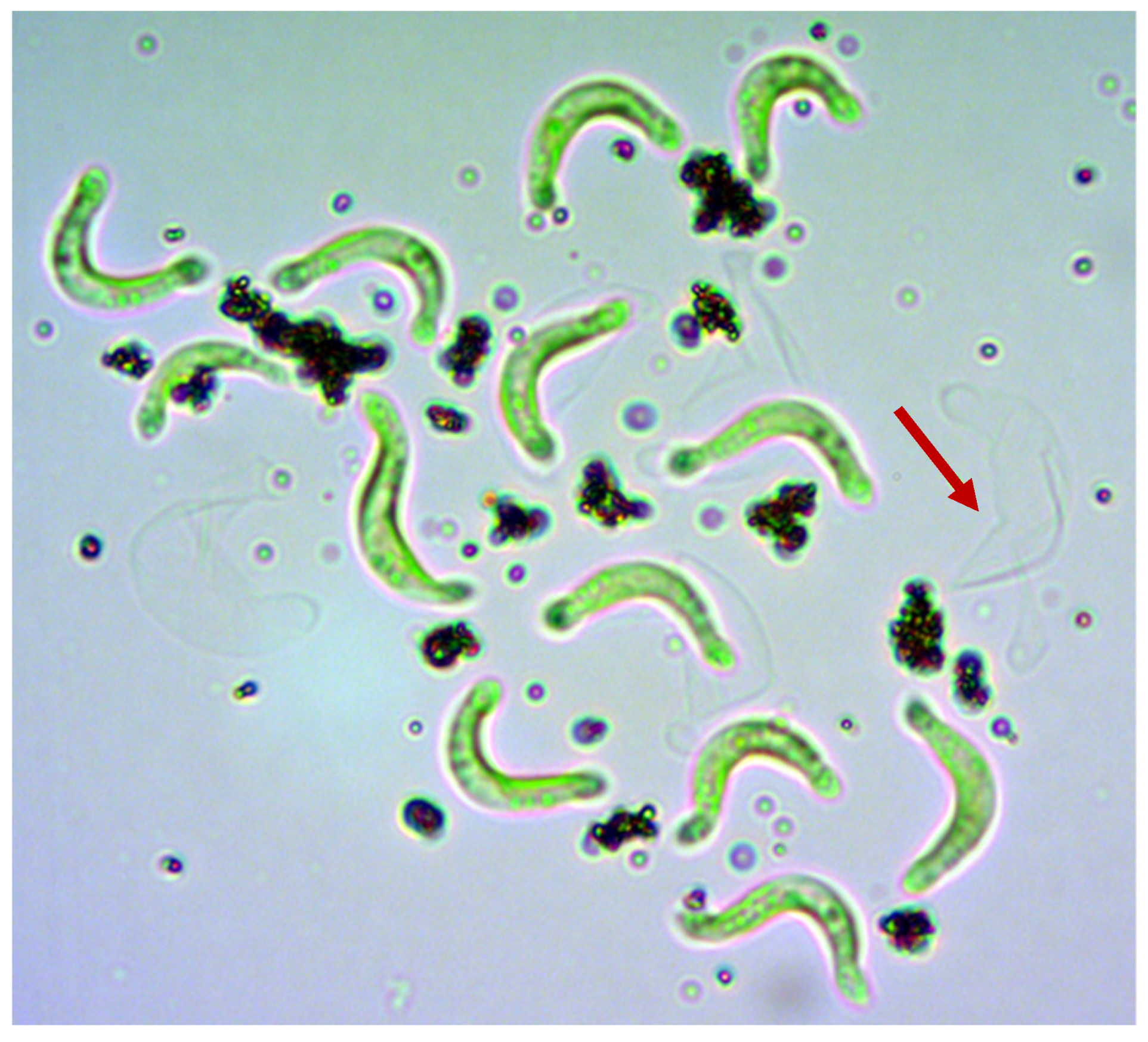
2.4.1. Attachment of ENMs to Algae by Light Microscopy
2.4.2. Attachment of ENMs to Algae by Electron Microscopy (SEM-EDX)
2.5. Statistical Evaluation
3. Results
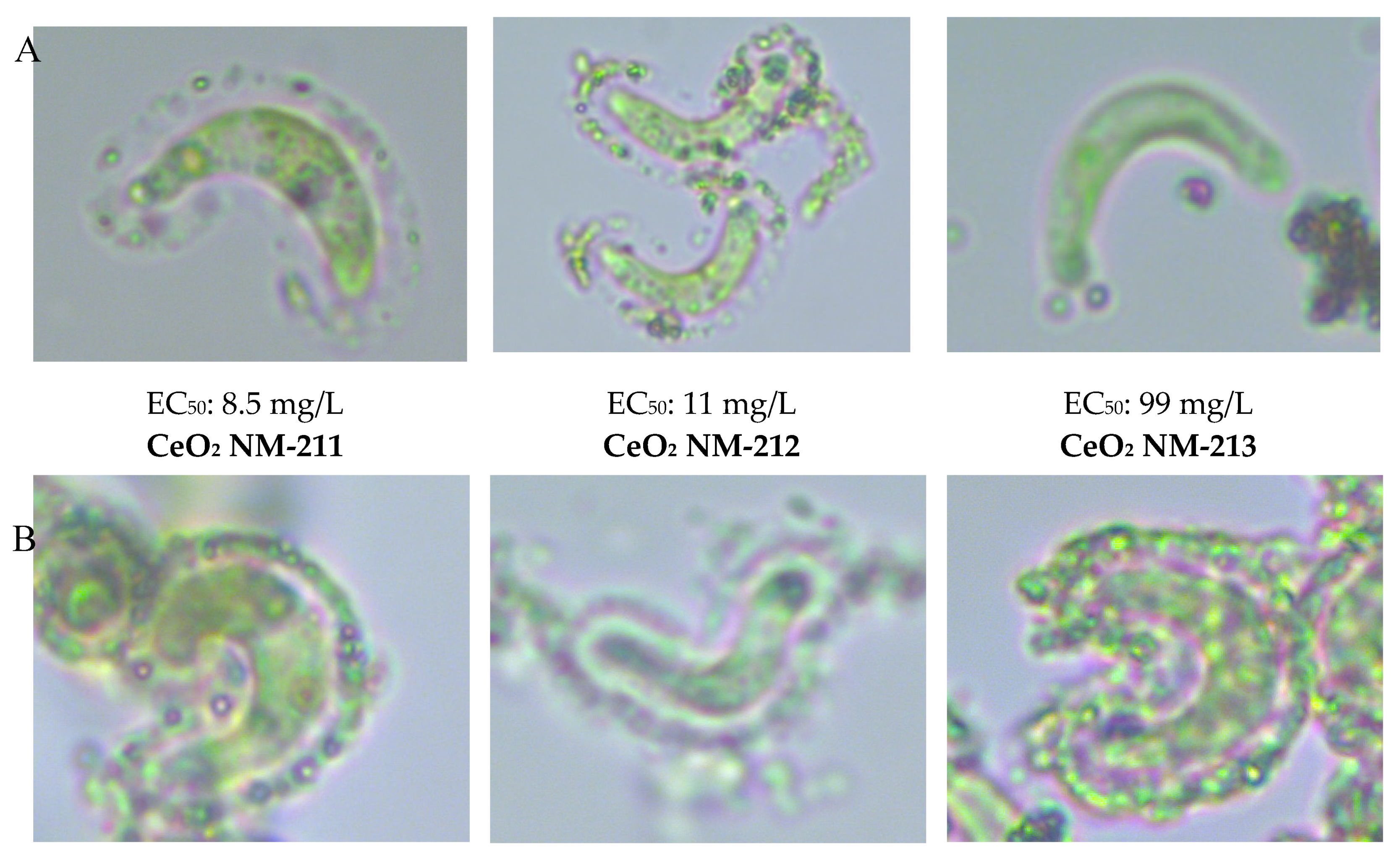
3.1. Attachment of CeO2
3.1.1. Light Microscopy
3.1.2. SEM-EDX Analysis
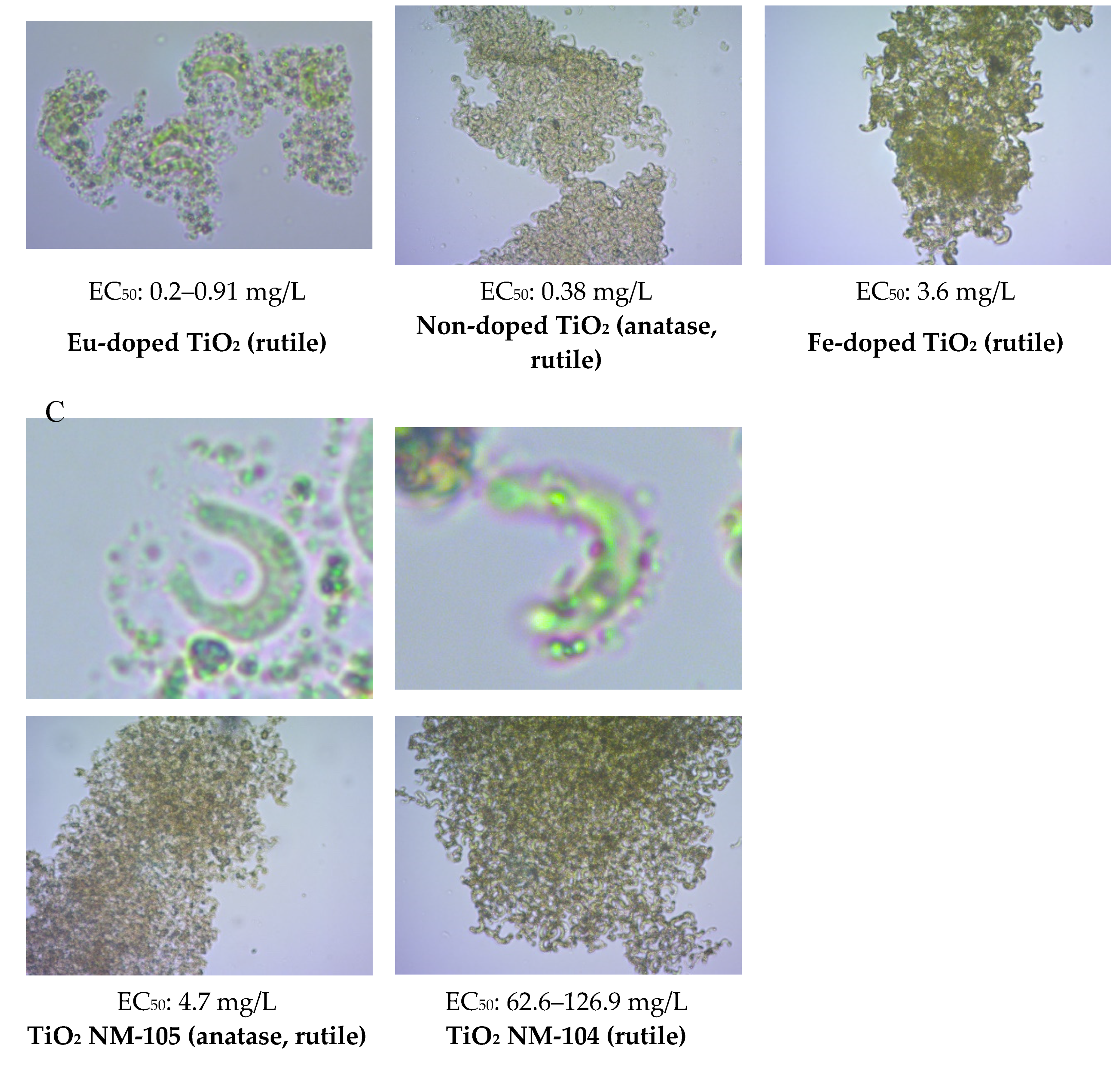
3.2. Attachment of TiO2
3.3. Growth Inhibition Tests
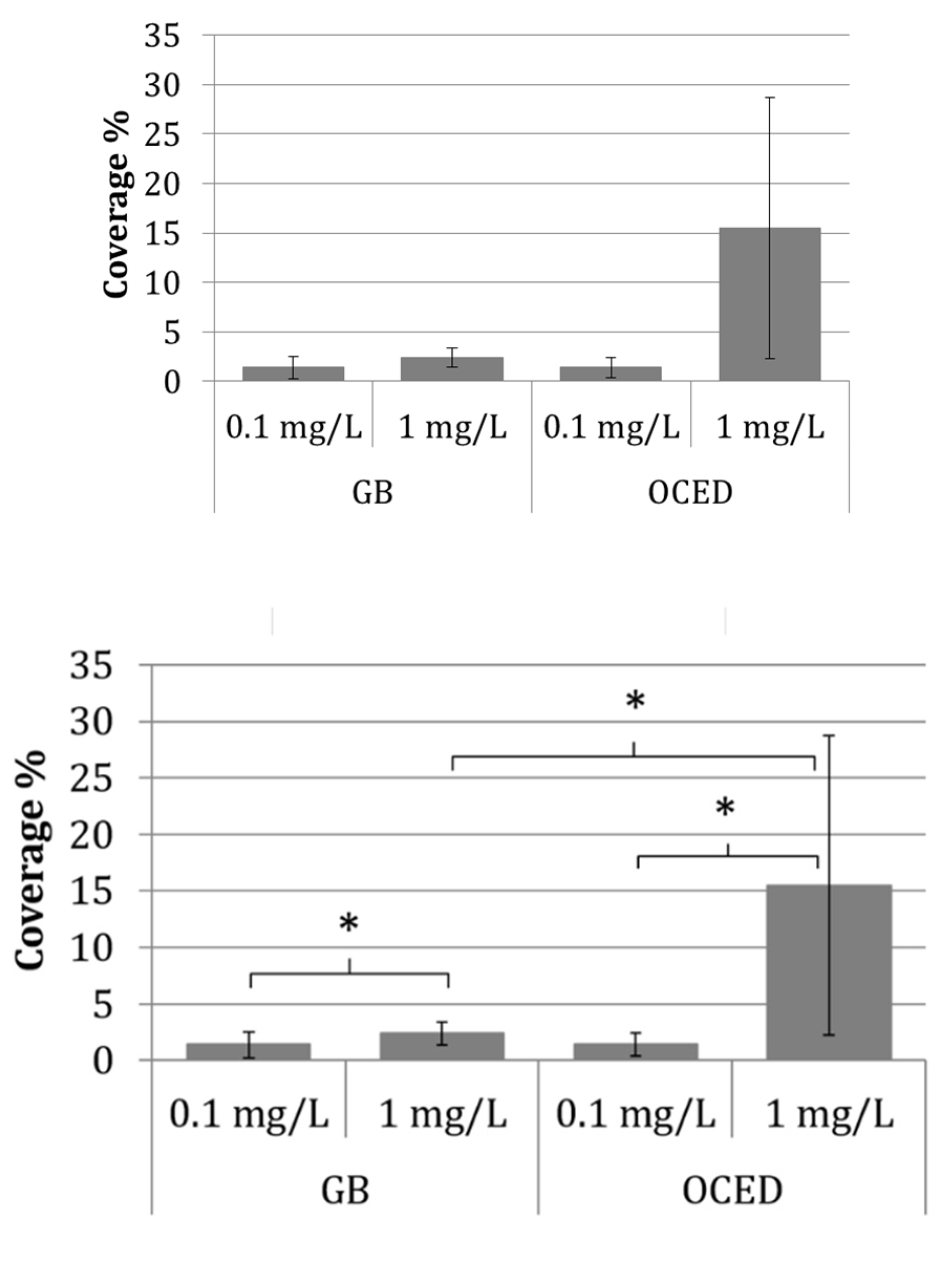
3.4. Relationship between Attachment Behavior and Ecotoxicity
4. Discussion
4.1. Attachment Behavior
4.2. Read-Across and Grouping Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ECHA. Guidance on Information Requirements and Chemical Safety Assessment—Appendix R.6.1 for Nanomaterials Applicable to the Guidance on QSARs and Grouping of Chemicals; ECHA: Helsinki, Finland, 2017. [Google Scholar]
