Dopant-Free Triazatruxene-Based Hole Transporting Materials with Three Different End-Capped Acceptor Units for Perovskite Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
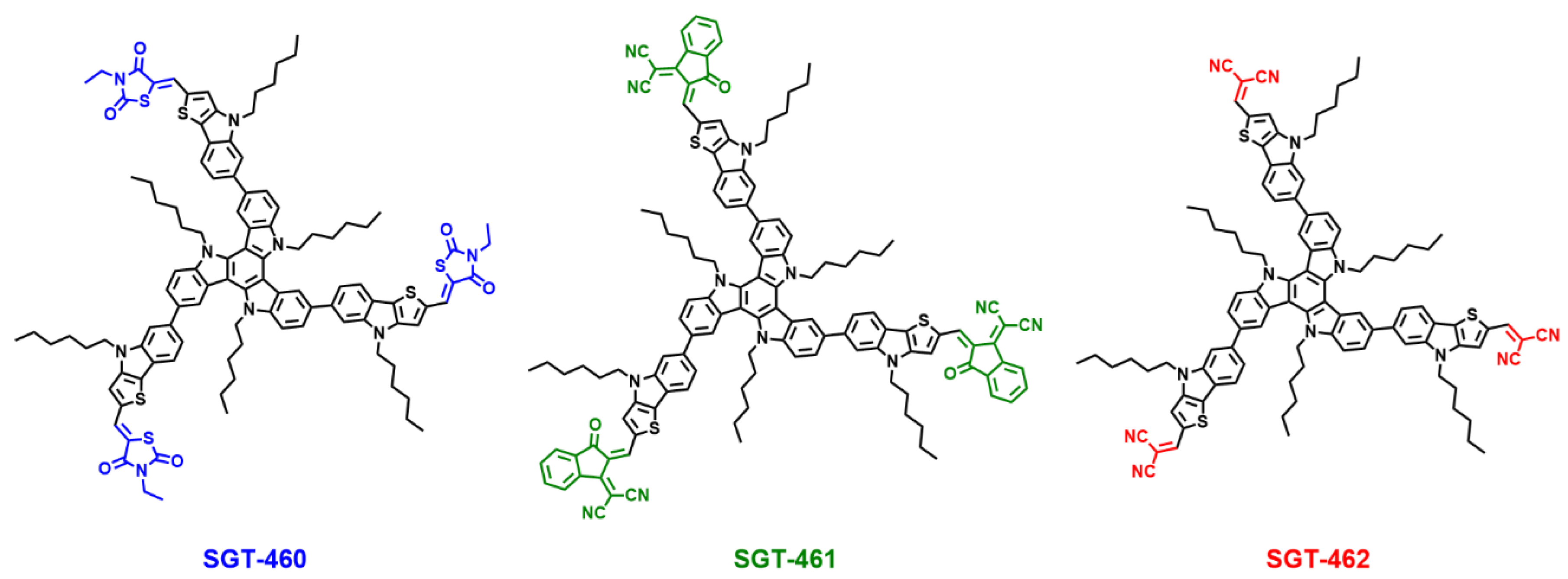
2.1. Synthesis
2.2. Photophysical and Electrochemical Properties
2.3. Thermal Analysis
2.4. Hole Mobility Analysis
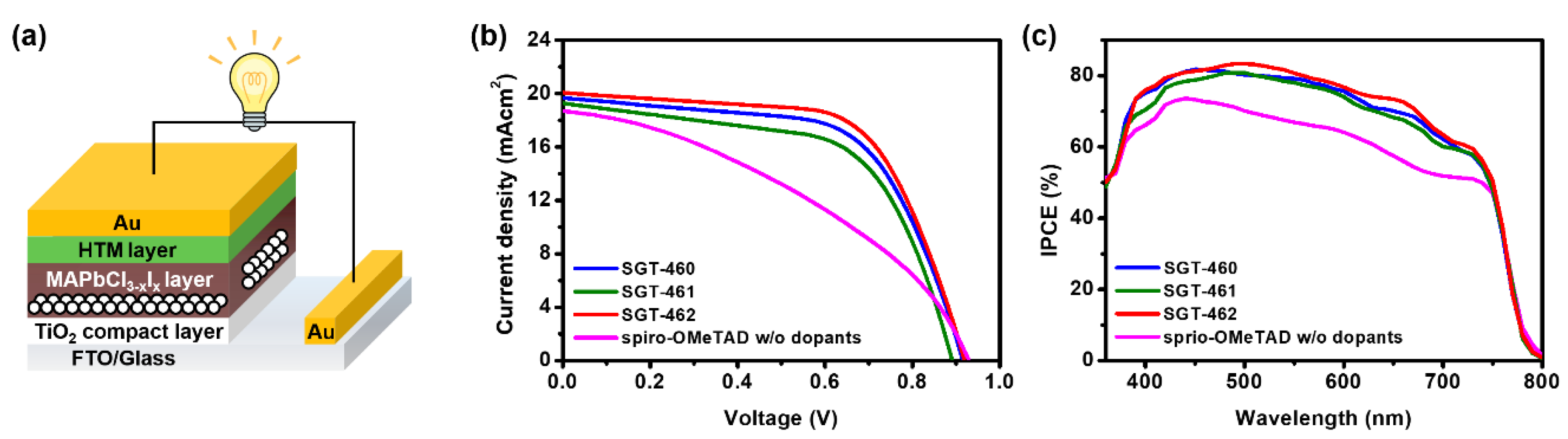
2.5. Photovoltaic Properties
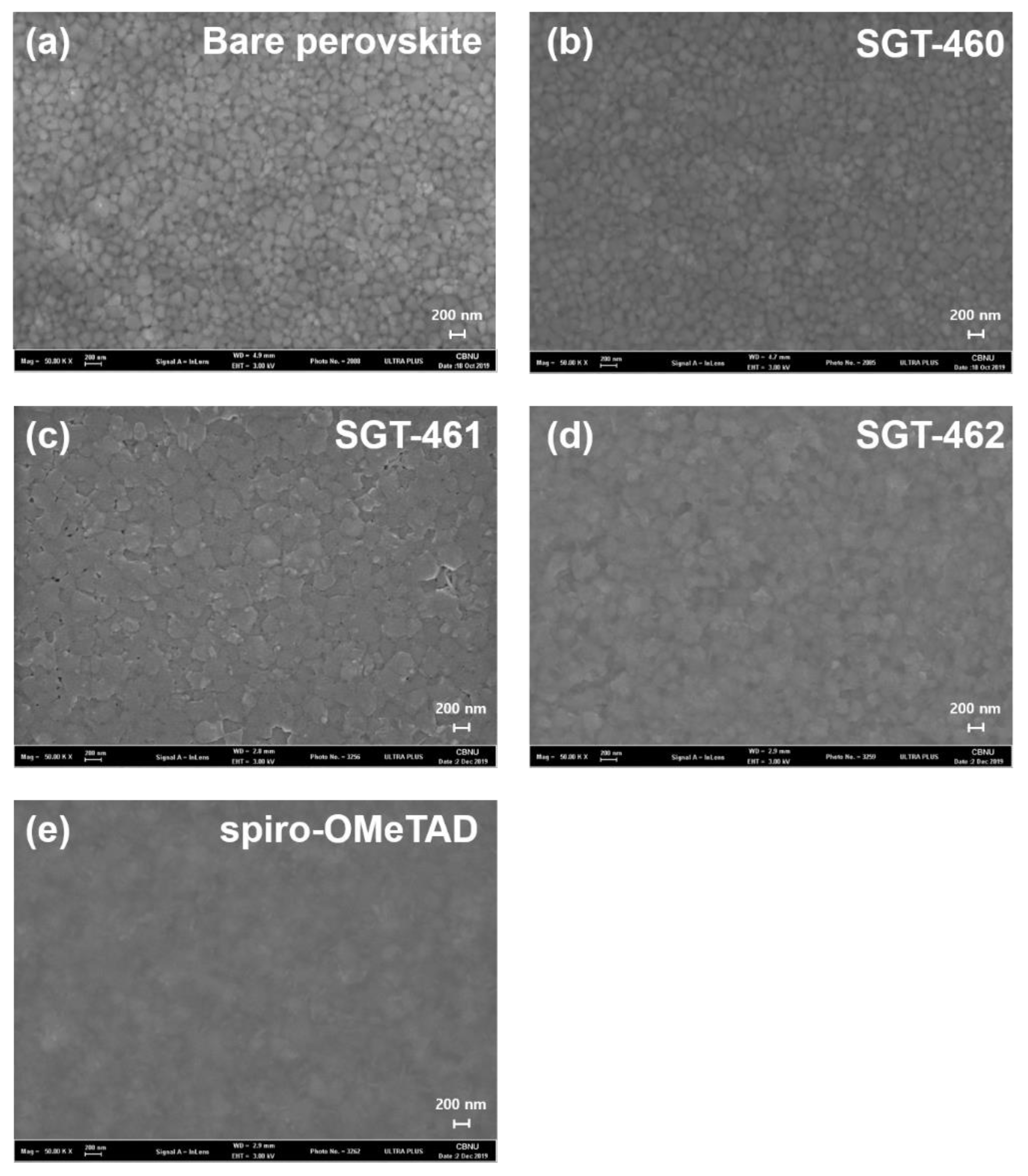
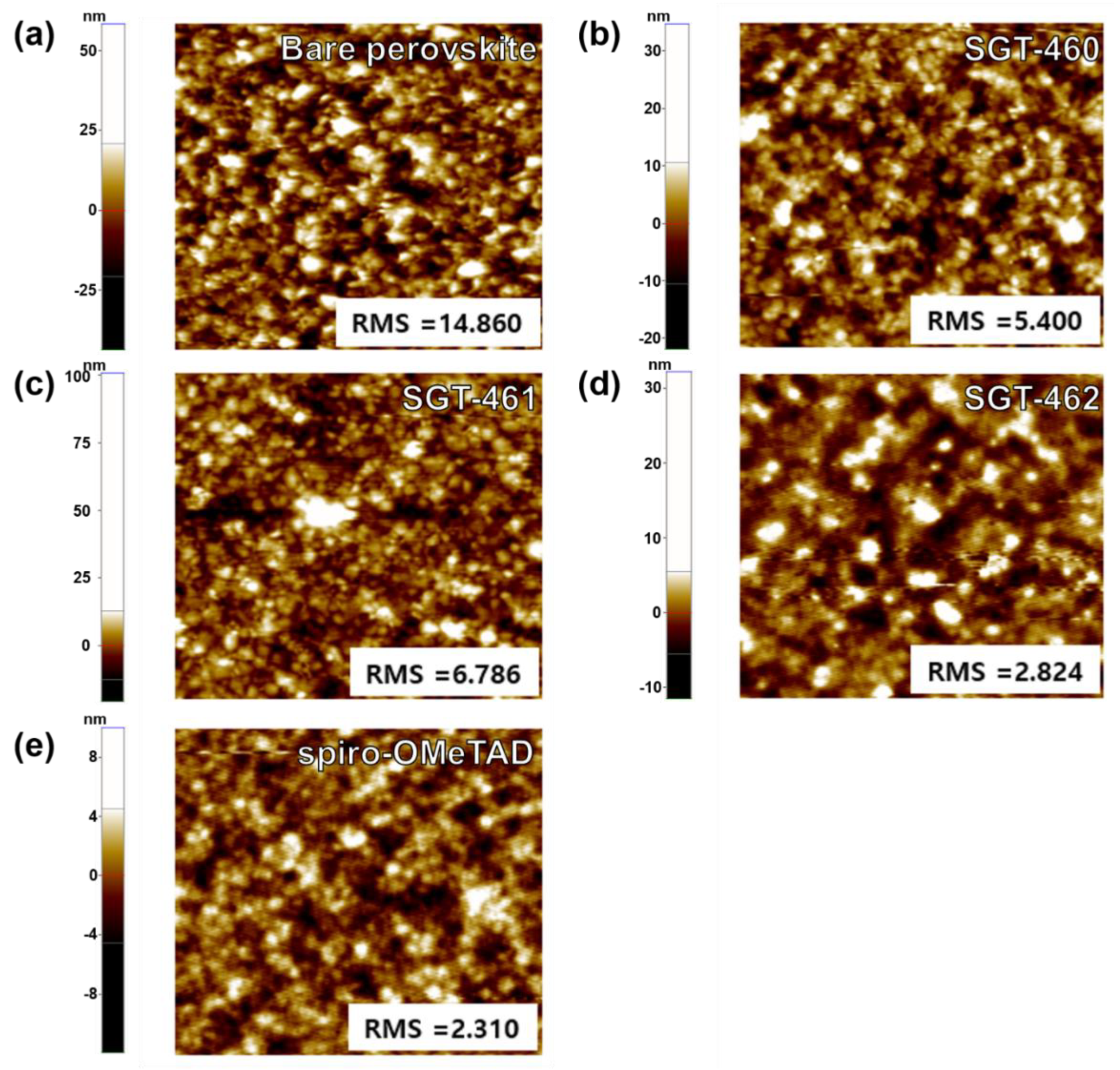
2.6. Surface Morphology Observation of HTM Films
2.7. Time-Resolved Photoluminescence Measurements
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Jung, M.; Ji, S.-G.; Kim, G.; Seok, S.I. Perovskite precursor solution chemistry: From fundamentals to photovoltaic applications. Chem. Soc. Rev. 2019, 48, 2011–2038. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.-K.; Xue, J.; Yang, Y. A review of perovskites solar cell stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Seok, S.I.; Grätzel, M.; Park, N.-G. Methodologies toward highly efficient perovskite solar cells. Small 2018, 14, 1704177. [Google Scholar] [CrossRef]
- NREL Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 6 April 2020).
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Yin, W.-J.; Yang, J.-H.; Kang, J.; Yan, Y.; Wei, S.-H. Halide perovskite materials for solar cells: A theoretical review. J. Mater. Chem. A 2015, 3, 8926–8942. [Google Scholar] [CrossRef]
- Calió, L.; Kazim, S.; Grätzel, M.; Ahmad, S. Hole-transport materials for perovskite solar cells. Angew. Chem. Int. Ed. 2016, 55, 14522–14545. [Google Scholar] [CrossRef]
