Structural Study of Nano-Sized Gahnite (ZnAl2O4): From the Average to the Local Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Synthesis
2.2. Data Collection
2.3. Data Treatment
2.4. TEM Analysis
3. Results
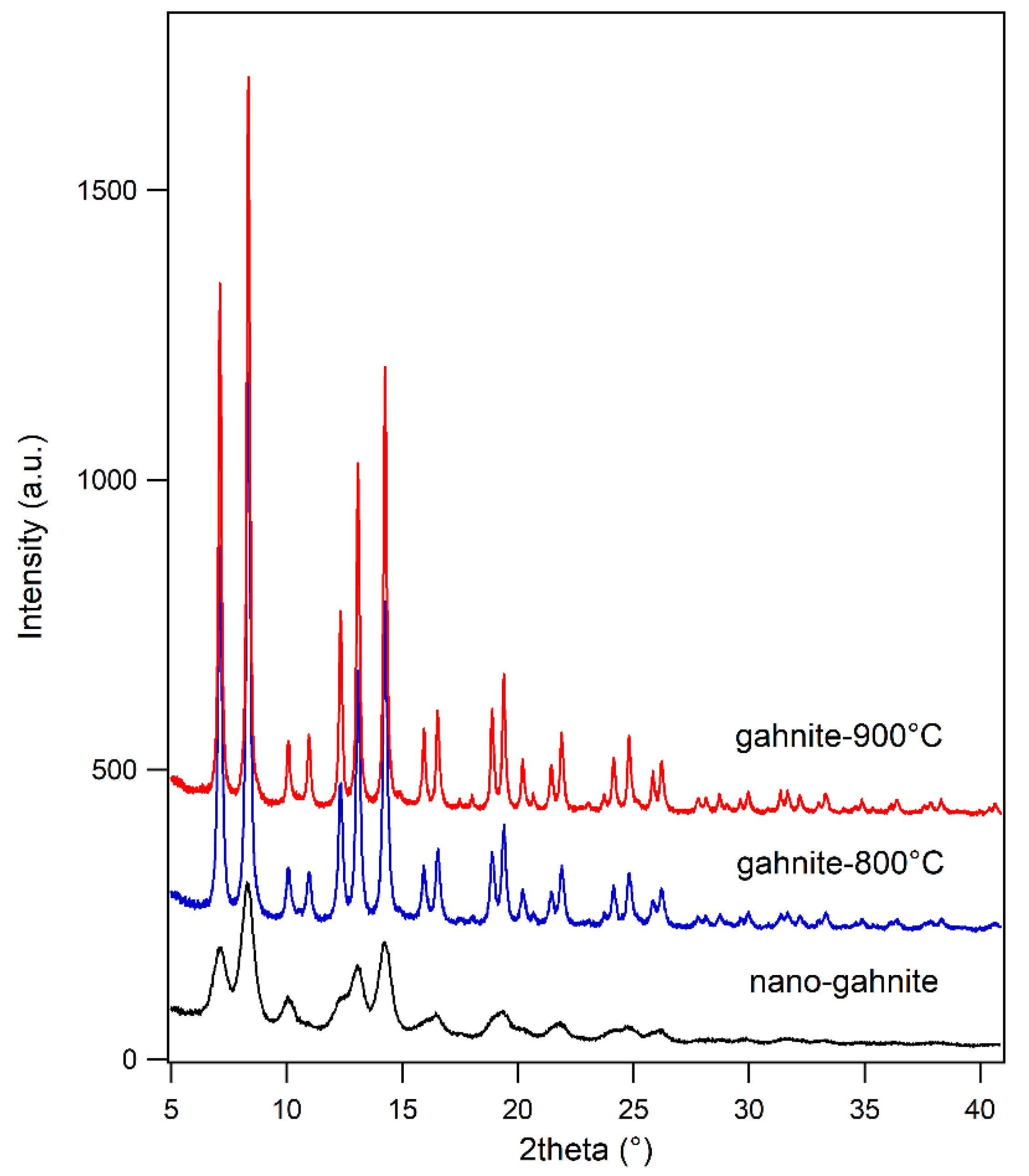
3.1. Average Structure
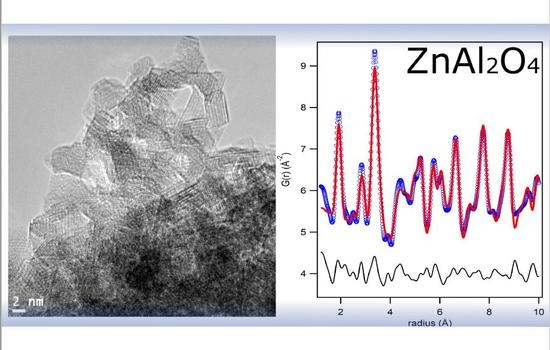
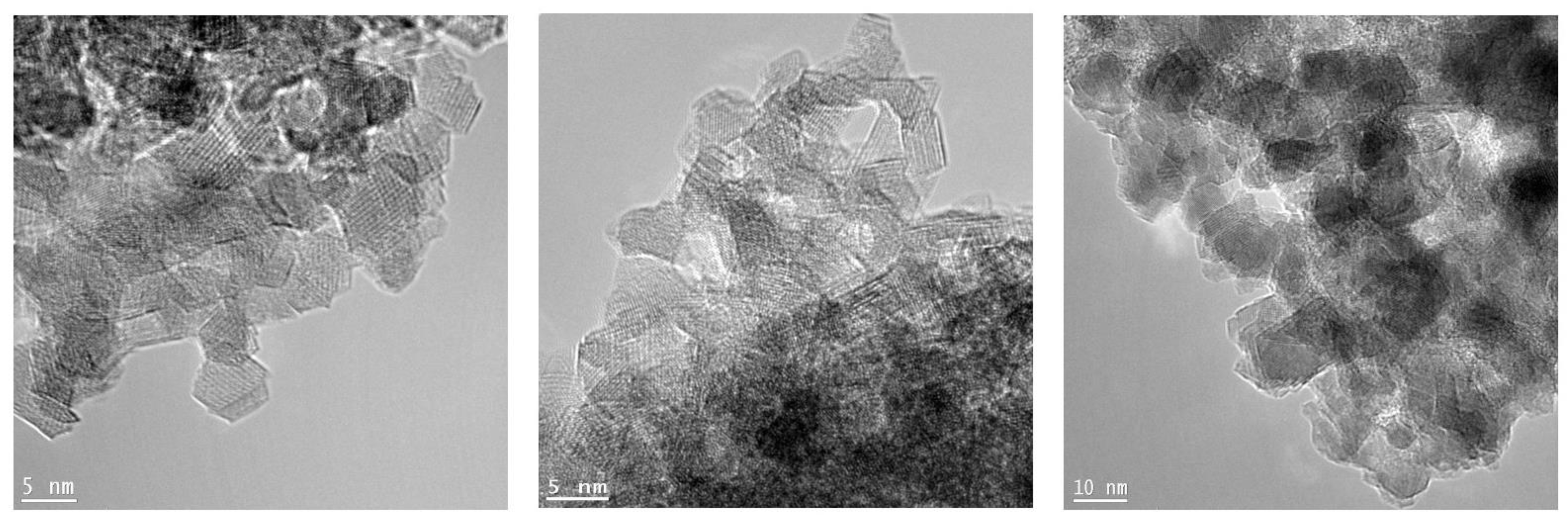
3.2. TEM and PDF Analysis: Grain Size Determination
3.3. Local Structure
4. Conclusions
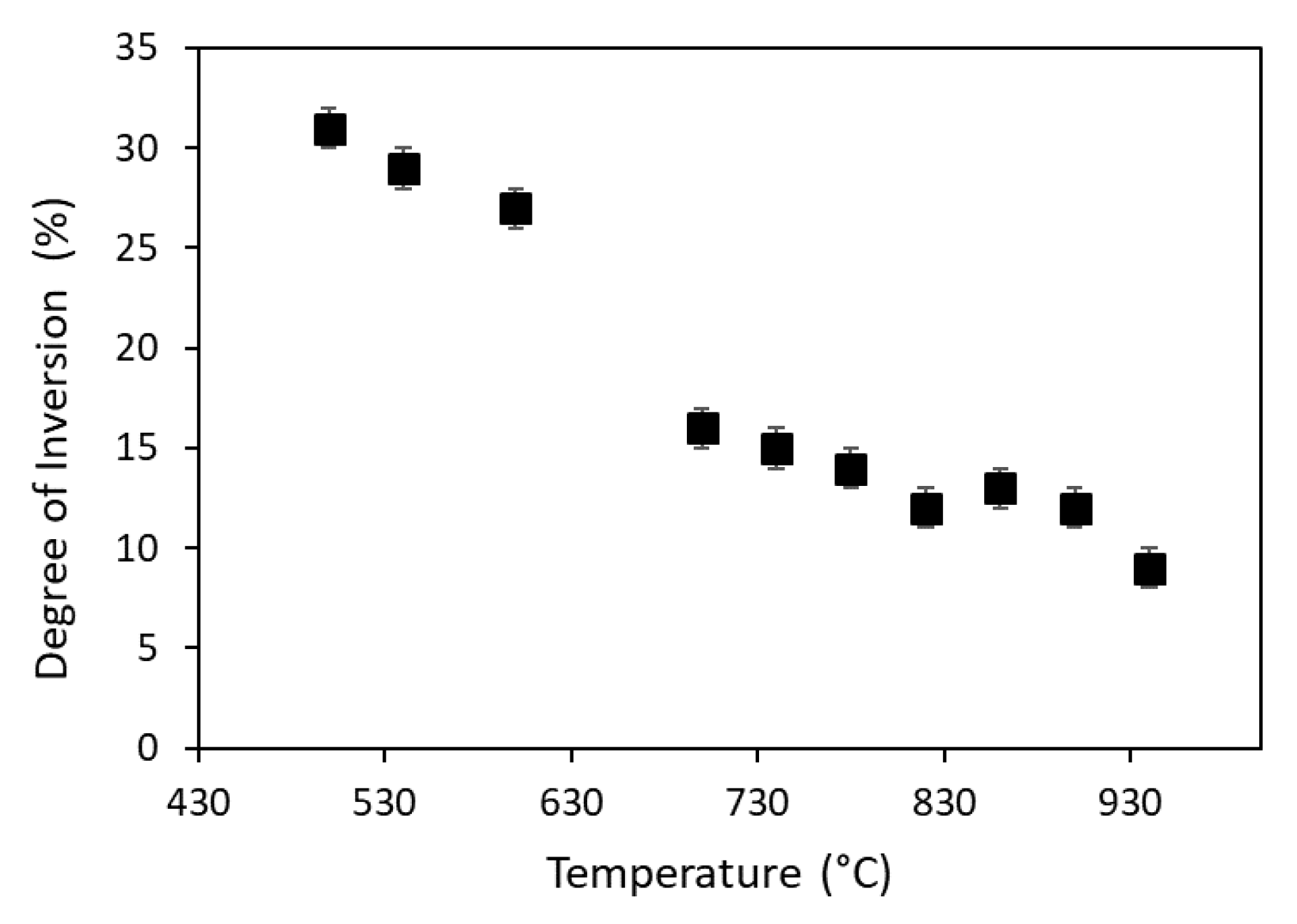
- The calcination temperature (up to 900 °C) induces an increase in the particle size, as proved by the peak broadening trend, and a decrease in the degree of inversion. This last effect is also observable with in situ data collection as a function of temperature.
- TEM analysis reveals the nanosize of the investigated materials increasing with the calcination temperature. In addition, images prove the good crystallinity of the sample, even in those powders smaller than 2 nm, and the lack of evident defects at the medium scale.
- The local structure shows some differences from the average structure, as expected. In particular, a new structural model was proposed, with more degree of freedom for the oxygen coordinates, which fits better with the PDF data. The goodness of fit improves with grain size, while the deviation from the ideal (xxx) oxygen coordinates increases.
- It is suggested that particular properties exhibit from nanocrystalline gahnite are more related to a disorder of the oxygen atoms resulting from the inversion grade of the spinel rather than from interstitial Zn2+.