- Arts, J.H.E.; Irfan, M.-A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Neubauer, N.; Petry, T.; Sauer, U.G.; et al. Case studies putting the decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) into practice. Regul. Toxicol. Pharmacol. 2016, 76, 234–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oomen, G.A.; Bleeker, A.E.; Bos, M.P.; Van Broekhuizen, F.; Gottardo, S.; Groenewold, M.; Hristozov, D.; Hund-Rinke, K.; Irfan, M.-A.; Marcomini, A.; et al. Grouping and Read-Across Approaches for Risk Assessment of Nanomaterials. Int. J. Environ. Res. Public Health 2015, 12, 13415–13434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hund-Rinke, K.; Schlich, K.; Kühnel, D.; Hellack, B.; Kaminski, H.; Nickel, C. Grouping concept for metal and metal oxide nanomaterials with regard to their ecotoxicological effects on algae, daphnids and fish embryos. NanoImpact 2018, 9, 52–60. [Google Scholar] [CrossRef]
- Kuehnel, D.; Nickel, C.; Zalm, E.V.D.; Kussatz, C.; Herrchen, M.; Meisterjahn, B.; Hund-Rinke, K. Closing gaps for environmental risk screening of engineered nanomaterials. NanoIMPACT 2019, 15, 100173. [Google Scholar] [CrossRef]
- Metzler, D.M.; Li, M.H.; Erdem, A.; Huang, C.P. Responses of algae to photocatalytic nano-TiO2 particles with an emphasis on the effect of particle size. Chem. Eng. J. 2011, 170, 538–546. [Google Scholar] [CrossRef]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef]
