Reagent-Free Colorimetric Assay for Galactose Using Agarose Gel Entrapping Nanoceria and Galactose Oxidase
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Agarose Gel Composites Entrapping Nanoceria Only (Agarose_Nanoceria) and Both Gal Ox and Nanoceria (Agarose_Nanoceria + Gal Ox)
2.3. Determination of H2O2 Using Agarose_Nanoceria
2.4. Determination of Galactose Using Agarose_Nanoceria + Gal Ox
3. Results and Discussion
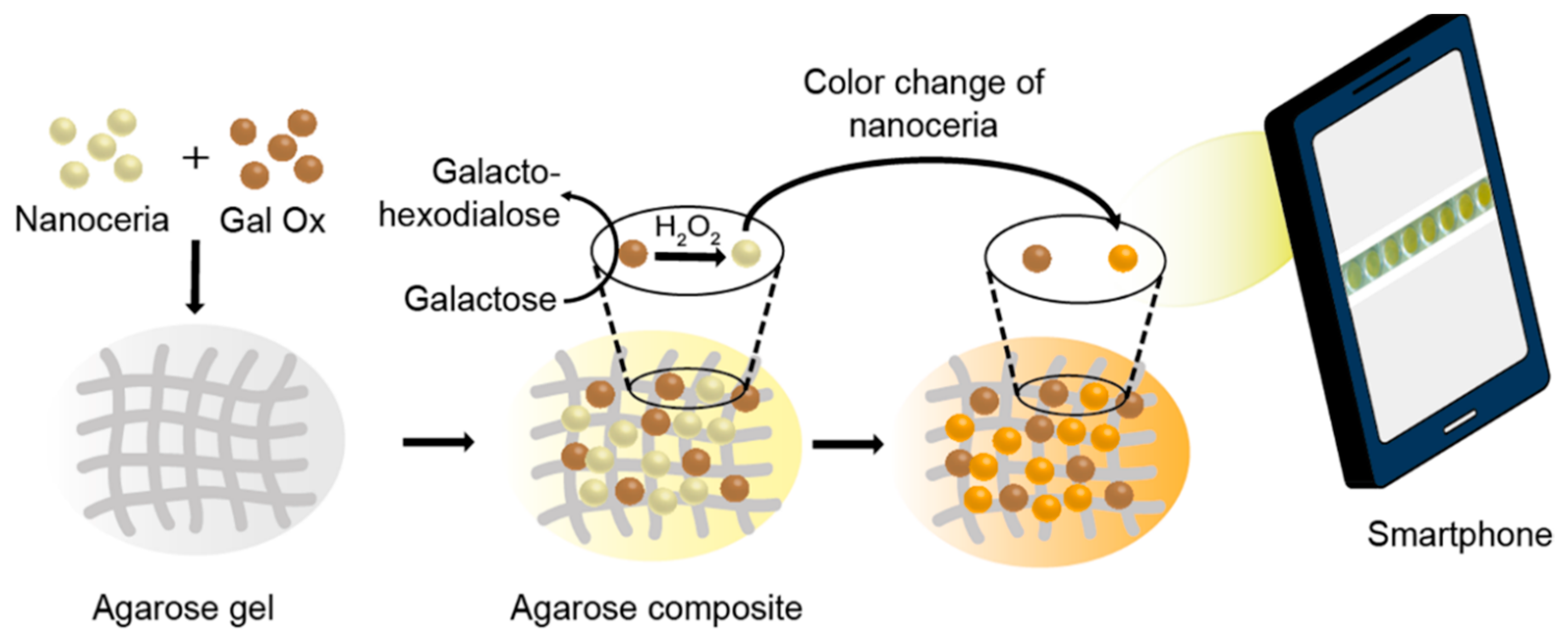
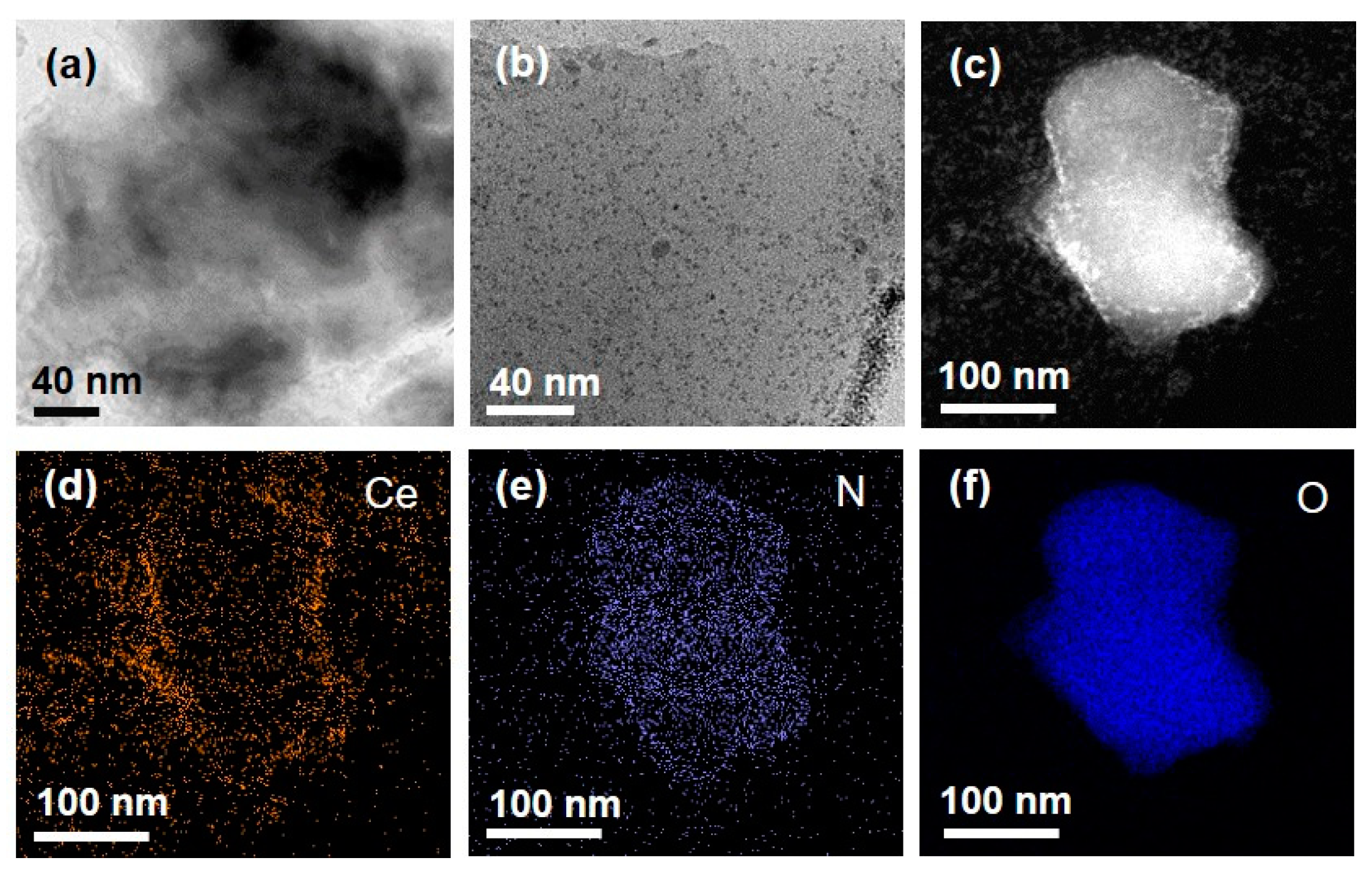
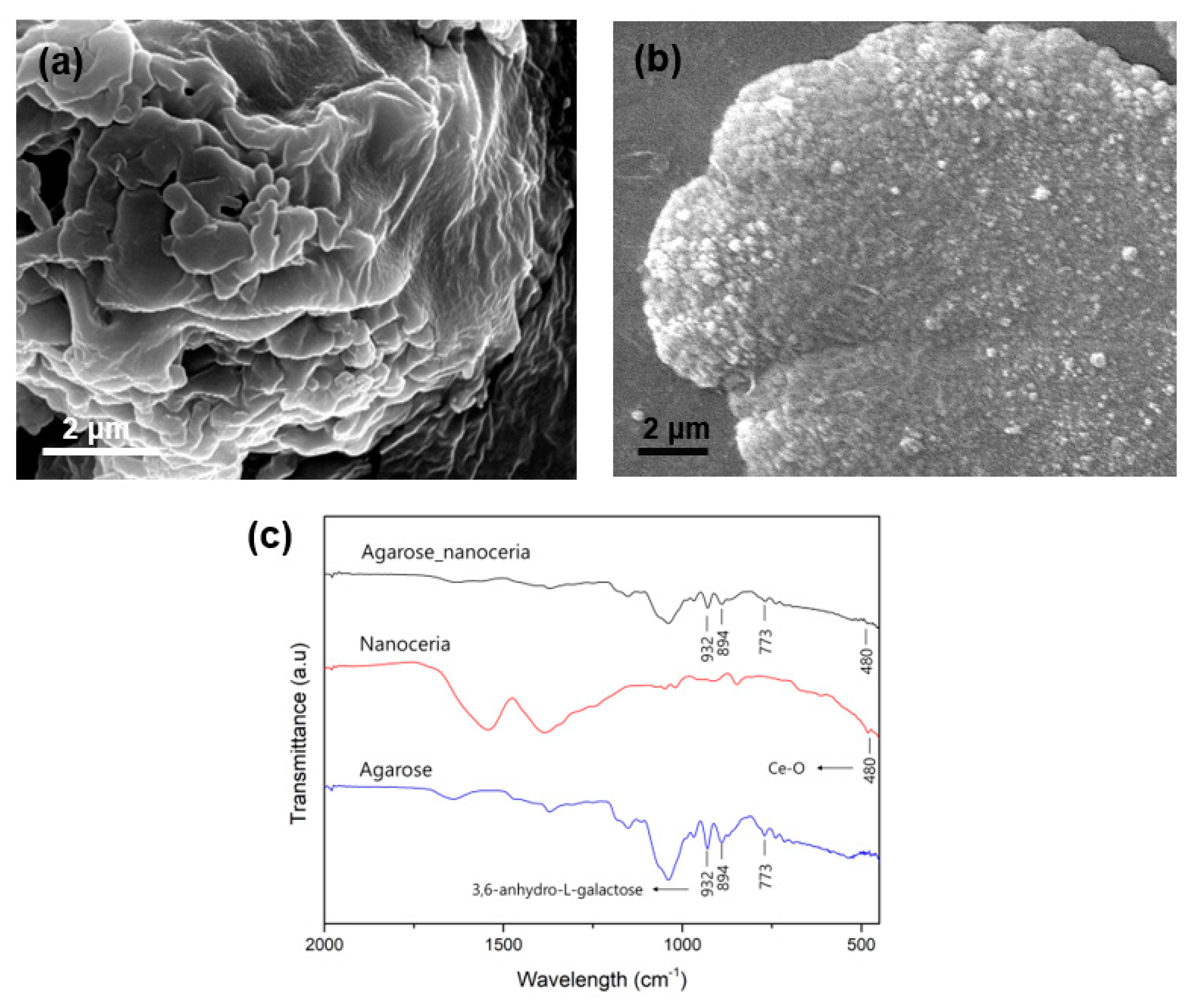
3.1. Construction of Agarose Composite for Colorimetric Determination of Galactose
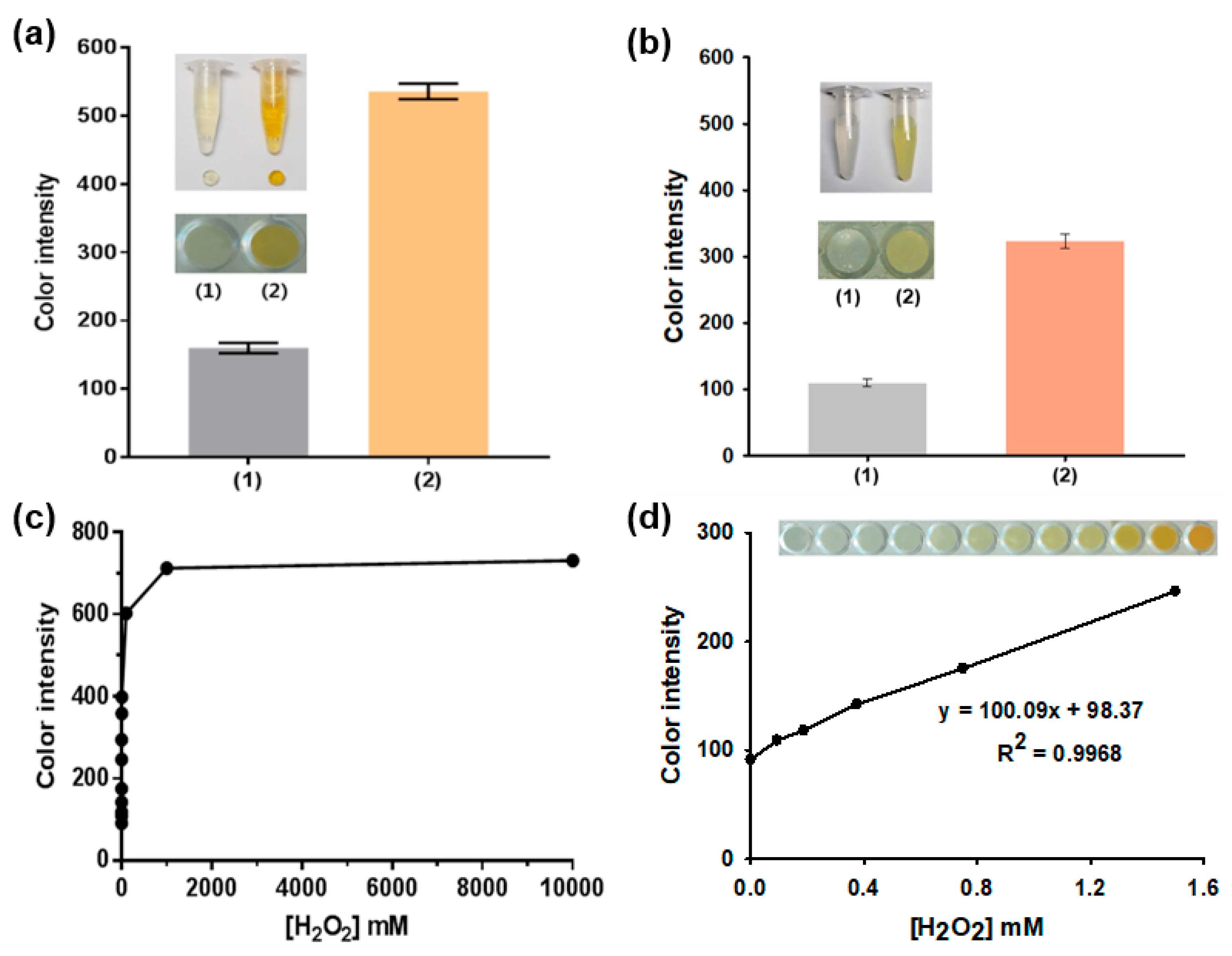
3.2. Vivid Colorimetric Responses of Agarose_Nanoceria toward H2O2
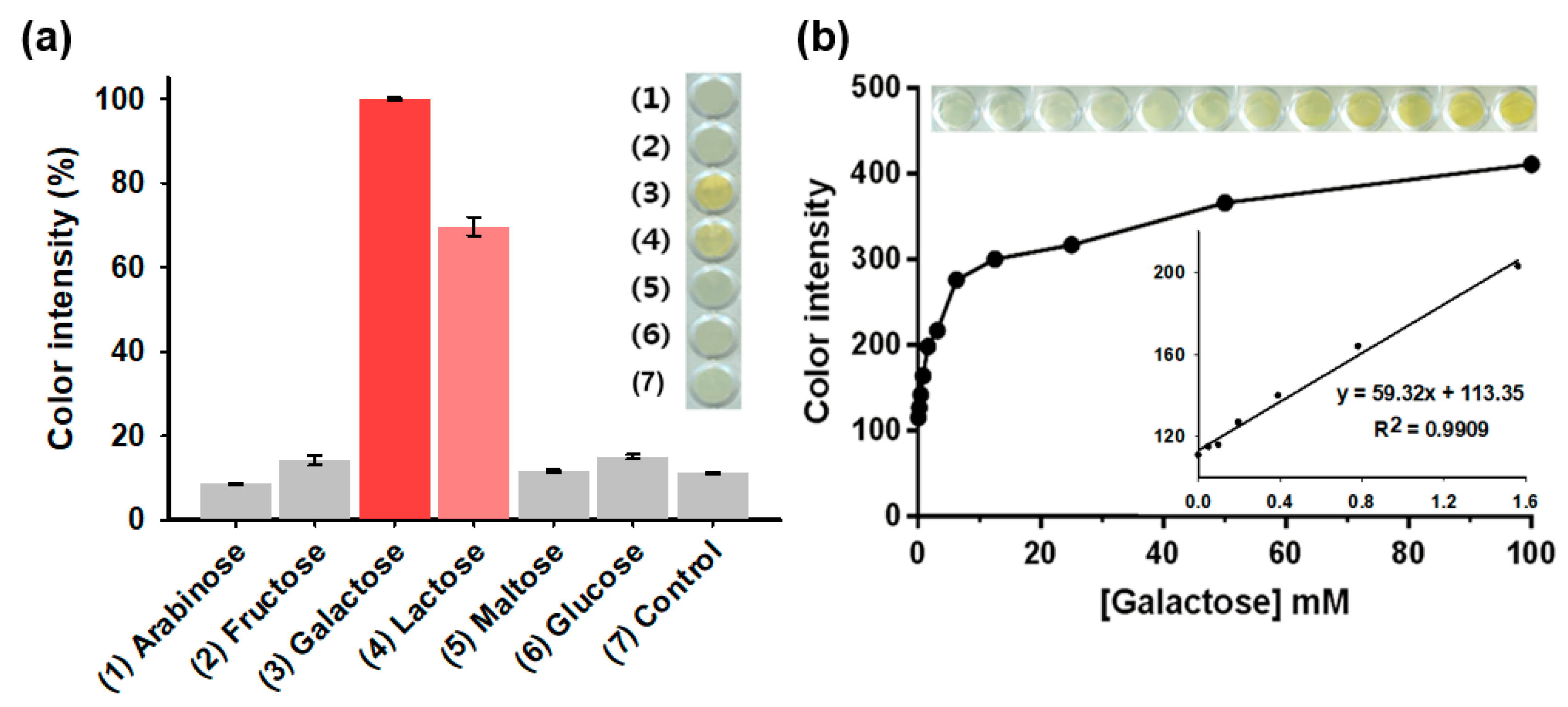
3.3. Analytical Capabilities of the Agarose Composite: Specificity, Linearity, Sensitivity, and Precision for Galactose Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weese, S.J.; Gosnell, K.; West, P.; Gropper, S.S. Galactose content of baby food meats: Considerations for infants with galactosemia. J. Am. Diet. Assoc. 2003, 103, 373–375. [Google Scholar] [PubMed]
