Supercapacitor Performance of Nickel-Cobalt Sulfide Nanotubes Decorated Using Ni Co-Layered Double Hydroxide Nanosheets Grown in Situ on Ni Foam
Abstract
:1. Introduction
2. Experimental Details
2.1. Chemicals
2.2. Fabrication of NCS Nanotubes on the Substrate
2.3. Fabrication of NCS@NCOH Core-Shell Nanotube Arrays
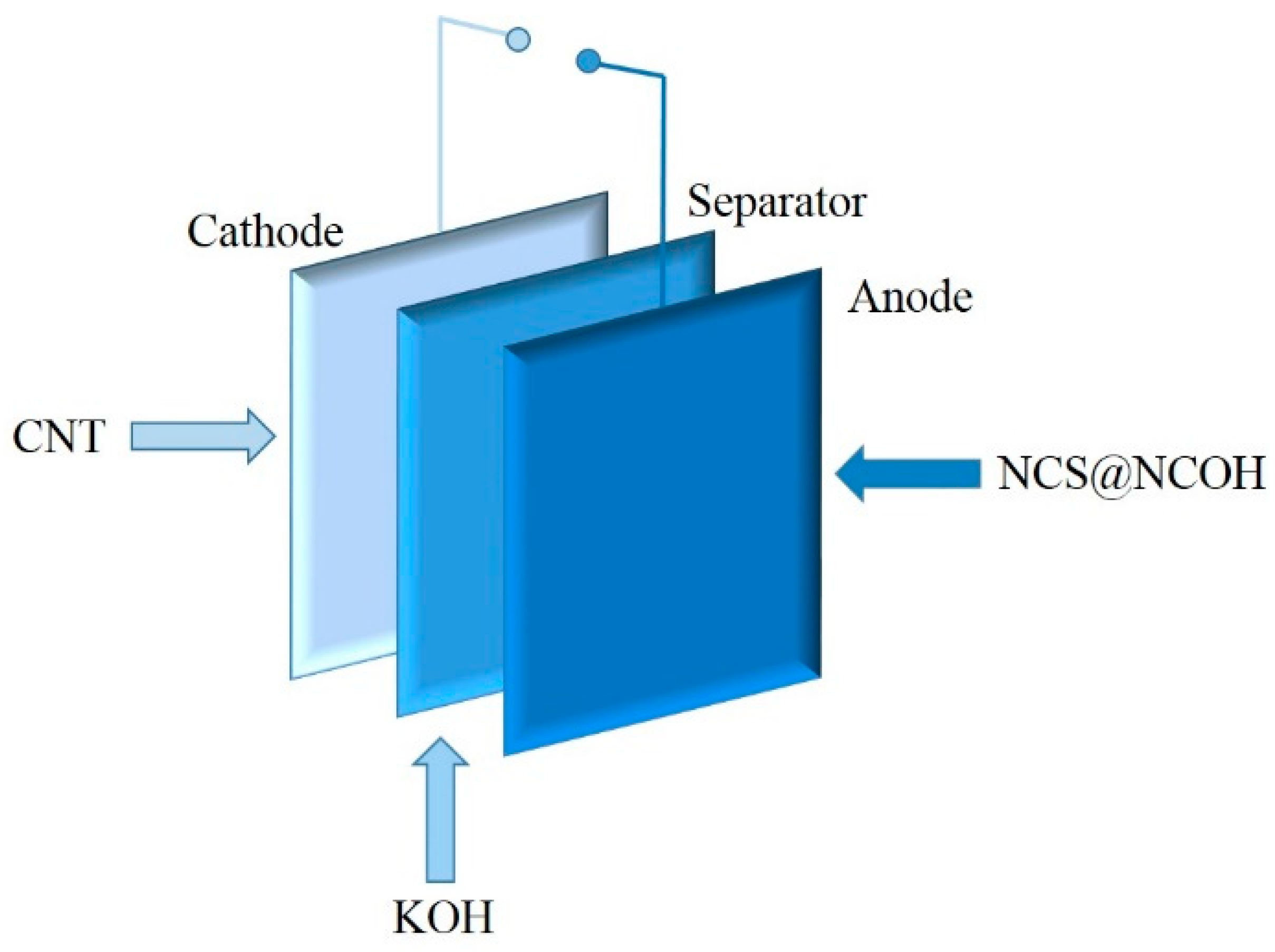
2.4. NCS@NCOH//AC Asymmetric Supercapacitor (ASC) Device Fabrication
2.5. Material Characterization
2.6. Electrochemical Experiments
3. Results and Discussion
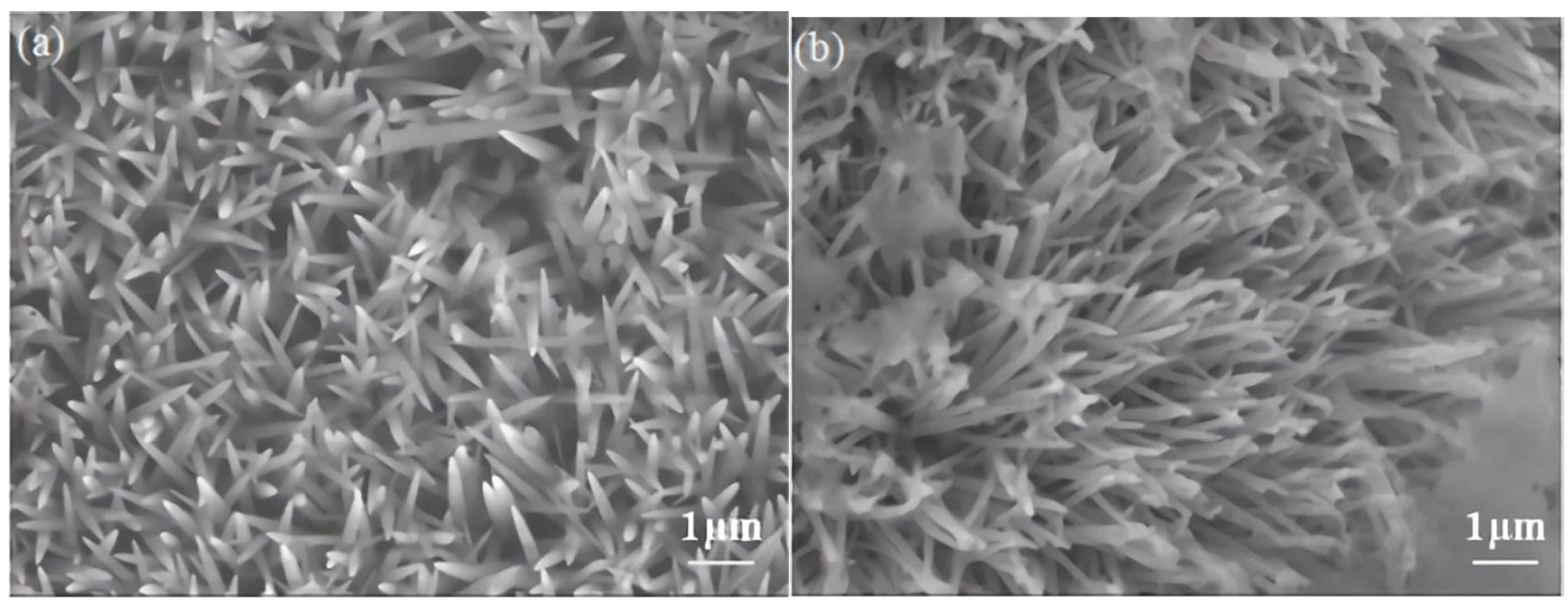
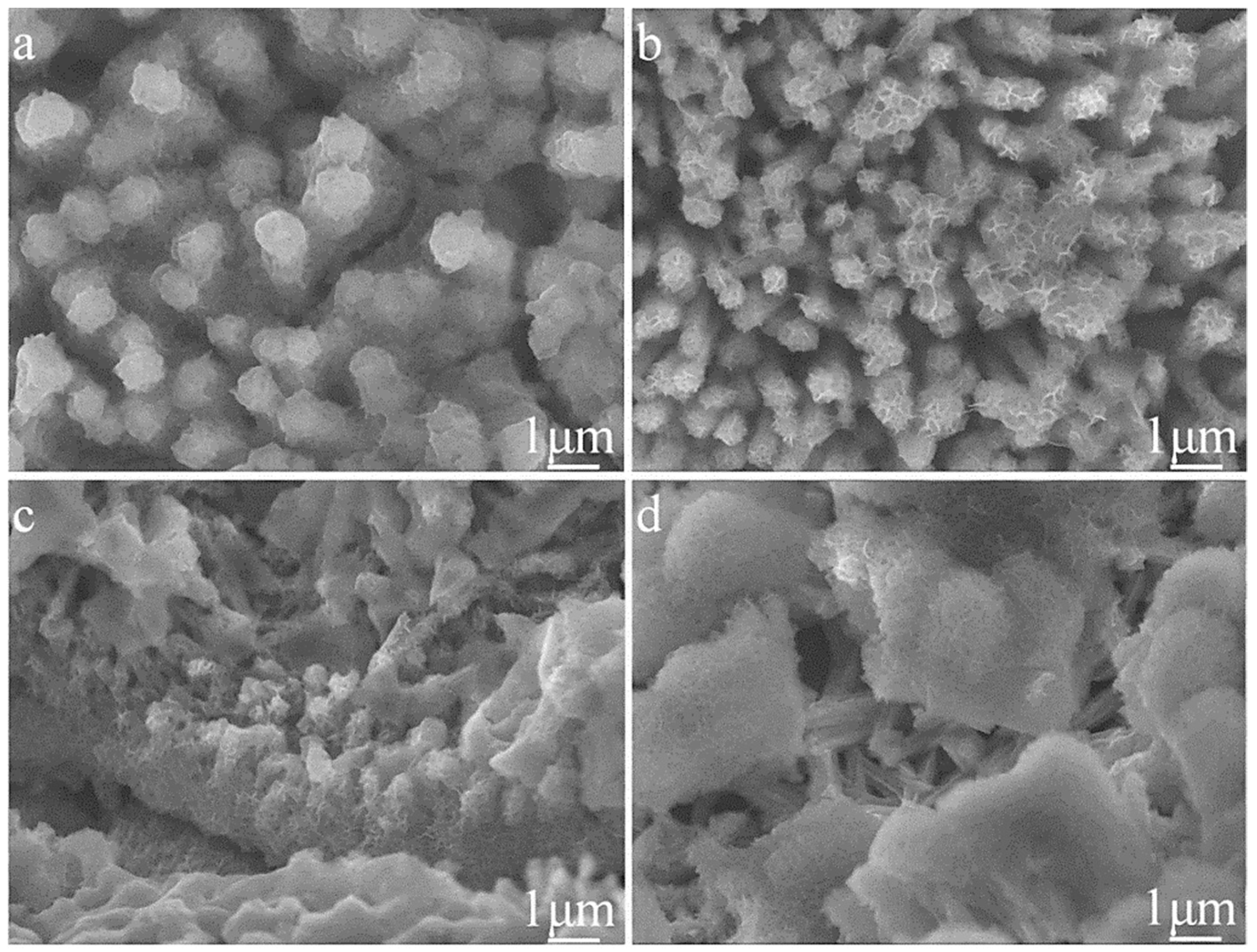
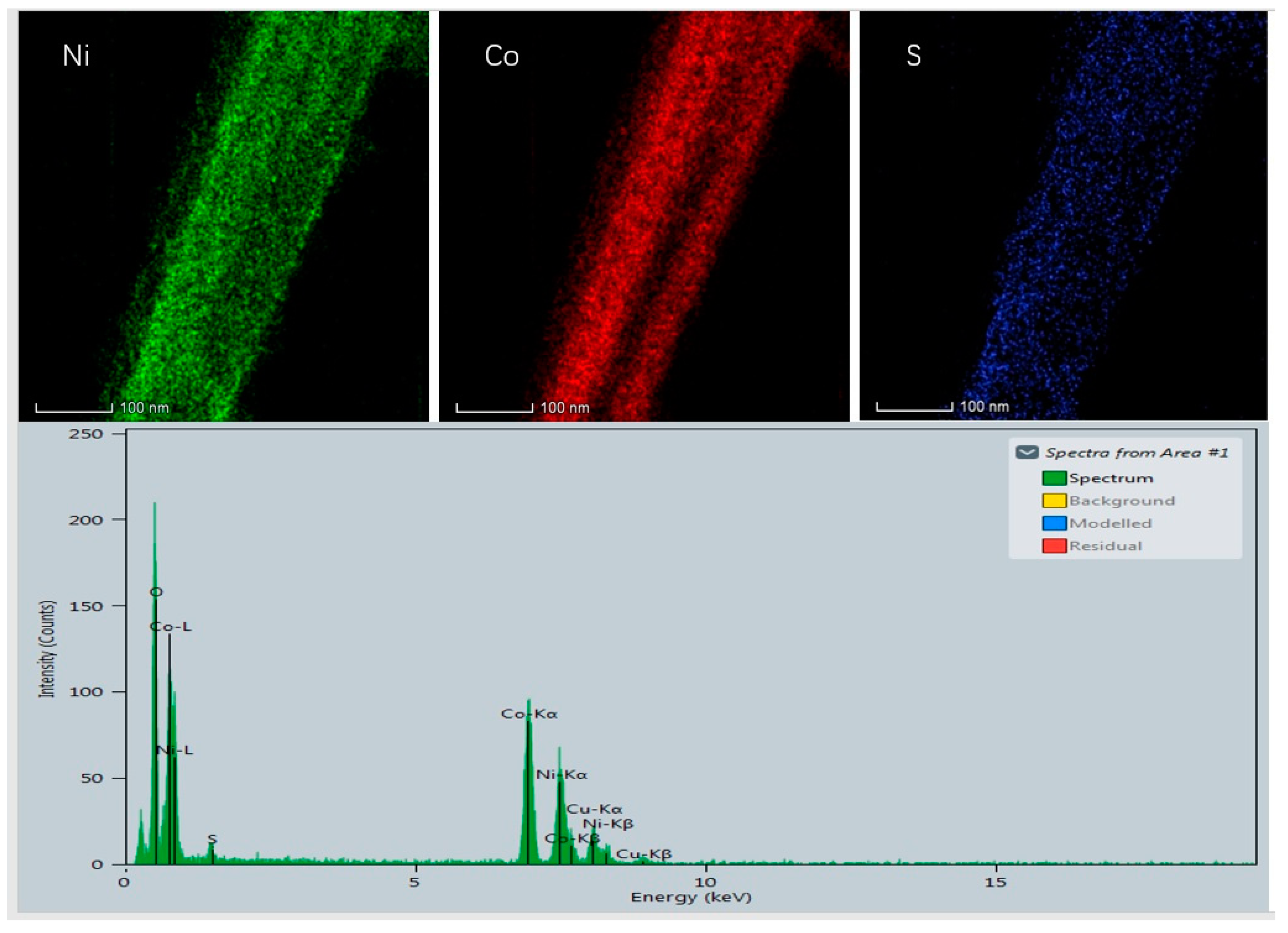
3.1. Sample Characterizations
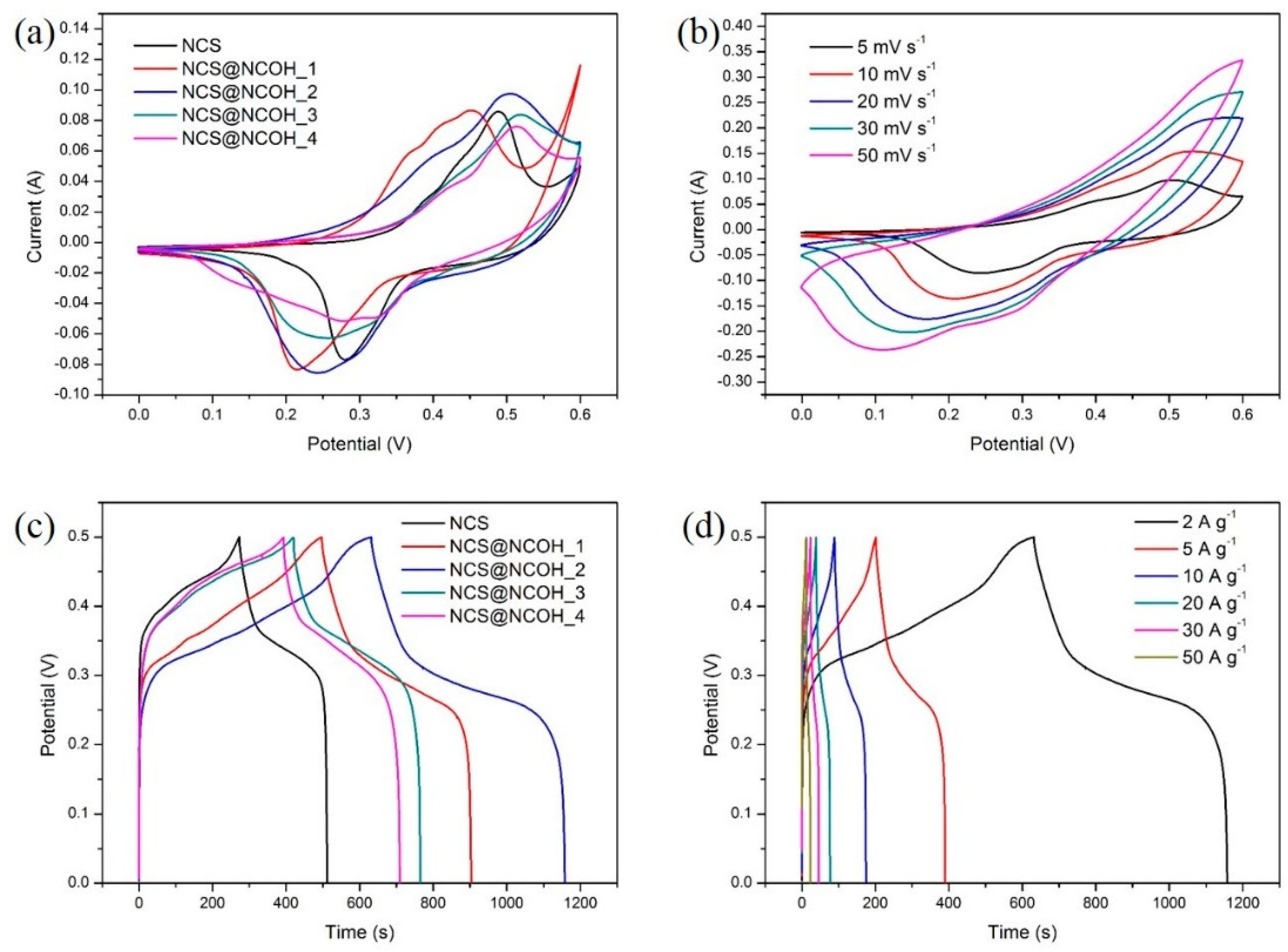
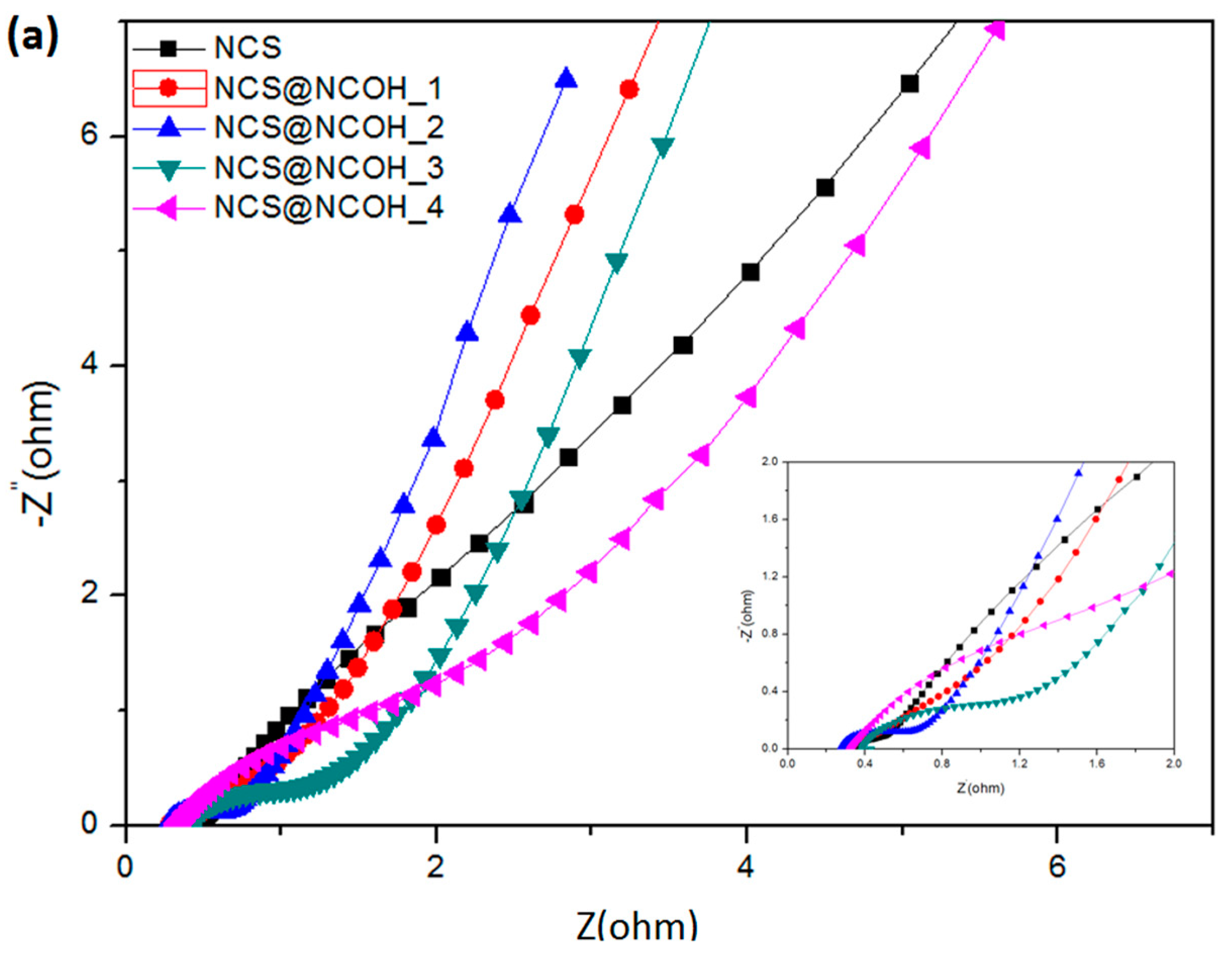
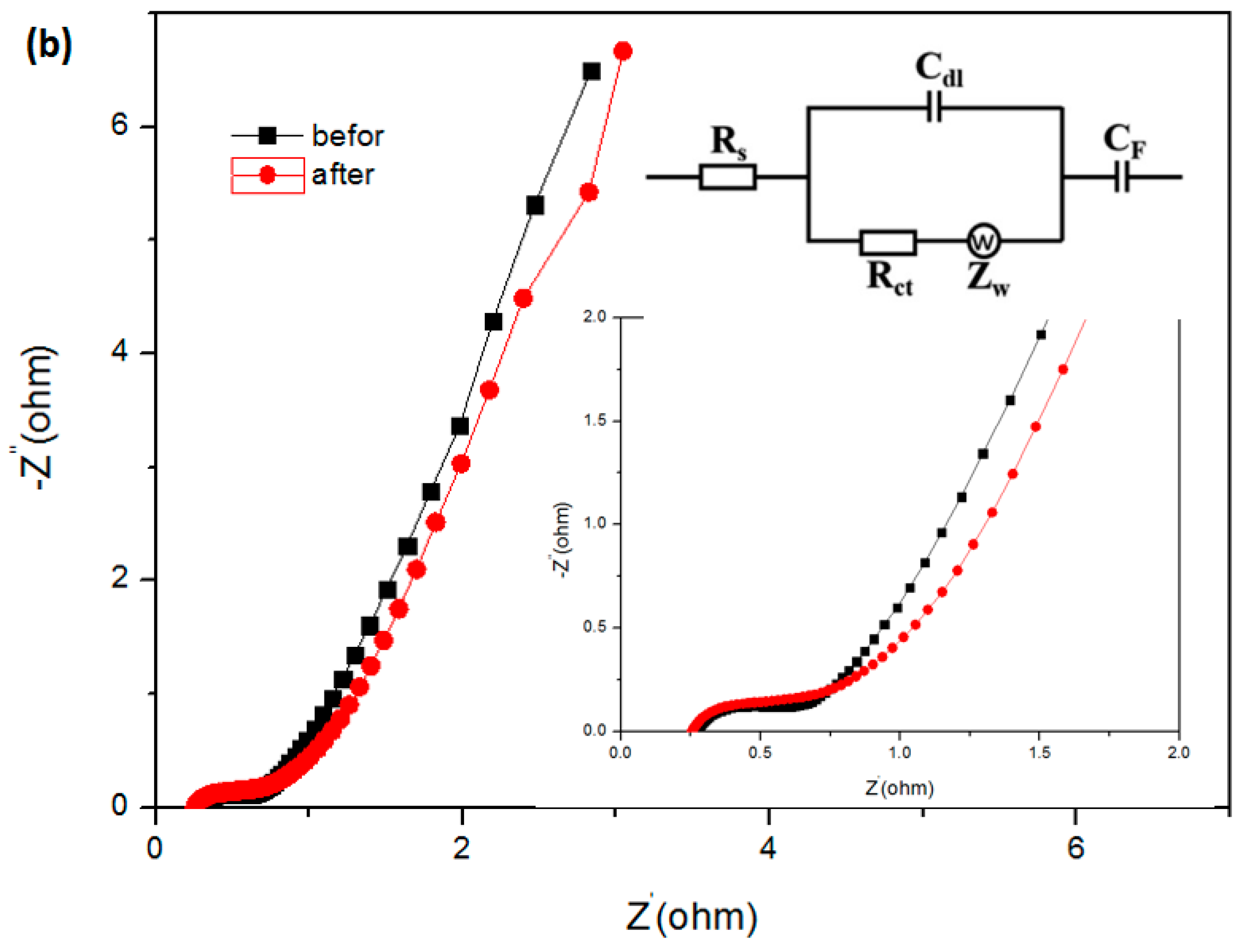
3.2. Electrochemical Characterizations of the Working Electrode
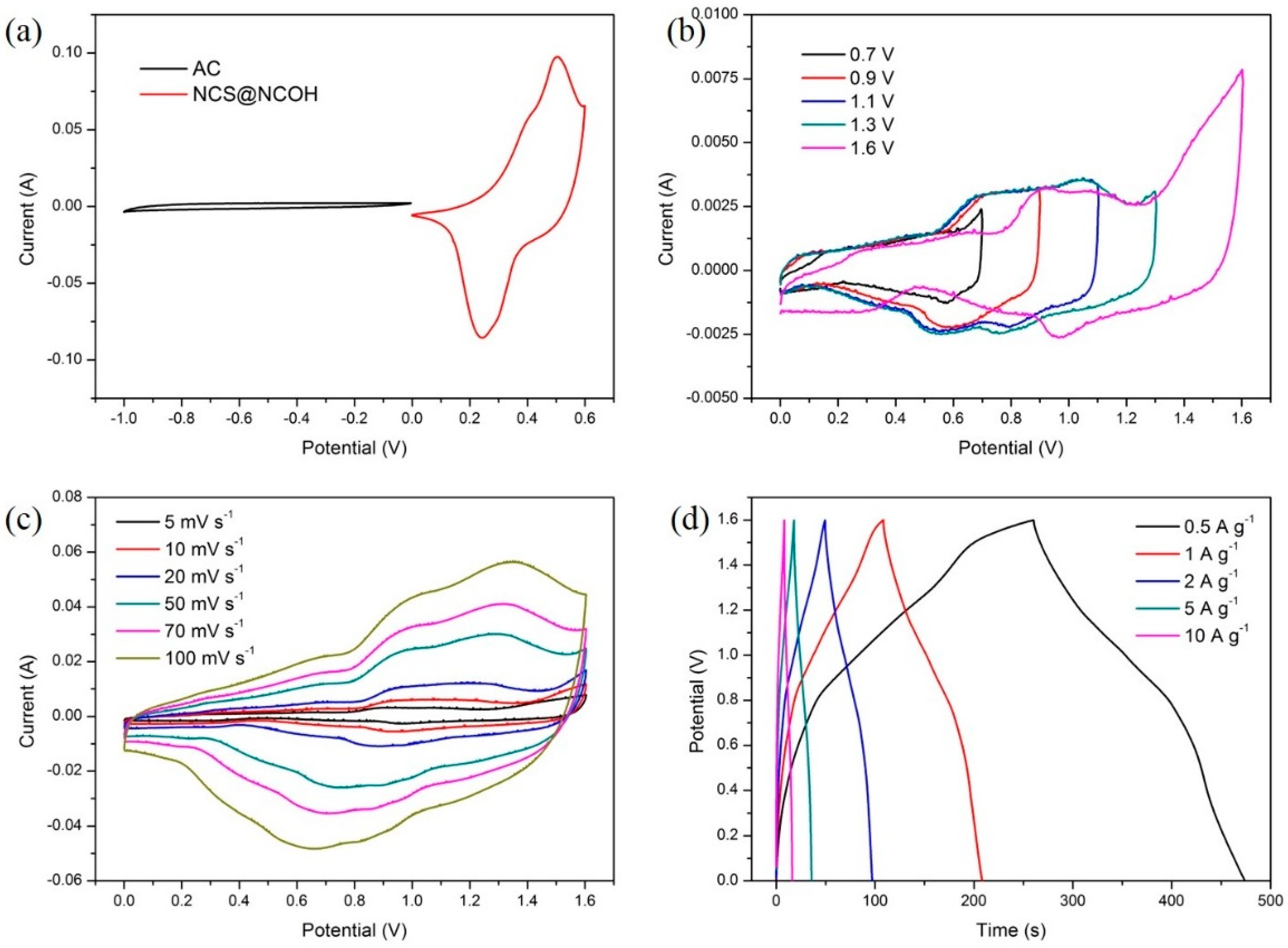
3.3. Performance Evaluation of Asymmetry Supercapacitor Based on Active Carbon and NCS@NCOH
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Williams, J.H.; DeBenedictis, A.; Ghanadan, R.; Mahone, A.; Moore, J.; Morrow, W.R.; Price, S.; Torn, M.S. The technology path to deep greenhouse gas emissions cuts by 2050: The pivotal