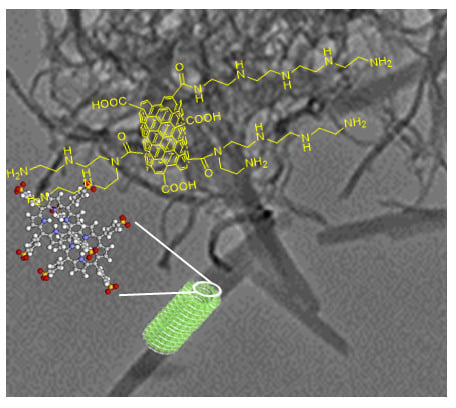
Novel Nanohybrids Based on Supramolecular Assemblies of Meso-tetrakis-(4-sulfonatophenyl) Porphyrin J-aggregates and Amine-Functionalized Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
Synthesis and Characterization of Amine Multiwalled Carbon Nanotubes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poudel, Y.R.; Li, W. Synthesis, properties, and applications of carbon nanotubes filled with foreign materials: A review. Mater. Today Phys. 2018, 7, 7–34. [Google Scholar] [CrossRef]
- Marchesan, S.; Kostarelos, K.; Bianco, A.; Prato, M. The winding road for carbon nanotubes in nanomedicine. Mater. Today 2015, 18, 12–19. [Google Scholar] [CrossRef]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Bourgognon, M.; Wang, J.T.W.; Al-Jamal, K.T. Functionalised carbon nanotubes: From intracellular uptake and cell-related toxicity to systemic brain delivery. J. Control. Release 2016, 241, 200–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, K.; Saito, N. Biocompatibility and Carcinogenicity of Carbon Nanotubes as Biomaterials. Nanomaterials 2020, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Battigelli, A.; Ménard-Moyon, C.; Da Ros, T.; Prato, M.; Bianco, A. Endowing carbon nanotubes with biological and biomedical properties by chemical modifications. Adv. Drug Deliv. Rev. 2013, 65, 1899–1920. [Google Scholar] [CrossRef]
- Iannazzo, D.; Mazzaglia, A.; Scala, A.; Pistone, A.; Galvagno, S.; Lanza, M.; Riccucci, C.; Ingo, G.M.; Colao, I.; Sciortino, M.T.; et al. β-Cyclodextrin-grafted on multiwalled carbon nanotubes as versatile nanoplatform for entrapment of guanine-based drugs. Colloids Surf. B Biointerfaces 2014, 123, 264–270. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Scala, A.; Sortino, G.; Zagami, R.; Zhu, Y.; Sciortino, M.T.; Pennisi, R.; Pizzo, M.M.; Neri, G.; Grassi, G.; et al. Intracellular trafficking and therapeutic outcome of multiwalled carbon nanotubes modified with cyclodextrins and polyethylenimine. Colloids Surf. B Biointerfaces 2018, 163, 55–63. [Google Scholar] [CrossRef]
- Piperno, A.; Scala, A.; Mazzaglia, A.; Neri, G.; Pennisi, R.; Sciortino, M.T.; Grassi, G. Cellular Signaling Pathways Activated by Functional Graphene Nanomaterials. Int. J. Mol. Sci. 2018, 19, 3365. [Google Scholar] [CrossRef] [Green Version]
- Piperno, A.; Mazzaglia, A.; Scala, A.; Pennisi, R.; Zagami, R.; Neri, G.; Torcasio, S.M.; Rosmini, C.; Mineo, P.G.; Potara, M.; et al. Casting Light on Intracellular Tracking of a New Functional Graphene-Based MicroRNA Delivery System by FLIM and Raman Imaging. ACS Appl. Mater. Interfaces 2019, 11, 46101–46111. [Google Scholar] [CrossRef]
- Vialla, F.; Delport, G.; Chassagneux, Y.; Roussignol, P.; Lauret, J.S.; Voisin, C. Diameter-selective non-covalent functionalization of carbon nanotubes with porphyrin monomers. Nanoscale 2016, 8, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Sprafke, J.K.; Stranks, S.D.; Warner, J.H.; Nicholas, R.J.; Anderson, H.L. Noncovalent Binding of Carbon Nanotubes by Porphyrin Oligomers. Angew. Chem. Int. Ed. 2011, 50, 2313–2316. [Google Scholar] [CrossRef] [PubMed]