- Ozkaleli, M.; Erdem, A. Biotoxicity of TiO2 Nanoparticles on Raphidocelis subcapitata Microalgae Exemplified by Membrane Deformation. Int. J. Environ. Res. Public Health 2018, 15, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Zhou, L.; Liu, Y.; Deng, S.; Wu, H.; Wang, G. Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii. Ecotoxicol. Environ. Saf. 2012, 84, 155–162. [Google Scholar] [CrossRef]
- Rasmussen, K.; Mast, J.; De Temmerman, P.-J.; Verleysen, E.; Waegeneers, N.; van Steen, F.; Pizzolon, J.; de Temmerman, L.; Van Doren, E.; Jensen, K.A.; et al. Titanium Dioxide, NM-100, NM-101, NM-102, NM-103, NM-104, NM-105: Characterisation and Physico-Chemical Properties; European Commission, Joint Research Centre, Insitute for Health and Consumer Production: Ispra, Italy, 2014. [Google Scholar]
- Singh, C.; Friedrichs, S.; Ceccone, G.; Gibson, N.; Jensen, K.A.; Levin, M.; Infante, H.G.; Carlender, D.; Rasmussen, K. Cerium Dioxide, NM-211, NM-212, NM-213. Characterisation and Test item Preparation. JRC Repository: NM-Series of Representative Manufactured Nanomaterials; Publications Office of the European Union: Ispra, Italy, 2014. [Google Scholar]
- OECD Guideline 201. OECD Guidelines for the Testing of Chemicals. Test Guideline 201: Freshwater Alga and Cyanobacterial, Growth Inhibition Test; Organisation for Economic Co-operation and Development: Paris, France, 2011; Available online: http://www.oecd-ilibrary.org/docserver/download/9720101e.pdf?expires=1497009075&id=id&accname=guest&checksum=B05A403DAB471E46DEDD582972FEDABE (accessed on 20 March 2018).