- The polyhedral volume for octahedra decreases, with respect to the one for the crystallographic structure, while, at the same time, the tetrahedral volumes increase. The degree of distortion of the octahedra is much larger for the two more crystalline samples than for the nanosized one: this may be partly due to the exchange between two different sized cations (Zn2+ and Al3+) and to the enlarged degree of freedom for the oxygen coordinates.
- The goodness of fit for proposed model on the local scale increases with the sample crystallinity, showing the model, though with a larger distortion of the polyhedra, is more suitable for the samples with a larger particle size.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levy, D.; Pavese, A.; Sani, A.; Pischedda, V. Structure and compressibility of synthetic ZnAl2O4 (gahnite) under high-pressure conditions, from synchrotron X-ray powder diffraction. Phys. Chem. Miner. 2001, 28, 612–618. [Google Scholar] [CrossRef]
- Cormack, A.N.; Lewis, G.V.; Parker, S.C.; Catlow, C.R.A. On the Cation Distribution of Spinels. J. Phys. Chem. Solids 1988, 49, 53–57. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Navrotsky, A. Simple Spinels—Crystallographic Parameters, Cation Radii, Lattice Energies, and Cation Distribution. Am. Mineral. 1983, 68, 181–194. [Google Scholar]
- Cooley, R.F.; Reed, J.S. Equilibrium Cation Distribution in NiAl2O4, CuAl2O4, and ZnAl2O4 Spinels. J. Am. Ceram. Soc. 1972, 55, 395–398. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Dollase, W.A. Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr2O4, ZnCr2O4, Fe3O4 and the temperature dependence of the cation distribution in ZnAl2O4. Phys. Chem. Miner. 1994, 20, 541–555. [Google Scholar] [CrossRef]
- Mathur, S.; Veith, M.; Haas, M.; Shen, A.; Lecerf, N.; Huch, V.; Hufner, S.; Haberkorn, R.; Beck, H.P.; Jilavi, M. Single-source sol-gel synthesis of nanocrystalline ZnAl2O4: Structural and optical properties. J. Am. Ceram. Soc. 2001, 84, 1921–1928. [Google Scholar] [CrossRef]
- Lucchesi, S.; Della Giusta, A.; Russo, U. Cation distribution in natural Zn-aluminate spinels. Mineral. Mag. 1998, 62, 41–54. [Google Scholar] [CrossRef]
- van der Laag, N.J.; Snel, M.D.; Magusin, P.C.M.M.; de With, G. Structural, elastic, thermophysical and dielectric properties of zinc aluminate (ZnAl2O4). J. Eur. Ceram. Soc. 2004, 24, 2417–2424. [Google Scholar] [CrossRef]