- Holton, J. Galactose disorders: An overview. J. Inherit. Metab. Dis. 1990, 13, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Holden, H.M.; Rayment, I.; Thoden, J.B. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J. Biol. Chem. 2003, 278, 43885–43888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.-S.; Kwon, H.-J.; Yoon, H.-R.; Lee, Y.-M.; Choi, T.-Y.; Hong, S.-P. A pulsed amperometric detection method of galactose 1-phosphate for galactosemia diagnosis. Anal. Biochem. 2008, 376, 200–205. [Google Scholar] [CrossRef]
- Bosch, A.M. Classical galactosaemia revisited. J. Inherit. Metab. Dis. 2006, 29, 516–525. [Google Scholar] [CrossRef]
- Bennett, M.J. Galactosemia diagnosis gets an upgrade. Clin. Chem. 2010, 56, 690–692. [Google Scholar] [CrossRef]
- Paigen, K.; Pacholec, F.; Levy, H.L. A new method of screening for inherited disorders of galactose metabolism. J. Lab. Clin. Med. 1982, 99, 895–907. [Google Scholar]
- Woo, M.-A.; Kim, M.I.; Cho, D.; Park, H.G. Cell-based galactosemia diagnosis system based on a galactose assay using a bioluminescent Escherichia coli array. Anal. Chem. 2013, 85, 11083–11089. [Google Scholar] [CrossRef]