- Shi, T.; Schins, R.P.F.; Knaapen, A.M.; Kuhlbusch, T.; Pitz, M.; Heinrich, J.; Borm, P.J. Hydroxyl radical generation by electron paramagnetic resonance as a new method to monitor ambient particulate matter composition. J. Environ. Monit. 2003, 5, 550–556. [Google Scholar] [CrossRef]
- Lipovsky, A.; Tzitrinovich, Z.; Friedmann, H.; Applerot, G.; Gedanken, A.; Lubart, R. EPR study of visible light-induced ROS generation by nanoparticles of ZnO. J. Phys. Chem. C 2009, 113, 15997–16001. [Google Scholar] [CrossRef]
- Hund-Rinke, K.; Baun, A.; Cupi, D.; Fernandes, T.F.; Handy, R.; Kinross, J.H.; Navas, J.M.; Peijnenburg, W.; Schlich, K.; Shaw, B.J.; et al. Regulatory ecotoxicity testing of nanomaterials—Proposed modifications of OECD test guidelines based on laboratory experience with silver and titanium dioxide nanoparticles. Nanotoxicology 2016, 10, 1442–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faust, M.R.; Altenburger, R.; Bödeker, W.; Grimme, L. Algentoxizitätstest mit synchronisierten kulturen. Schriftenreihe Ver. Wasser Boden Lufthyg. 1992, 89, 311–329. [Google Scholar]
- Rohde, F. Entwicklung eines Nichtlinearen Algorithmus zur Automatischen Elastischen Registrierung Kor-Relativer Mikroskopiedaten. Master’s Thesis, Leipzig University of Applied Sciences, Leipzig, Germany, 2018. [Google Scholar]
- Rohde, F.; Braumann, U.-D.; Schmidt, M. Correlia: An ImageJ plug-in to register and visualise multi-modal correlative micrographs. in preparation. In Proceedings of the Berlin Microscope Conference, Berlin, Germany, 1–5 September 2019. [Google Scholar]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Maršálek, B.; Rojíčková, R. Stress Factors Enhancing Production of Algal Exudates: A Potential Self-Protective Mechanism? Z. Naturforsch 1996, 51, 646–650. [Google Scholar] [CrossRef]
- Maršálek, B.; Zahradníčková, H.; Hronková, M. Extracellular Production of Abscisic Acid by Soil Algae under Salt, Acid or Drought Stress. Z. Naturforsch 1992, 47, 701–704. [Google Scholar] [CrossRef]
- Iswarya, V.; Sharma, V.; Chandrasekaran, N.; Mukherjee, A. Impact of tetracycline on the toxic effects of titanium dioxide (TiO2) nanoparticles towards the freshwater algal species, Scenedesmus obliquus. Aquat. Toxicol. 2017, 193, 168–177. [Google Scholar] [CrossRef]
- Miao, A.J.; Schwehr, K.A.; Xu, C.; Zhang, S.J.; Luo, Z.P.; Quigg, A.; Santschi, P.H. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ. Pollut. 2009, 157, 3034–3041. [Google Scholar] [CrossRef]
- Morelli, E.; Gabellieri, E.; Bonomini, A.; Tognotti, D.; Grassi, G.; Corsi, I. TiO2 nanoparticles in seawater: Aggregation and interactions with the green alga Dunaliella tertiolecta. Ecotoxicol. Environ. Saf. 2018, 148, 184–193. [Google Scholar] [CrossRef]
- Wang, C.Q.; Dong, D.M.; Zhang, L.W.; Song, Z.W.; Hua, X.Y.; Guo, Z.Y. Response of Freshwater Biofilms to Antibiotic Florfenicol and Ofloxacin Stress: Role of Extracellular Polymeric Substances. Int. J. Environ. Res. Public Health 2019, 16, 715. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.F.; Liu, S.X.; Liu, N.; Zhang, H.; Zhou, Q.Z.; Ge, F. Accelerated productions and physicochemical characterizations of different extracellular polymeric substances from Chiorella vulgaris with nano-ZnO. Sci. Total Environ. 2019, 658, 582–589. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Khairy, H.M.; El-Shenody, R. Algal production of extra and intra-cellular polysaccharides as an adaptive response to the toxin crude extract of Microcystis aeruginosa. Iran. J. Environ. Health Sci. Eng. 2012, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Naveed, S.; Li, C.; Lu, X.; Chen, S.; Yin, B.; Zhang, C.; Ge, Y. Microalgal extracellular polymeric substances and their interactions with metal(loid)s: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1769–1802. [Google Scholar] [CrossRef]