- Hawash, Z.; Ono, L.K.; Qi, Y. Recent advances in spiro-MeOTAD hole transport material and its applications in organic–inorganic halide perovskite solar cells. Adv. Mater. Interfaces 2018, 5, 1700623. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Sun, L. Recent progress on hole-transporting materials for emerging organometal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500213. [Google Scholar] [CrossRef]
- Kang, S.H.; Lu, C.; Zhou, H.; Choi, S.; Kim, J.; Kim, H.K. Novel π-extended porphyrin-based hole-transporting materials with triarylamine donor units for high performance perovskite solar cells. Dyes Pigment. 2019, 163, 734–739. [Google Scholar] [CrossRef]
- Urieta-Mora, J.; García-Benito, I.; Molina-Ontoria, A.; Martín, N. Hole transporting materials for perovskite solar cells: A chemical approach. Chem. Soc. Rev. 2018, 47, 8541–8571. [Google Scholar] [CrossRef]
- Rakstys, K.; Igci, C.; Nazeeruddin, M.K. Efficiency vs. stability: Dopant-free hole transporting materials towards stabilized perovskite solar cells. Chem. Sci. 2019, 10, 6748–6769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Choi, I.T.; Kim, J.; Kim, H.K. Simple synthesis and molecular engineering of low-cost and star-shaped carbazole-based hole transporting materials for highly efficient perovskite solar cells. J. Mater. Chem. A 2017, 5, 20263–20276. [Google Scholar] [CrossRef]
- Kang, M.S.; Sung, S.D.; Choi, I.T.; Kim, H.; Hong, M.; Kim, J.; Lee, W.I.; Kim, H.K. Novel carbazole-based hole-transporting materials with star-shaped chemical structures for perovskite-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 22213–22217. [Google Scholar] [CrossRef]