role of electricity. Science 2012, 335, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Harris, N.L.; Brown, S.; Hagen, S.C.; Saatchi, S.S.; Petrova, S.; Salas, W.; Hansen, M.C.; Potapov, P.V.; Lotsch, A. Baseline map of carbon emissions from deforestation in tropical regions. Science 2012, 336, 1573–1576. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, J.; Li, C.Z. Mesoporous carbon incorporated metal oxide nanomaterials as supercapacitor electrodes. Adv. Mater. 2012, 24, 4197–4202. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Tan, H.; Cai, R.; Shi, W.; Li, C.; Mhaisalkar, S.G.; Srinivasan, M.; Ramakrishna, S.; Yan, Q. MS 2 (M = Co and Ni) hollow spheres with tunable interiors for high-performance supercapacitors and photovoltaics. Adv. Funct. Mater. 2014, 24, 2155–2162. [Google Scholar] [CrossRef]
- Amaresh, S.; Karthikeyan, K.; Jang, I.C.; Lee, Y.S. Single-step microwave mediated synthesis of the CoS2 anode material for high rate hybrid supercapacitors. J. Mater. Chem. A 2014, 2, 11099–11106. [Google Scholar] [CrossRef]
- Luo, F.; Li, J.; Yuan, H.; Xiao, D. Rapid synthesis of three-dimensional flower-like cobalt sulfide hierarchitectures by microwave assisted heating method for high-performance supercapacitors. Electrochim. Acta 2014, 123, 183–189. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, C.; Zhang, D.; Zhao, J.; Zheng, S.; Su, H.; Wei, F.; Yuan, B.; Fernandezb, C. Facile synthesis of a nickel sulfide (NiS) hierarchical flower for the electrochemical oxidation of H2O2 and the methanol oxidation reaction (MOR). J. Electrochem. Soc. 2017, 164, B92–B96. [Google Scholar] [CrossRef] [Green Version]
- Raj, C.J.; Kim, B.C.; Cho, W.J.; Lee, W.G.; Seo, Y.; Yu, K.H. Electrochemical capacitor behavior of copper sulfide (CuS) nanoplatelets. J. Alloys Compd. 2014, 586, 191–196. [Google Scholar]
- Falola, B.D.; Wiltowski, T.; Suni, I.I. Electrodeposition of MoS2 for charge storage in electrochemical supercapacitors. J. Electrochem. Soc. 2016, 163, D568–D574. [Google Scholar] [CrossRef] [Green Version]
- Khawula, T.N.; Raju, K.; Franklyn, P.J.; Sigalas, I.; Ozoemena, K.I. The effects of morphology re-arrangements on the pseudocapacitive properties of mesoporous molybdenum disulfide (MoS2) nanoflakes. J. Electrochem. Soc. 2016, 163, A1927–A1935. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Musharavati, F.; Sun, J.; Jaber, F.; Zalnezhad, E.; Hui, K.N.; Hui, K.S. Investigation of the electrochemical properties of CoAl-layered double hydroxide/Ni(OH)2. J. Electrochem. Soc. 2018, 165, A407–A415. [Google Scholar] [CrossRef] [Green Version]