- Zhou, K.; Hu, Y.; Zhang, L.; Yang, K.; Lin, D. The role of exopolymeric substances in the bioaccumulation and toxicity of Ag nanoparticles to algae. Sci. Rep. 2016, 6, 32998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.; Matzke, M.; Kroll, A.; Read, D.S.; Svendsen, C.; Crossley, A. Toxic interactions of different silver forms with freshwater green algae and cyanobacteria and their effects on mechanistic endpoints and the production of extracellular polymeric substances. Environ. Sci.-Nano 2016, 3, 396–408. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, J.; Wang, P.; Wang, C.; Wang, X.; You, G. Influence of extracellular polymeric substances on cell-NPs heteroaggregation process and toxicity of cerium dioxide NPs to Microcystis aeruginosa. Environ. Pollut. 2018, 242, 1206–1216. [Google Scholar] [CrossRef]
- Chiu, M.H.; Khan, Z.A.; Garcia, S.G.; Le, A.D.; Kagiri, A.; Ramos, J.; Tsai, S.M.; Drobenaire, H.W.; Santschi, P.H.; Quigg, A.; et al. Effect of Engineered Nanoparticles on Exopolymeric Substances Release from Marine Phytoplankton. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, S.; Lakshmi, D.S.; Bhuvaneshwari, M.; Iswarya, V.; Mrudula, P.; Chandrasekaran, N.; Mukherjee, A. Antifouling activities of pristine and nanocomposite chitosan/TiO2/Ag films against freshwater algae. RSC Adv. 2017, 7, 27645–27655. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, K.; Rauscher, H.; Kearns, P.; González, M.; Riego Sintes, J. Developing OECD test guidelines for regulatory testing of nanomaterials to ensure mutual acceptance of test data. Regul. Toxicol. Pharmacol. 2019, 104, 74–83. [Google Scholar] [CrossRef]
- OECD. Draft-Guidance Document on Aquatic and Sediment Toxicological Testing of Nanomaterials; OECD: Paris, France, 2019. [Google Scholar]
- Environmental Protection Agency of United States. Technical Overview of Ecological Risk Assessment—Analysis Phase: Ecological Effects Characterization. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/technical-overview-ecological-risk-assessment-0#Ecotox (accessed on 12 December 2019).
- Li, K.; Qian, J.; Wang, P.; Wang, C.; Fan, X.; Lu, B.; Tian, X.; Jin, W.; He, X.; Guo, W. Toxicity of Three Crystalline TiO2 Nanoparticles in Activated Sludge: Bacterial Cell Death Modes Differentially Weaken Sludge Dewaterability. Environ. Sci. Technol. 2019, 53, 4542–4555. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhou, K.; Zhang, L.; Yang, K.; Lin, D. Distinct effects of soluble and bound exopolymeric substances on algal bioaccumulation and toxicity of anatase and rutile TiO2 nanoparticles. Environ. Sci. Nano 2018, 5, 720–729. [Google Scholar] [CrossRef]
- ECHA. Guidance on Information Requirements and Chemical Safety Assessment Chapter R.6: QSARs and Grouping of Chemicals; ECHA: Helsinki, Finland, 2008; Available online: https://echa.europa.eu/documents/10162/13632/information_requirements_r6_en.pdf/77f49f81-b76d-40ab-8513-4f3a533b6ac9 (accessed on 7 June 2019).
- ECHA. Appendix for Nanoforms Applicable to the Guicance on Registration and Substance Identification; Draft (Public) Version 1.0.; ECHA: Helsinki, Finland, 2019. [Google Scholar]



| Nanomaterial | Primary Particle Size (SEM/TEM) (nm) | Surface Area (m2/g) 1 | Agglomerate Size–Z Average (nm) (DLS) 2 | Zeta-Potential (mV) 2 | Reactivity (DMPO) 2,3 | Crystalline Structure | Coating |