- Hu, O.Y.-P.; Hu, T.-M.; Tang, H.-S. Determination of galactose in human blood by high-performance liquid chromatography: Comparison with an enzymatic method and application to the pharmacokinetic study of galactose in patients with liver dysfunction. J. Pharm. Sci. 1995, 84, 231–235. [Google Scholar]
- Nishimura, Y.; Tajima, G.; Bahagia, A.D.; Sakamoto, A.; Ono, H.; Sakura, N.; Naito, K.; Hamakawa, M.; Yoshii, C.; Kubota, M. Differential diagnosis of neonatal mild hypergalactosaemia detected by mass screening: Clinical significance of portal vein imaging. J. Inherit. Metab. Dis. 2004, 27, 11–18. [Google Scholar] [CrossRef]
- Ning, C.; Segal, S. Plasma galactose and galactitol concentration in patients with galactose-1-phosphate uridyltransferase deficiency galactosemia: Determination by gas chromatography/mass spectrometry. Metabolism 2000, 49, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Diepenbrock, F.; Heckler, R.; Schickling, H.; Engelhard, T.; Bock, D.; Sander, J. Colorimetric determination of galactose and galactose-1-phosphate from dried blood. Clin. Biochem. 1992, 25, 37–39. [Google Scholar] [CrossRef]