- Newman, S.P.; Jones, W.; O’Connor, P.; Stamires, D.N. Synthesis of the 3R(2) polytype of a hydrotalcite-like mineral. J. Mater. Chem. 2002, 12, 153–155. [Google Scholar] [CrossRef]
- Ulibarri, M.A.; Pavlovic, I.; Barriga, C.; Hermosın, M.C.; Cornejo, J. Adsorption of anionic species on hydrotalcite-like compounds: Effect of interlayer anion and crystallinity. Appl. Clay Sci. 2001, 18, 17–27. [Google Scholar] [CrossRef]
- Leroux, F.; Besse, J.P. Polymer interleaved layered double hydroxide: A new emerging class of nanocomposites. Chem. Mater. 2001, 13, 3507–3515. [Google Scholar] [CrossRef]
- De Vos, D.E.; Wahlen, J.; Sels, B.F.; Jacobs, P.A. Peroxide reactions with anion-exchanged layered double hydroxide catalysts. Synlett 2002, 2002, 367–380. [Google Scholar] [CrossRef]
- Choy, J.H.; Kwak, S.Y.; Jeong, Y.J.; Park, J.S. Inorganic layered double hydroxides as nonviral vectors. Angew. Chem. Int. Ed. 2000, 39, 4042–4045. [Google Scholar] [CrossRef]
- Choy, J.H.; Kwak, S.Y.; Park, J.S.; Jeong, Y.J.; Portier, J. Intercalative nanohybrids of nucleoside monophosphates and DNA in layered metal hydroxide. J. Am. Chem. Soc. 1999, 121, 1399–1400. [Google Scholar] [CrossRef]