|---|---|---|---|---|---|---|---|
| CeO2 NM-211 | 4–15 | 66 | 442 ± 85 | −19.8 | 0.81 | Cubic cereonite | Uncoated |
| CeO2 NM-212 | 40 | 27 | 831 ± 209 | −20.4 | 0.96 | Cubic cereonite | Uncoated |
| CeO2 NM-213 | 35 | 4 | 1042 ± 178 | −25.9 | 1.1 | Cubic cereonite | Uncoated |
| TiO2 NM-104 | 30 | 60 | 1596 ± 498 | −0.9 | 1.1 UV activation: 1.6 | Rutile | Al2O3 (6%) coating and glycerol (1%) functionalization |
| TiO2 NM-105 | 21 | 51 | 1409 ± 533 | −2.4 | 1.0 UV activation: 20.8 | 14% Rutile 86% anatase | Uncoated |
| TiO2 Eu-doped | 19 (BET) | 148 | 1612 ± 384 | −23.1 | 0.7 UV activation: 1.4 | Mainly rutile | Uncoated |
| TiO2 Fe-doped | 10 (BET) | 63 | 1866 ± 106 | −21.3 | 1.0 UV activation: 1.4 | Mainly rutile | Uncoated |
| TiO2 non-doped | 15 | 78 | 743 ± 859 | −22 | 0.7 UV activation: 1.5 | 9% Rutile, 91% anatase | Uncoated |
| GB Medium (µmol/L) | OECD Medium (µmol/L) | |
|---|---|---|
| KNO3 | 8000 | – |
| NaCl | 8000 | – |
| MgSO4 * 7 H2O | 1000 | 60.9 |
| Na2HPO4 * 2 H2O | 1000 | – |
| NaH2PO4 * H2O | 3000 | – |
| CaCl2 * 2 H2O | 100 | 122 |
| MnCl2 * 4 H2O | 2.5 | 2.1 |
| H3BO3 | 8 | 2.99 |
| ZnSO4 * 7 H2O | 0.7 | – |
| Na2MoO4 * 2 H2O | 0.016 | 0.0289 |
| FeEDTA 1 | 25 | – |
| NH4Cl | – | 280 |
| KH2PO4 | – | 9.19 |
| MgCl2 * 6 H2O | – | 59 |
| ZnCl2 | – | 0.022 |
| CoCl2 * 6 H2O | – | 0.0063 |
| CuCl2 * 2 H2O | – | 0.00006 |
| Na2EDTA * 2 H2O | – | 0.269 |
| FeCl3 * 6 H2O | – | 0.237 |
| Ionic strength | 24.349 | 1.602 |
| Light Microscopy | Electron Microscopy |
|---|---|
| Time and cost efficient | Time and cost intensive |
| No fixation and preparation | Fixation and preparation of samples may lead to artifacts (e.g., particles may be washed off) |
| Qualitative | Quantification of attachment possible |
| No identification of elements (may lead to artifacts) | Element identification ensures particle identity |
| Lower resolution compared to electron microscopy | Lower concentrations of particles and smaller particles / agglomerates can be detected |
| Nanomaterials | EC50 (mg/L) | EC50 (mg/L) Published Data 2 | EC50 (mg/L) 5 GB |
|---|---|---|---|
| CeO2-NM-211 | Not performed | 8.5 [7.7–9.3] | Not performed |
| CeO2 NM-212 | 10.9 [9.9–11.9] 4 1.8 [n.d.] 5 | 5.6 [3.0–10.4] | >100 |
| CeO2 NM-213 | 98.7 [96.5–101.4] 4 | 43.8 [n.d.] 3 | Not performed |
| TiO2 NM-104 | 126.9 [95.0 ± 190.4] 4 | 62.6 [42.6–106] | Not performed |
| TiO2 NM-105 | Not performed | 4.7 [3.5–5.5] | Not performed |
| TiO2 Eu-doped | 0.36 [0.34 ± 0.38] 4 0.29 [n.d.] 5 | 0.91 [0.75–1.10] | >100 |
| TiO2 Fe-doped | Not performed | 3.6 [2.6–4.8] | Not performed |
| TiO2 Non-doped | 0.06 [n.d.] 5 | 0.38 [0.33–0.43] | >100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hund-Rinke, K.; Sinram, T.; Schlich, K.; Nickel, C.; Dickehut, H.P.; Schmidt, M.; Kühnel, D. Attachment Efficiency of Nanomaterials to Algae as an Important Criterion for Ecotoxicity and Grouping. Nanomaterials 2020, 10, 1021. https://doi.org/10.3390/nano10061021
Hund-Rinke K, Sinram T, Schlich K, Nickel C, Dickehut HP, Schmidt M, Kühnel D. Attachment Efficiency of Nanomaterials to Algae as an Important Criterion for Ecotoxicity and Grouping. Nanomaterials. 2020; 10(6):1021. https://doi.org/10.3390/nano10061021
Chicago/Turabian StyleHund-Rinke, Kerstin, Tim Sinram, Karsten Schlich, Carmen Nickel, Hanna Paula Dickehut, Matthias Schmidt, and Dana Kühnel. 2020. "Attachment Efficiency of Nanomaterials to Algae as an Important Criterion for Ecotoxicity and Grouping" Nanomaterials 10, no. 6: 1021. https://doi.org/10.3390/nano10061021