- Vizuete, M.; Gómez-Escalonilla, M.J.; Fierro, J.L.G.; Atienzar, P.; García, H.; Langa, F. Double-Wall Carbon Nanotube–Porphyrin Supramolecular Hybrid: Synthesis and Photophysical Studies. ChemPhysChem 2014, 15, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Magadur, G.; Lauret, J.-S.; Alain-Rizzo, V.; Voisin, C.; Roussignol, P.; Deleporte, E.; Delaire, J.A. Excitation Transfer in Functionalized Carbon Nanotubes. ChemPhysChem 2008, 9, 1250–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Bork, M.A.; Riedy, K.J.; McMillin, D.R.; Choi, J.H. Understanding Photophysical Interactions of Semiconducting Carbon Nanotubes with Porphyrin Chromophores. J. Phys. Chem. C 2014, 118, 11612–11619. [Google Scholar] [CrossRef]
- Hasobe, T.; Fukuzumi, S.; Kamat, P.V. Ordered Assembly of Protonated Porphyrin Driven by Single-Wall Carbon Nanotubes. J- and H-Aggregates to Nanorods. J. Am. Chem. Soc. 2005, 127, 11884–11885. [Google Scholar] [CrossRef]
- Aurisicchio, C.; Marega, R.; Corvaglia, V.; Mohanraj, J.; Delamare, R.; Vlad, D.A.; Kusko, C.; Dutu, C.A.; Minoia, A.; Deshayes, G.; et al. CNTs in Optoelectronic Devices: New Structural and Photophysical Insights on Porphyrin-DWCNTs Hybrid Materials. Adv. Funct. Mater. 2012, 22, 3209–3222. [Google Scholar] [CrossRef]
- Devaramani, S.; Shinger, M.I.; Ma, X.; Yao, M.; Zhang, S.; Qin, D.; Lu, X. Porphyrin aggregates decorated MWCNT film for solar light harvesting: Influence of J- and H-aggregation on the charge recombination resistance, photocatalysis, and photoinduced charge transfer kinetics. Phys. Chem. Chem. Phys. 2017, 19, 18232–18242. [Google Scholar] [CrossRef]
- Lutsyk, P.; Piryatinski, Y.; Shandura, M.; AlAraimi, M.; Tesa, M.; Arnaoutakis, G.E.; Melvin, A.A.; Kachkovsky, O.; Verbitsky, A.; Rozhin, A. Self-Assembly for Two Types of J-Aggregates: Cis-Isomers of Dye on the Carbon Nanotube Surface and Free Aggregates of Dye trans-Isomers. J. Phys. Chem. C 2019, 123, 19903–19911. [Google Scholar] [CrossRef]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef]
- Song, X.; Zhang, R.; Liang, C.; Chen, Q.; Gong, H.; Liu, Z. Nano-assemblies of J-aggregates based on a NIR dye as a multifunctional drug carrier for combination cancer therapy. Biomaterials 2015, 57, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.; Huang, H.; Kang, H.; Zhan, Y.; Wang, D.; Zhou, Y.; Geng, J.; Kilian, H.I.; Stiles, W.; Razi, A.; et al. Highly-Soluble Cyanine J-aggregates Entrapped by Liposomes for In Vivo Optical Imaging around 930 nm. Theranostics 2019, 9, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, M.; Ng, K.K.; Huynh, E.; Chan, H.; Charron, D.M.; Chen, J.; Muhanna, N.; Foster, F.S.; Wilson, B.C.; Zheng, G. Stable J-aggregation enabled dual photoacoustic and fluorescence nanoparticles for intraoperative cancer imaging. Nanoscale 2016, 8, 12618–12625. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Zhang, D.; Li, L.-L.; Qiao, Z.-Y.; Zhang, J.-C.; Zhao, Y.-X.; Qi, G.-B.; Wan, D.; Pan, J.; Wang, H. In Situ Construction and Characterization of Chlorin-Based Supramolecular Aggregates in Tumor Cells. ACS Appl. Mater. Interfaces 2016, 8, 22875–22883. [Google Scholar] [CrossRef]
- Cheng, M.H.Y.; Harmatys, K.M.; Charron, D.M.; Chen, J.; Zheng, G. Stable J-Aggregation of an aza-BODIPY-Lipid in a Liposome for Optical Cancer Imaging. Angew. Chem. Int. Ed. 2019, 58, 13394–13399. [Google Scholar] [CrossRef]
- Gandini, S.C.M.; Gelamo, E.L.; Itri, R.; Tabak, M. Small angle X-ray scattering study of meso-tetrakis (4-sulfonatophenyl) porphyrin in aqueous solution: A self-aggregation model. Biophys. J. 2003, 85, 1259–1268. [Google Scholar] [CrossRef] [Green Version]