- Pham, H.D.; Jain, S.M.; Li, M.; Wang, Z.-K.; Manzhos, S.; Feron, K.; Pitchaimuthu, S.; Liu, Z.; Motta, N.; Durrant, J.R.; et al. All-rounder low-cost dopant-free D-A-D hole-transporting materials for efficient indoor and outdoor performance of perovskite solar cells. Adv. Electron. Mater. 2020, 6, 1900884. [Google Scholar] [CrossRef]
- Oh, S.; Khan, N.; Jin, S.-M.; Tran, H.; Yoon, N.; Song, C.E.; Lee, H.K.; Shin, W.S.; Lee, J.-C.; Moon, S.-J.; et al. Alkyl side-chain dependent self-organization of small molecule and its application in high-performance organic and perovskite solar cells. Nano Energy 2020, 72, 104708. [Google Scholar] [CrossRef]
- Kazim, S.; Ramos, F.J.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M.; Ahmad, S. A dopant free linear acene derivative as a hole transport material for perovskite pigmented solar cells. Energy Environ. Sci. 2015, 8, 1816–1823. [Google Scholar] [CrossRef]
- Lu, C.; Paramasivam, M.; Park, K.; Kim, C.H.; Kim, H.K. Phenothiazine functionalized multifunctional A−π–D−π–D−π–A-type hole-transporting materials via sequential C–H arylation approach for efficient and stable perovskite solar cells. ACS Appl. Mater. Interfaces 2019, 11, 14011–14022. [Google Scholar] [CrossRef]
- Azmi, R.; Nam, S.Y.; Sinaga, S.; Akbar, Z.A.; Lee, C.-L.; Yoon, S.C.; Jung, I.H.; Jang, S.-Y. High-performance dopant-free conjugated small molecule-based hole-transport materials for perovskite solar cells. Nano Energy 2018, 44, 191–198. [Google Scholar] [CrossRef]
- Lin, Y.-D.; Abate, S.Y.; Chung, H.-C.; Liau, K.-L.; Tao, Y.-T.; Chow, T.J.; Sun, S.-S. Donor–acceptor–donor type cyclopenta[2,1-b;3,4-b′]dithiophene derivatives as a new class of hole transporting materials for highly efficient and stable perovskite solar cells. ACS Appl. Energy Mater. 2019, 2, 7070–7082. [Google Scholar] [CrossRef]