- Da Silva, A.A.; Goncalves, A.D.; Davolos, M.R. Characterization of nanosized ZnAl2O4 spinel synthesized by the sol-gel method. J. Sol-Gel Sci. Techn. 2009, 49, 101–105. [Google Scholar] [CrossRef]
- Jagadeeshwaran, C.; Murugaraj, R. Impact of the sintering temperature on the structural, optical and electrical properties of zinc aluminate. J. Mater. Sci. Mater. Electron. 2019, 30, 15683–15692. [Google Scholar] [CrossRef]
- Ciupina, V.; Carazeanu, I.; Prodan, G. Characterization of ZnAl2O4 nanocrystals prepared by coprecipitation and microemulsion techniques. J. Optoelectron. Adv. M. 2004, 6, 1317–1322. [Google Scholar]
- Kaliyannan, G.V.; Palanisamy, S.V.; Palanisamy, M.; Chinnasamy, M.; Somasundaram, S.; Nagarajan, N.; Rathanasamy, R. Utilization of 2D gahnite nanosheets as highly conductive, transparent and light trapping front contact for silicon solar cells. App. Nanosci. 2019, 9, 1427–1437. [Google Scholar] [CrossRef]
- Joffy, P.J.; Sreeja, V.G.; Devasia, S.; Anila, E.I. Spectral and nonlinear optical characterization of blue light emitting gahnite nanorods synthesized through radiation assisted sol gel method. Solid State Sci. 2019, 96, 105947. [Google Scholar] [CrossRef]
- Cornu, L.; Gaudon, M.; Jubera, V. ZnAl2O4 as potential sensor: Variation of luminescence with thermal history. J. Mater. Chem. C 2013, 1, 5419–5428. [Google Scholar] [CrossRef] [Green Version]
- Billinge, S.J.L.; Thorpe, M.F. Local Structure from Diffraction; Plenum Publishing Corp.: New York, NY, USA, 1998. [Google Scholar]
- Billinge, S.J.L.; Kanatzidis, M.G. Beyond crystallography: The study of disorder, nanocrystallinity and crystallographically challenged materials with pair distribution functions. Chem. Commun. 2004, 7, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Egami, T.; Billinge, S.J.L. Underneath the Bragg Peaks: Structural Analysis of Complex Materials, 2nd ed.; Pergamon Materials Series; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Canu, G.; Confalonieri, G.; Deluca, M.; Curecheriu, L.; Buscaglia, M.T.; Asandulesa, M.; Horchidan, N.; Dapiaggi, M.; Mitoseriu, L.; Buscaglia, V. Structure-property correlations and origin of relaxor behaviour in BaCexTi1-xO3. Acta Mater. 2018, 152, 258–268. [Google Scholar] [CrossRef]
- Masadeh, A.S.; Bozin, E.S.; Farrow, C.L.; Paglia, G.; Juhas, P.; Billinge, S.J.L.; Karkamkar, A.; Kanatzidis, M.G. Quantitative size-dependent structure and strain determination of CdSe nanoparticles using atomic pair distribution function analysis. Phys. Rev. B 2007, 76, 115413. [Google Scholar] [CrossRef] [Green Version]