- Collini, E.; Ferrante, C.; Bozio, R.; Lodi, A.; Ponterini, G. Large third-order nonlinear optical response of porphyrin J-aggregates oriented in self-assembled thin films. J. Mater. Chem. 2006, 16, 1573–1578. [Google Scholar] [CrossRef]
- Collini, E.; Ferrante, C.; Bozio, R. Strong Enhancement of the Two-Photon Absorption of Tetrakis(4-sulfonatophenyl)porphyrin Diacid in Water upon Aggregation. J. Phys. Chem. B 2005, 109, 2–5. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Donato, M.G.; Villari, V.; Micali, N.; Romeo, A.; Scolaro, L.M. Surfactant-like behavior of short-chain alcohols in porphyrin aggregation. J. Phys. Chem. B 2009, 113, 11173–11178. [Google Scholar] [CrossRef]
- Zagami, R.; Castriciano, M.A.; Romeo, A.; Trapani, M.; Pedicini, R.; Monsù Scolaro, L. Tuning supramolecular chirality in nano and mesoscopic porphyrin J-aggregates. Dyes Pigments 2017, 142, 255–261. [Google Scholar] [CrossRef]
- Scolaro, L.M.; Romeo, A.; Castriciano, M.A.; Micali, N. Unusual optical properties of porphyrin fractal J-aggregates. Chem. Commun. 2005, 3018–3020. [Google Scholar] [CrossRef]
- Villari, V.; Fazio, B.; De Luca, G.; Trapani, M.; Romeo, A.; Scolaro, L.M.; Castriciano, M.A.; Mazzaglia, A.; Micali, N. Scattering enhancement in colloidal metal-organic composite aggregates. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 13–16. [Google Scholar] [CrossRef]
- Villari, V.; Fazio, B.; Micali, N.; De Luca, G.; Corsaro, C.; Romeo, A.; Scolaro, L.M.; Castriciano, M.A.; Mazzaglia, A. Light scattering enhancement in porphyrin nanocomposites. In Proceedings of the International School of Physics Enrico Fermi; Mallamace, F., Stanley, H., Eds.; IOS: Amsterdam, The Netherlands; SIF: Bologna, Italy, 2012; pp. 335–340. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Romeo, A.; Scolaro, L.M. Aggregation of meso-tetrakis(4-sulfonatophenyl)porphyrin on polyethyleneimine in aqueous solutions and on a glass surface. J. Porphyr. Phthalocyanines 2002, 6, 431–438. [Google Scholar] [CrossRef]
- Micali, N.; Villari, V.; Romeo, A.; Castriciano, M.A.; Scolaro, L.M. Evidence of the early stage of porphyrin aggregation by enhanced Raman scattering and fluorescence spectroscopy. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2007, 76. [Google Scholar] [CrossRef] [PubMed]
- Castriciano, M.A.; Trapani, M.; Romeo, A.; Depalo, N.; Rizzi, F.; Fanizza, E.; Patanè, S.; Monsù Scolaro, L. Influence of Magnetic Micelles on Assembly and Deposition of Porphyrin J-Aggregates. Nanomaterials 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapani, M.; Castriciano, M.A.; Romeo, A.; De Luca, G.; Machado, N.; Howes, B.D.; Smulevich, G.; Scolaro, L.M. Nanohybrid Assemblies of Porphyrin and Au-10 Cluster Nanoparticles. Nanomaterials 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapani, M.; De Luca, G.; Romeo, A.; Castriciano, M.A.; Scolaro, L.M. Spectroscopic investigation on porphyrins nano-assemblies onto gold nanorods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 343–349. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Leone, N.; Cardiano, P.; Manickam, S.; Scolaro, L.M.; Lo Schiavo, S. A new supramolecular polyhedral oligomeric silsesquioxanes (POSS)-porphyrin nanohybrid: Synthesis and spectroscopic characterization. J. Mater. Chem. C 2013, 1, 4746–4753. [Google Scholar] [CrossRef]