- Rakstys, K.; Paek, S.; Gao, P.; Gratia, P.; Marszalek, T.; Grancini, G.; Cho, K.T.; Genevicius, K.; Jankauskas, V.; Pisula, W.; et al. Molecular engineering of face-on oriented dopant-free hole transporting material for perovskite solar cells with 19% PCE. J. Mater. Chem. A 2017, 5, 7811–7815. [Google Scholar] [CrossRef]
- Yang, X.; Xi, J.; Sun, Y.; Zhang, Y.; Zhou, G.; Wong, W.-Y. A dopant-free twisted organic small-molecule hole transport material for inverted planar perovskite solar cells with enhanced efficiency and operational stability. Nano Energy 2019, 64, 103946. [Google Scholar] [CrossRef]
- Zhang, F.; Yi, C.; Wei, P.; Bi, X.; Luo, J.; Jacopin, G.; Wang, S.; Li, X.; Xiao, Y.; Zakeeruddin, S.M.; et al. A novel dopant-free triphenylamine based molecular “Butterfly” hole-transport material for highly efficient and stable perovskite solar cells. Adv. Energy Mater. 2016, 6, 1600401. [Google Scholar] [CrossRef]
- Pham, H.D.; Jain, S.M.; Li, M.; Manzhos, S.; Feron, K.; Pitchaimuthu, S.; Liu, Z.; Motta, N.; Wang, H.; Durrant, J.R.; et al. Dopant-free novel hole-transporting materials based on quinacridone dye for high-performance and humidity-stable mesoporous perovskite solar cells. J. Mater. Chem. A 2019, 7, 5315–5323. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhao, X.; Yi, C.; Bi, D.; Bi, X.; Wei, P.; Liu, X.; Wang, S.; Li, X.; Zakeeruddin, S.M.; et al. Dopant-free star-shaped hole-transport materials for efficient and stable perovskite solar cells. Dyes Pigm. 2017, 136, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, L.J.; Huang, P.; Zhou, Y.; Zhu, Y.Y.; Yuan, N.Y.; Ding, J.N.; Zhang, Z.G.; Li, Y.F. A