- Confalonieri, G.; Buscaglia, V.; Capitani, G.; Canu, G.; Rotiroti, N.; Bernasconi, A.; Pavese, A.; Dapiaggi, M. Local distortion and octahedral tilting in BaCexTi1-xO3 perovskite. J. Appl. Crystallogr. 2018, 51, 1283–1294. [Google Scholar] [CrossRef]
- Coduri, M.; Brunelli, M.; Scavini, M.; Allieta, M.; Masala, P.; Capogna, L.; Fischer, H.E.; Ferrero, C. Rare Earth doped ceria: A combined X-ray and neutron pair distribution function study. Z. Kristallogr. 2012, 227, 272–279. [Google Scholar] [CrossRef]
- Chen, X.Y.; Ma, C.; Zhang, Z.J.; Wang, B.N. Ultrafine gahnite (ZnAl2O4) nanocrystals: Hydrothermal synthesis and photoluminescent properties. Mater. Sci. Eng. B-Adv. 2008, 151, 224–230. [Google Scholar] [CrossRef]
- Fitch, A.N. The high resolution powder diffraction beam line at ESRF. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, A.; Wright, J.; Harker, N. Total scattering experiments on glass and crystalline materials at the ESRF on the ID11 Beamline. Powder Diffr. 2015, 30, S2–S8. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System “GSAS”; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Juhas, P.; Davis, T.; Farrow, C.L.; Billinge, S.J.L. PDFgetX3: A rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Crystallogr. 2013, 46, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Confalonieri, G.; Dapiaggi, M.; Sommariva, M.; Gateshki, M.; Fitch, A.N.; Bernasconi, A. Comparison of total scattering data from various sources: The case of a nanometric spinel. Powder Diffr. 2015, 30, S65–S69. [Google Scholar] [CrossRef]
- Farrow, C.L.; Juhas, P.; Liu, J.W.; Bryndin, D.; Bozin, E.S.; Bloch, J.; Proffen, T.; Billinge, S.J.L. PDFfit2 and PDFgui: Computer programs for studying nanostructure in crystals. J. Phys.-Condens. Mat. 2007, 19, 335219. [Google Scholar] [CrossRef] [Green Version]
- Confalonieri, G.; Buscaglia, V.; Canu, G.; Buscaglia, M.T.; Dapiaggi, M. The local and average structure of Ba(Ti, Ce)O3 perovskite solid solution: Effect of cerium concentration and particle size. J. Synchrotron Radiat. 2019, 26, 1280–1287. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]


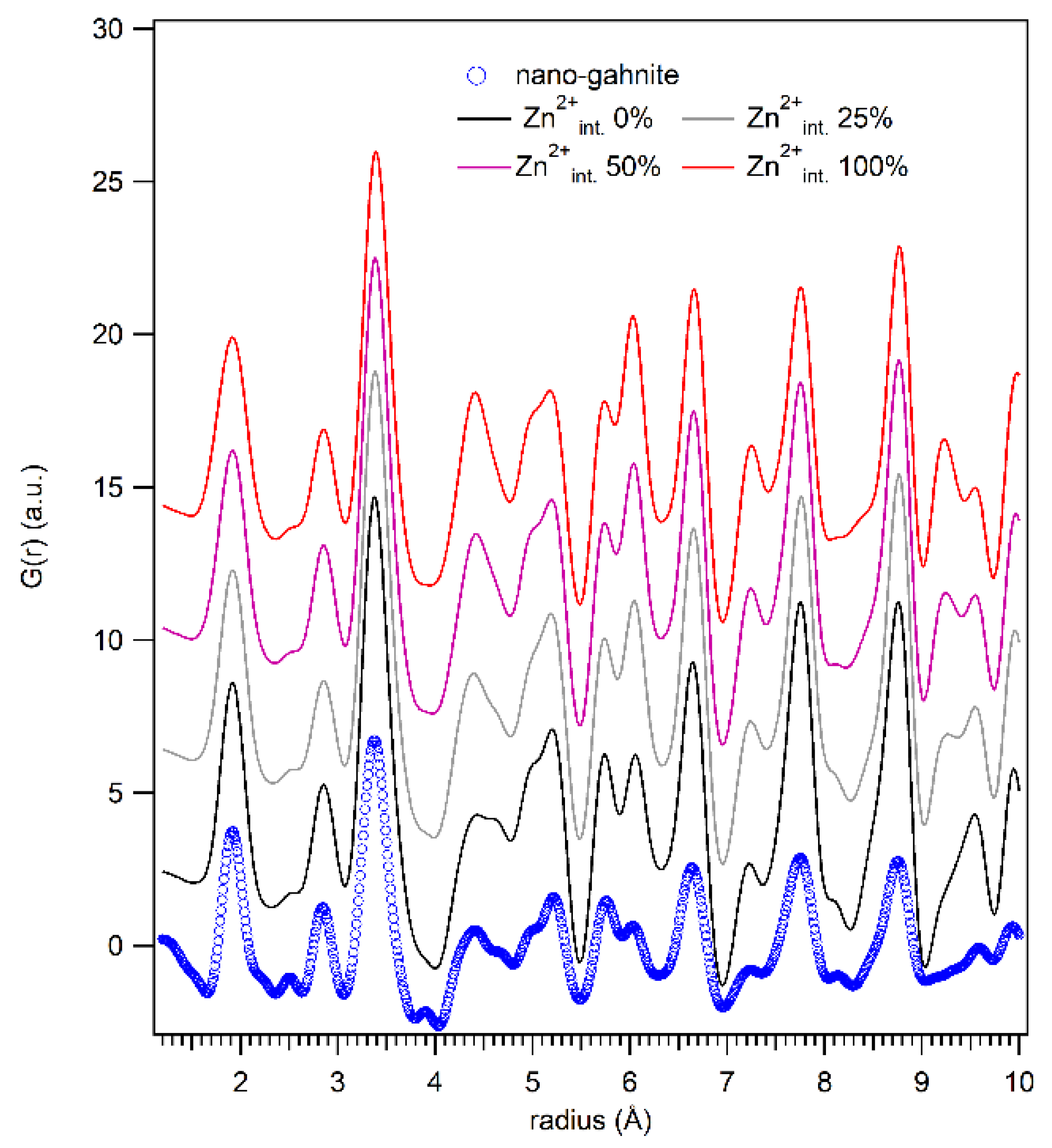
| Atom | x/a | y/b | z/c | Occupancy Factor | UISO (Å2) |
|---|---|---|---|---|---|
| Zn1 | 0.125 | 0.125 | 0.125 | 0.865(4) | 0.0038(1) |
| Zn2 | 0.5 | 0.5 | 0.5 | 0.054(2) | 0.0038(1) |
| Al1 | 0.125 | 0.125 | 0.125 | 0.135(4) | 0.0058(3) |