- Villari, V.; Mazzaglia, A.; Trapani, M.; Castriciano, M.A.; De Luca, G.; Romeo, A.; Scolaro, L.M.; Micali, N. Optical enhancement and structural properties of a hybrid organic-inorganic ternary nanocomposite. J. Phys. Chem. C 2011, 115, 5435–5439. [Google Scholar] [CrossRef]
- Hollingsworth, J.V.; Richard, A.J.; Vicente, M.G.H.; Russo, P.S. Characterization of the Self-Assembly of meso-Tetra(4-sulfonatophenyl)porphyrin (H2TPPS4–) in Aqueous Solutions. Biomacromolecules 2012, 13, 60–72. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Romeo, A.; Villari, V.; Micali, N.; Scolaro, L.M. Structural rearrangements in 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin J-aggregates under strongly acidic conditions. J. Phys. Chem. B 2003, 107, 8765–8771. [Google Scholar] [CrossRef]
- Romeo, A.; Castriciano, M.A.; Occhiuto, I.; Zagami, R.; Pasternack, R.F.; Scolaro, L.M. Kinetic control of chirality in porphyrin J-aggregates. J. Am. Chem. Soc. 2014, 136, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Occhiuto, I.G.; Zagami, R.; Trapani, M.; Bolzonello, L.; Romeo, A.; Castriciano, M.A.; Collini, E.; Monsù Scolaro, L. The role of counter-anions in the kinetics and chirality of porphyrin J-aggregates. Chem. Commun. 2016, 52, 11520–11523. [Google Scholar] [CrossRef] [PubMed]
- Occhiuto, I.; De Luca, G.; Trapani, M.; Scolaro, L.M.; Pasternack, R.F. Peripheral Stepwise Degradation of a Porphyrin J-Aggregate. Inorg. Chem. 2012, 51, 10074–10076. [Google Scholar] [CrossRef]
- Trapani, M.; Occhiuto, I.G.; Zagami, R.; De Luca, G.; Castriciano, M.A.; Romeo, A.; Scolaro, L.M.; Pasternack, R.F. Mechanism for Copper(II)-Mediated Disaggregation of a Porphyrin J-Aggregate. ACS Omega 2018, 3, 18843–18848. [Google Scholar] [CrossRef]
- Liu, R.; Tang, J.; Xu, Y.; Zhou, Y.; Dai, Z. Nano-sized Indocyanine Green J-aggregate as a One-component Theranostic Agent. Nanotheranostics 2017, 1, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Qu, K.; Xu, H.; Zhao, C.; Ren, J.; Qu, X. Amine-linker length dependent electron transfer between porphyrins and covalent amino-modified single-walled carbon nanotubes. RSC Adv. 2011, 1, 632–639. [Google Scholar] [CrossRef]
- Chen, J.; Collier, C.P. Noncovalent Functionalization of Single-Walled Carbon Nanotubes with Water-Soluble Porphyrins. J. Phys. Chem. B 2005, 109, 7605–7609. [Google Scholar] [CrossRef]
- Cardiano, P.; Fazio, E.; Lazzara, G.; Manickam, S.; Milioto, S.; Neri, F.; Mineo, P.G.; Piperno, A.; Lo Schiavo, S. Highly untangled multiwalled carbon nanotube@polyhedral oligomeric silsesquioxane ionic hybrids: Synthesis, characterization and nonlinear optical properties. Carbon 2015, 86, 325–337. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Ménard-Moyon, C.; Fabbro, C.; Prato, M.; Bianco, A. One-Pot Triple Functionalization of Carbon Nanotubes. Chem. Eur. J. 2011, 17, 3222–3227. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.; Angela Castriciano, M.; Scolaro, L.M. Spectroscopic and kinetic investigations on porphyrin J-aggregates induced by polyamines. J. Porphyr. Phthalocyanines 2010, 14, 713–721. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Collings, P.J. Resonance Light-Scattering—A New Technique for Studying Chromophore Aggregation. Science 1995, 269, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Tuci, G.; Vinattieri, C.; Luconi, L.; Ceppatelli, M.; Cicchi, S.; Brandi, A.; Filippi, J.; Melucci, M.; Giambastiani, G. “Click” on Tubes: A Versatile Approach towards Multimodal Functionalization of SWCNTs. Chem. Eur. J. 2012, 18, 8454–8463. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Piperno, A.; Ferlazzo, A.; Pistone, A.; Milone, C.; Lanza, M.; Cimino, F.; Speciale, A.; Trombetta, D.; Saija, A.; et al. Functionalization of multi-walled carbon nanotubes with coumarin derivatives and their biological evaluation. Organ. Biomol. Chem. 2012, 10, 1025–1031. [Google Scholar] [CrossRef]