- Kim, M.I.; Shim, J.; Li, T.; Woo, M.-A.; Cho, D.; Lee, J.; Park, H.G. Colorimetric quantification of galactose using a nanostructured multi-catalyst system entrapping galactose oxidase and magnetic nanoparticles as peroxidase mimetics. Analyst 2012, 137, 1137–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanyong, P.; Krampa, F.D.; Aniweh, Y.; Awandare, G.A. Enzyme-based amperometric galactose biosensors: A review. Microchim. Acta 2017, 184, 3663–3671. [Google Scholar] [CrossRef] [Green Version]
- Kanyong, P.; Pemberton, R.M.; Jackson, S.K.; Hart, J.P. Amperometric screen-printed galactose biosensor for cell toxicity applications. Anal. Lett. 2016, 49, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Singhal, R.; Malhotra, B.D.; Sehgal, N.; Kumar, A. Langmuir-Blodgett film based biosensors for estimation of galactose in milk. Electrochim. Acta 2004, 49, 2479–2485. [Google Scholar] [CrossRef]
- Kim, D.H.; Hur, J.; Park, H.G.; Kim, M.I. Reagentless colorimetric biosensing platform based on nanoceria within an agarose gel matrix. Biosens. Bioelectron. 2017, 93, 226–233. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef] [Green Version]