simple and dopant-free hole-transporting material based on (2-ethylhexyl)-9H-carbazole for efficient planar perovskite solar cells. J. Mater. Chem. C 2017, 5, 12752–12757. [Google Scholar] [CrossRef]
- Wu, J.; Liu, C.; Hu, M.; Deng, X.; Tan, W.; Tian, Y.; Xu, B. Polystyrene with a methoxytriphenylamine-conjugated-thiophene moiety side-chain as a dopant-free hole-transporting material for perovskite solar cells. J. Mater. Chem. A 2018, 6, 13123–13132. [Google Scholar] [CrossRef]
- Li, H.; Fu, K.; Hagfeldt, A.; Grätzel, M.; Mhaisalkar, S.G.; Grimsdale, A.C. A Simple 3,4-ethylenedioxythiophene based hole-transporting material for perovskite solar cells. Angew. Chem. Int. Ed. 2014, 53, 4085–4088. [Google Scholar] [CrossRef]
- Cui, B.-B.; Yang, N.; Shi, C.; Yang, S.; Shao, J.-Y.; Han, Y.; Zhang, L.; Zhang, Q.; Zhong, Y.-W.; Chen, Q. Naphtho[1,2-b:4,3-b′]dithiophene-based hole transporting materials for high-performance perovskite solar cells: Molecular engineering and opto-electronic properties. J. Mater. Chem. A 2018, 6, 10057–10063. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, J.; Hua, Y.; Liu, P.; Wang, L.; Ruan, C.; Li, Y.; Boschloo, G.; Johansson, E.M.J.; Kloo, L.; et al. Tailor-Making Low-Cost Spiro[fluorene-9,9′-xanthene]-Based 3D Oligomers for Perovskite Solar Cells. Chem 2017, 2, 676–687. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.; Thakur, U.K.; Lee, D.; Wenping, Y.; Kim, D.; Lee, S.; Ahn, T.K.; Park, H.J.; Kim, B.G. N-phenylindole-diketopyrrolopyrrole-containing narrow band-gap materials for dopant-free hole transporting layer of perovskite solar cell. Org. Electron. 2016, 37, 134–140. [Google Scholar] [CrossRef]