- Madhavan, D.; Pitchumani, K. Photodimerisation of enones in a clay microenvironment. Photochem. Photobiol. Sci. 2002, 1, 991–995. [Google Scholar] [CrossRef]
- Li, Q.; Deng, L.; Kim, J.K.; Zhu, Y.Q.; Holmes, S.M.; Perez-Page, M.; Eichhorn, S.J. Growth of carbon nanotubes on electrospun cellulose fibers for high performance supercapacitors. J. Electrochem. Soc. 2017, 164, A3220–A3228. [Google Scholar] [CrossRef] [Green Version]
- Yeager, M.P.; Su, D.; Marinković, N.S.; Teng, X. Pseudocapacitive NiO fine nanoparticles for supercapacitor reactions. J. Electrochem. Soc. 2012, 159, A1598–A1603. [Google Scholar] [CrossRef]
- Thota, R.; Ganesh, V. Electrodeposition of Hierarchical Nanosheet Arrays of NiCo2S4 onto a Polymer Substrate: A New High Power Flexible Battery Electrode. J. Electrochem. Soc. 2017, 164, A3793–A3803. [Google Scholar] [CrossRef]
- Li, H.; Musharavati, F.; Zalenezhad, E.; Chen, X.; Hui, K.N.; Hui, K.S. Electrodeposited Ni-Co layered double hydroxides on titanium carbide as a binder-free electrode for supercapacitors. Electrochim. Acta 2018, 261, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Xia, C.; Alshareef, H.N. One-step electrodeposited nickel cobalt sulfide nanosheet arrays for high-performance asymmetric supercapacitors. ACS Nano 2014, 8, 9531–9541. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.U.; Manthiram, A. Synthesis of nickel sulfides in aqueous solutions using sodium dithionite. Inorg. Chem. 2001, 40, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zou, R.; Li, W.; Liu, Q.; Liu, X.; An, L.; Hu, J. Design and synthesis of 3D interconnected mesoporous NiCo2O4@ CoxNi1− x(OH)2 core–shell nanosheet arrays with large areal capacitance and high rate performance for supercapacitors. J. Mater. Chem. A 2014, 2, 10090–10097. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L.; Wan, H.; Qi, T.; Xia, D. Highly conductive NiCo2S4 urchin-like nanostructures for high-rate pseudocapacitors. Nanoscale 2013, 5, 8879–8883. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, K.; Wang, D.; Chu, J.; Li, J.; Zhao, L.; Ding, C.; Du, Y.; Jia, X.; Wen, G. Hierarchically constructed NiCo2S4@ Ni(1−x)Cox(OH)2 core/shell nanoarrays and their application in energy storage. Nanotechnology 2016, 27, 235402. [Google Scholar] [CrossRef]
- Luo, L.; He, B.; Kong, W.; Wang, Z. NiCo2S4/Ni–Co layered double hydroxide nanocomposite prepared by a vapor-phase hydrothermal method for electrochemical capacitor application. J. Alloys Compd. 2017, 705, 349–355. [Google Scholar] [CrossRef]
- Kong, W.; Lu, C.; Zhang, W.; Pu, J.; Wang, Z. Homogeneous core–shell NiCo2S4 nanostructures supported on nickel foam for supercapacitors. J. Mater. Chem. A 2015, 3, 12452–12460. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, H.; Liu, Y.; Zhao, Y.; Zhang, B. Hierarchical polypyrrole nanotubes@ NiCo2S4 nanosheets core-shell composites with improved electrochemical performance as supercapacitors. Electrochim. Acta 2017, 258, 182–191. [Google Scholar] [CrossRef]
- Tiruneh, S.N.; Kang, B.K.; Kwag, S.H.; Lee, Y.; Kim, M.; Yoon, D.H. Synergistically active NiCo2S4 nanoparticles coupled with holey defect graphene hydrogel for high-performance solid-state supercapacitors. Chem. Eur. J. 2018, 24, 3263–3270. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.S.; Chien, P.Y.; Lin, J.Y.; Chou, S.W.; Wu, W.K.; Li, P.H.; Wu, K.Y.; Lin, T.W. Hierarchically structured Ni3S2/carbon nanotube composites as high performance cathode materials for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 12168–12174. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Du, X.; Ma, X.; Hao, X.; Xue, C.; Zhu, H.; Li, S. Controllable synthesis of NiCo LDH nanosheets for fabrication of high-performance supercapacitor electrodes. Electroanalysis 2017, 29, 1286–1293. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, C.; Ang, L.; Musharavati, F.; Jaber, F.; Hui, L.; Zalnezhad, E.; Bae, S.; Hui, K.S.; Hui, K.N. Supercapacitor Performance of Nickel-Cobalt Sulfide Nanotubes Decorated Using Ni Co-Layered Double Hydroxide Nanosheets Grown in Situ on Ni Foam. Nanomaterials 2020, 10, 584. https://doi.org/10.3390/nano10030584
Xin C, Ang L, Musharavati F, Jaber F, Hui L, Zalnezhad E, Bae S, Hui KS, Hui KN. Supercapacitor Performance of Nickel-Cobalt Sulfide Nanotubes Decorated Using Ni Co-Layered Double Hydroxide Nanosheets Grown in Situ on Ni Foam. Nanomaterials. 2020; 10(3):584. https://doi.org/10.3390/nano10030584
Chicago/Turabian StyleXin, Chen, Li Ang, Farayi Musharavati, Fadi Jaber, Li Hui, Erfan Zalnezhad, Sungchul Bae, Kwan San Hui, and Kwun Nam Hui. 2020. "Supercapacitor Performance of Nickel-Cobalt Sulfide Nanotubes Decorated Using Ni Co-Layered Double Hydroxide Nanosheets Grown in Situ on Ni Foam" Nanomaterials 10, no. 3: 584. https://doi.org/10.3390/nano10030584