- Scholes, F.H.; Soste, C.; Hughes, A.E.; Hardin, S.G.; Curtis, P.R. The role of hydrogen peroxide in the deposition of cerium-based conversion coatings. Appl. Surf. Sci. 2006, 253, 1770–1780. [Google Scholar] [CrossRef]
- Feng, X.; Sayle, D.C.; Wang, Z.L.; Paras, M.S.; Santora, B.; Sutorik, A.C.; Sayle, T.X.; Yang, Y.; Ding, Y.; Wang, X.; et al. Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science 2006, 312, 1504–1508. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.; Mehta, G.; Meena, R.; Siddhanta, A.K. Hydrogel-forming agar-graft-PVP and κ-carrageenan-graft-PVP blends: Rapid synthesis and characterization. J. Appl. Polym. Sci. 2006, 102, 3654–3663. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Rao, K.S.; Kumar, A. Facile preparation of agarose–chitosan hybrid materials and nanocomposite ionogels using an ionic liquid via dissolution, regeneration and sol–gel transition. Green Chem. 2014, 16, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Das, J.; Han, J.W.; Choi, Y.-J.; Song, H.; Cho, S.-G.; Park, C.; Seo, H.G.; Kim, J.-H. Cationic lipid-nanoceria hybrids, a novel nonviral vector-mediated gene delivery into mammalian cells: Investigation of the cellular uptake mechanism. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- McDevitt, N.T.; Baun, W.L. Infrared absorption study of metal oxides in the low frequency region (700-240 cm−1). Spectrochim. Acta 1964, 20, 799–808. [Google Scholar] [CrossRef]
- Ford, J.D.; Haworth, J.C. The estimation of galactose in plasma using galactose oxidase. Clin. Chem. 1964, 10, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.U.; Song, S.; Kim, S.; Sim, S.J. A shape-code nanoplasmonic biosensor for multiplex detection of Alzheimer’s disease biomarkers. Biosens. Bioelectron. 2018, 101, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Cho, H.J.; Lee, D.H.; Song, J.H. Galactosemia detected by meonatal screening test. J. Korean Pediatr. Soc. 2003, 46, 440–446. [Google Scholar]
- Jensen, U.G.; Brandt, N.J.; Christensen, E.; Skovby, F.; Nørgaard-Pedersen, B.; Simonsen, H. Neonatal screening for galactosemia by quantitative analysis of hexose monophosphates using tandem mass spectrometry: A retrospective study. Clin. Chem. 2001, 47, 