- Wang, R.; Qiao, J.; He, B.; Tang, X.; Wu, F.; Zhu, L. Electron extraction layer based on diketopyrrolopyrrole/isoindigo to improve the efficiency of inverted perovskite solar cells. J. Mater. Chem. C 2018, 6, 8429–8434. [Google Scholar] [CrossRef]
- Wu, F.; Ji, Y.; Wang, R.; Shan, Y.; Zhu, L. Molecular engineering to enhance perovskite solar cell performance: Incorporation of benzothiadiazole as core unit for low cost hole transport materials. Dyes Pigment. 2017, 143, 356–360. [Google Scholar] [CrossRef]
- Wu, F.; Ji, Y.; Zhong, C.; Liu, Y.; Tan, L.; Zhu, L. Fluorine-substituted benzothiadiazole-based hole transport materials for highly efficient planar perovskite solar cells with a FF exceeding 80%. Chem. Commun. 2017, 53, 8719–8722. [Google Scholar] [CrossRef]
- Rakstys, K.; Abate, A.; Dar, M.I.; Gao, P.; Jankauskas, V.; Jacopin, G.; Kamarauskas, E.; Kazim, S.; Ahmad, S.; Grätzel, M.; et al. Triazatruxene-based hole transporting materials for highly efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 16172–16178. [Google Scholar] [CrossRef]
- Eom, Y.K.; Kang, S.H.; Choi, I.T.; Yoo, Y.; Kim, J.; Kim, H.K. Significant light absorption enhancement by a single heterocyclic unit change in the π-bridge moiety from thieno[3,2-b]benzothiophene to thieno[3,2-b]indole for high performance dye-sensitized and tandem solar cells. J. Mater. Chem. A 2017, 5, 2297–2308. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, Z.; Chen, Q.; Chen, H.; Chang, W.H.; Yang, Y.; Song, T.B.; Yang, Y. Perovskite solar cells employing dopant-free organic hole transport materials with tunable energy levels. Adv. Mater. 