- Grassi, G.; Scala, A.; Piperno, A.; Iannazzo, D.; Lanza, M.; Milone, C.; Pistone, A.; Galvagno, S. A facile and ecofriendly functionalization of multiwalled carbon nanotubes by an old mesoionic compound. Chem. Commun. 2012, 48, 6836–6838. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Carbone, A.; Saccà, A.; Donato, M.G.; Micali, N.; Romeo, A.; De Luca, G.; Scolaro, L.M. Optical and sensing features of TPPS4 J-aggregates embedded in Nafion® membranes: Influence of casting solvents. J. Mater. Chem. 2010, 20, 2882–2886. [Google Scholar] [CrossRef]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J- and H-aggregates of porphyrins with surfactants: Fluorescence, stopped flow and electron microscopy studies. J. Porphyr. Phthalocyanines 1998, 2, 369–376. [Google Scholar] [CrossRef]
- Maiti, N.C.; Ravikanth, M.; Mazumdar, S.; Periasamy, N. Fluorescence Dynamics of Noncovalently Linked Porphyrin Dimers, and Aggregates. J. Phys. Chem. B 1995, 99, 17192–17197. [Google Scholar] [CrossRef]
- Micali, N.; Villari, V.; Scolaro, L.M.; Romeo, A.; Castriciano, M.A. Light scattering enhancement in an aqueous solution of spermine-induced fractal J-aggregate composite. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005, 72. [Google Scholar] [CrossRef]
- Micali, N.; Villari, V.; Castriciano, M.A.; Romeo, A.; Scolaro, L.M. From fractal to nanorod porphyrin J-aggregates. concentration-induced tuning of the aggregate size. J. Phys. Chem. B 2006, 110, 8289–8295. [Google Scholar] [CrossRef] [PubMed]
- Trapani, M.; Romeo, A.; Parisi, T.; Sciortino, M.T.; Patanè, S.; Villari, V.; Mazzaglia, A. Supramolecular hybrid assemblies based on gold nanoparticles, amphiphilic cyclodextrin and porphyrins with combined phototherapeutic action. RSC Adv. 2013, 3, 5607–5614. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trapani, M.; Mazzaglia, A.; Piperno, A.; Cordaro, A.; Zagami, R.; Castriciano, M.A.; Romeo, A.; Monsù Scolaro, L. Novel Nanohybrids Based on Supramolecular Assemblies of Meso-tetrakis-(4-sulfonatophenyl) Porphyrin J-aggregates and Amine-Functionalized Carbon Nanotubes. Nanomaterials 2020, 10, 669. https://doi.org/10.3390/nano10040669
Trapani M, Mazzaglia A, Piperno A, Cordaro A, Zagami R, Castriciano MA, Romeo A, Monsù Scolaro L. Novel Nanohybrids Based on Supramolecular Assemblies of Meso-tetrakis-(4-sulfonatophenyl) Porphyrin J-aggregates and Amine-Functionalized Carbon Nanotubes. Nanomaterials. 2020; 10(4):669. https://doi.org/10.3390/nano10040669
Chicago/Turabian StyleTrapani, Mariachiara, Antonino Mazzaglia, Anna Piperno, Annalaura Cordaro, Roberto Zagami, Maria Angela Castriciano, Andrea Romeo, and Luigi Monsù Scolaro. 2020. "Novel Nanohybrids Based on Supramolecular Assemblies of Meso-tetrakis-(4-sulfonatophenyl) Porphyrin J-aggregates and Amine-Functionalized Carbon Nanotubes" Nanomaterials 10, no. 4: 669. https://doi.org/10.3390/nano10040669