1364–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Original Amount (mM) | Added Galactose Concentration (mM) | Expected Galactose Concentration (mM) | Measured a Galactose Concentration (mM) | SD b | CV c (%) | Recovery d (%) | |
|---|---|---|---|---|---|---|---|
| Normal | 0.060 | 0.139 | 0.199 | 0.188 | 0.012 | 6.38 | 94.47 |
| Boundary | 0.589 | 0.649 | 0.687 | 0.033 | 4.80 | 105.86 | |
| High | 1.333 | 1.393 | 1.377 | 0.095 | 6.90 | 98.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, P.T.; Ahn, H.T.; Kim, M.I. Reagent-Free Colorimetric Assay for Galactose Using Agarose Gel Entrapping Nanoceria and Galactose Oxidase. Nanomaterials 2020, 10, 895. https://doi.org/10.3390/nano10050895
Nguyen PT, Ahn HT, Kim MI. Reagent-Free Colorimetric Assay for Galactose Using Agarose Gel Entrapping Nanoceria and Galactose Oxidase. Nanomaterials. 2020; 10(5):895. https://doi.org/10.3390/nano10050895
Chicago/Turabian StyleNguyen, Phuong Thy, Hee Tae Ahn, and Moon Il Kim. 2020. "Reagent-Free Colorimetric Assay for Galactose Using Agarose Gel Entrapping Nanoceria and Galactose Oxidase" Nanomaterials 10, no. 5: 895. https://doi.org/10.3390/nano10050895