2016, 28, 440–446. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; You, J.; Zhang, Y.; Li, X. Solution-processed thermally stable amorphous films of small molecular hole injection/transport bi-functional materials and their application in high efficiency OLEDs. J. Mater. Chem. C 2015, 3, 11377–11384. [Google Scholar] [CrossRef]
- Malinauskas, T.; Tomkute-Luksiene, D.; Sens, R.; Daskeviciene, M.; Send, R.; Wonneberger, H.; Jankauskas, V.; Bruder, I.; Getautis, V. Enhancing thermal stability and lifetime of solid-state dye-sensitized solar cells via molecular engineering of the hole-transporting material spiro-OMeTAD. ACS Appl. Mater. Interfaces 2015, 7, 11107–11116. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fu, W.; Li, C.-Z.; Zhang, Z.; Qiu, W.; Shi, M.; Heremans, P.; Jen, A.K.Y.; Chen, H. Dopant-free hole-transporting material with a C3h symmetrical truxene core for highly efficient perovskite solar cells. J. Am. Chem. Soc. 2016, 138, 2528–2531. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ji, L.; Liu, R.; Zhang, C.; Mak, C.H.; Zou, X.; Shen, H.-H.; Leu, S.-Y.; Hsu, H.-Y. A review on morphology engineering for highly efficient and stable hybrid perovskite solar cells. J. Mater. Chem. A 2018, 6, 12842–12875. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, Y.; Lee, H.K.H.; Mu, C.; Zhang, T.; Zhang, L.; Wang, J.; Yan, H.; So, S.K.; Yang, S. Polyfluorene derivatives are high-performance organic hole-transporting materials for inorganic−organic hybrid perovskite solar cells. Adv. Funct. Mater. 2014, 24, 7357–7365. [Google Scholar] [CrossRef]
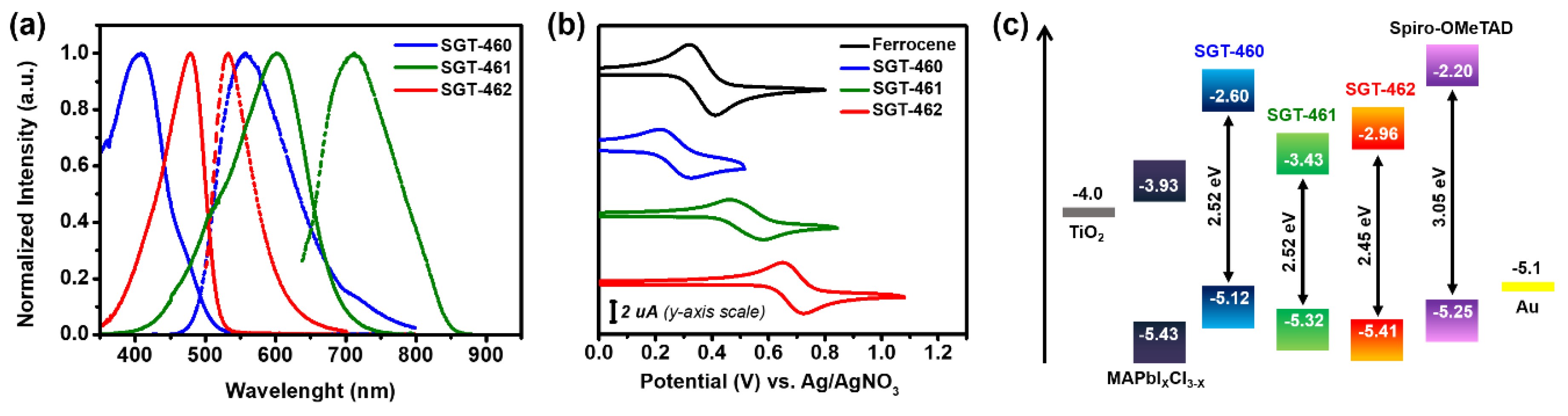