| Al2 | 0.5 | 0.5 | 0.5 | 0.946(2) | 0.0058(3) |
| O | 0.2613(1) | 0.2613(1) | 0.2613(1) | 1 | 0.0014(3) |
| Atom | x/a | y/b | z/c | Occupancy Factor | UISO (Å2) |
|---|---|---|---|---|---|
| Zn1 | 0.125 | 0.125 | 0.125 | 0.895(3) | 0.0039(1) |
| Zn2 | 0.5 | 0.5 | 0.5 | 0.038(2) | 0.0039(1) |
| Al1 | 0.125 | 0.125 | 0.125 | 0.105(3) | 0.0057(3) |
| Al2 | 0.5 | 0.5 | 0.5 | 0.962(2) | 0.0057(3) |
| O | 0.2631(1) | 0.2631(1) | 0.2631(1) | 1 | 0.0016(4) |
| Gahnite | Gahnite-800 °C | Gahnite-900 °C | |
|---|---|---|---|
| PDF (Å) | 27(3) | 62(9) | 69(11) |
| TEM (Å) | ~ 40 | ~ 70 | ~ 90 |
| Nanogahnite | Gahnite-800 °C | Gahnite-900 °C | |
|---|---|---|---|
| Cell Parameter (Å) | 8.092(8) | 8.069(7) | 8.062(6) |
| Zn Uiso (Å2) | 0.006(2) | 0.003(1) | 0.004(1) |
| Al Uiso (Å2) | 0.006(2) | 0.007(2) | 0.006(2) |
| O Uiso (Å2) | 0.008(5) | 0.009(3) | 0.009(3) |
| O x coordinate | 0.265(3) | 0.263(5) | 0.262(4) |
| O y coordinate | 0.265(3) | 0.269(3) | 0.269(3) |
| O z coordinate | 0.263(4) | 0.261(5) | 0.262(4) |
| Rw (%) | 22.9 | 16.3 | 14.8 |
| Bibliographic Structure [4] | Nanogahnite | Gahnite-800 °C | Gahnite-900 °C | |
|---|---|---|---|---|
| Octahedra | ||||
| Average bond length (Å) | 1.9226 | 1.9141 | 1.9090 | 1.9073 |
| Average polyhedral volume (Å3) | 9.3092 | 9.3105 | 9.8684 | 9.7433 |
| Distortion index | 0 | 0.00397 | 0.01396 | 0.01397 |
| Min bond length (Å) | 1.9226 | 1.91 | 1.87 | 1.87 |
| Max bond length (Å) | 1.9226 | 1.93 | 1.94 | 1.93 |
| Standard deviation | 0 | 0.097 | 0.02985 | 0.028 |
| Tetrahedra | ||||
| Average bond length (Å) | 1.9332 | 1.9529 | 1.9479 | 1.9462 |
| Average polyhedral volume (Å3) | 3.7079 | 4.0416 | 4.3559 | 4.1721 |
| Distortion index | 0 | 0 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Confalonieri, G.; Rotiroti, N.; Bernasconi, A.; Dapiaggi, M. Structural Study of Nano-Sized Gahnite (ZnAl2O4): From the Average to the Local Scale. Nanomaterials 2020, 10, 824. https://doi.org/10.3390/nano10050824
Confalonieri G, Rotiroti N, Bernasconi A, Dapiaggi M. Structural Study of Nano-Sized Gahnite (ZnAl2O4): From the Average to the Local Scale. Nanomaterials. 2020; 10(5):824. https://doi.org/10.3390/nano10050824
Chicago/Turabian StyleConfalonieri, Giorgia, Nicola Rotiroti, Andrea Bernasconi, and Monica Dapiaggi. 2020. "Structural Study of Nano-Sized Gahnite (ZnAl2O4): From the Average to the Local Scale" Nanomaterials 10, no. 5: 824. https://doi.org/10.3390/nano10050824