| HTM | λabs max (nm) | ε (M−1 cm−1) | λem max (nm) | Egopt 1 (eV) | Eox2 (V vs. NHE) | EHOMO3 (eV) | ELUMO4 (eV) | Hole Mobility 5 (cm2 V−1 s−1) |
|---|---|---|---|---|---|---|---|---|
| SGT-460 | 407 | 25390 | 557 | 2.52 | 0.512 | −5.12 | −2.60 | 7.59 × 10−5 |
| SGT-461 | 607 | 13244 | 713 | 1.89 | 0.816 | −5.32 | −3.43 | 5.13 × 10−4 |
| SGT-462 | 478 | 17424 | 532 | 2.45 | 0.910 | −5.41 | −3.03 | 7.61 × 10−4 |
| Spiro-OMeTAD 6 | 387 | 60530 | 418 | 3.05 | 0.751 | −5.25 | −2.20 | 7.59 × 10−5 |
| HTM | JSC (mA cm−2) | VOC (V) | FF (%) | PCE (%) 1 |
|---|---|---|---|---|
| SGT-460 | 19.57 ± 0.11 | 0.898 ± 3.02 | 61.5 ± 0.22 | 10.8 ± 0.07 |
| SGT-461 | 19.15 ± 0.12 | 0.887 ± 3.74 | 59.2 ± 0.27 | 10.1 ± 0.10 |
| SGT-462 | 20.01 ± 0.11 | 0.914 ± 4.83 | 63.2 ± 0.35 | 11.7 ± 0.06 |
| spiro-OMeTAD w/o dopants | 18.69 ± 0.14 | 0.929 ± 1.43 | 39.1 ± 0.1 | 6.8 ± 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kil, D.R.; Lu, C.; Ji, J.-M.; Kim, C.H.; Kim, H.K. Dopant-Free Triazatruxene-Based Hole Transporting Materials with Three Different End-Capped Acceptor Units for Perovskite Solar Cells. Nanomaterials 2020, 10, 936. https://doi.org/10.3390/nano10050936
Kil DR, Lu C, Ji J-M, Kim CH, Kim HK. Dopant-Free Triazatruxene-Based Hole Transporting Materials with Three Different End-Capped Acceptor Units for Perovskite Solar Cells. Nanomaterials. 2020; 10(5):936. https://doi.org/10.3390/nano10050936
Chicago/Turabian StyleKil, Da Rim, Chunyuan Lu, Jung-Min Ji, Chul Hoon Kim, and Hwan Kyu Kim. 2020. "Dopant-Free Triazatruxene-Based Hole Transporting Materials with Three Different End-Capped Acceptor Units for Perovskite Solar Cells" Nanomaterials 10, no. 5: 936. https://doi.org/10.3390/nano10050936






