An Ultrasensitive Non-Enzymatic Sensor for Quantitation of Anti-Cancer Substance Chicoric Acid Based on Bimetallic Nanoalloy with Polyetherimide-Capped Reduced Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
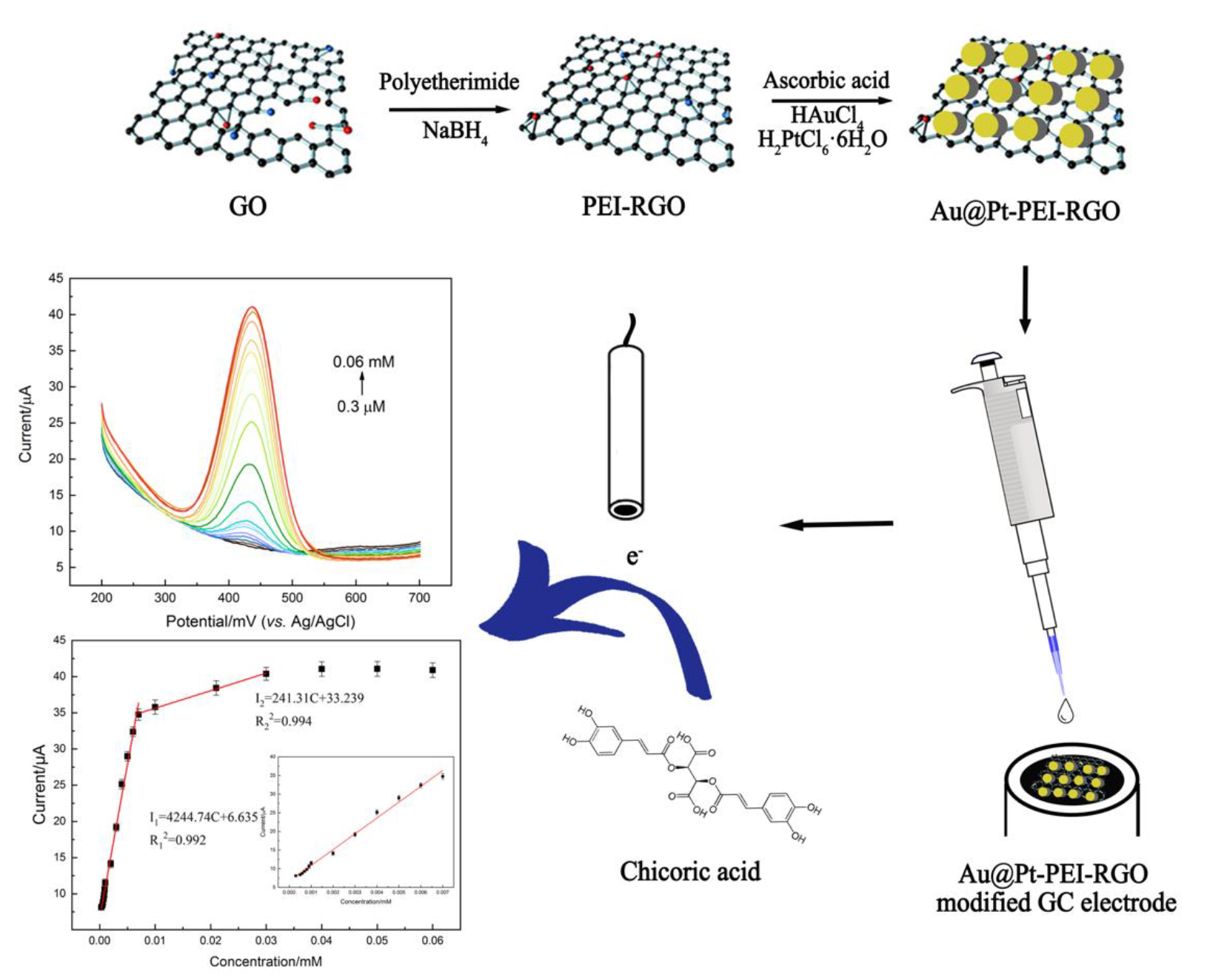
2.2. Preparation of PEI-Functionalized RGO
2.3. Preparation of Au@Pt-PEI-RGO
2.4. Preparation of Modified Electrodes
3. Results and Discussion
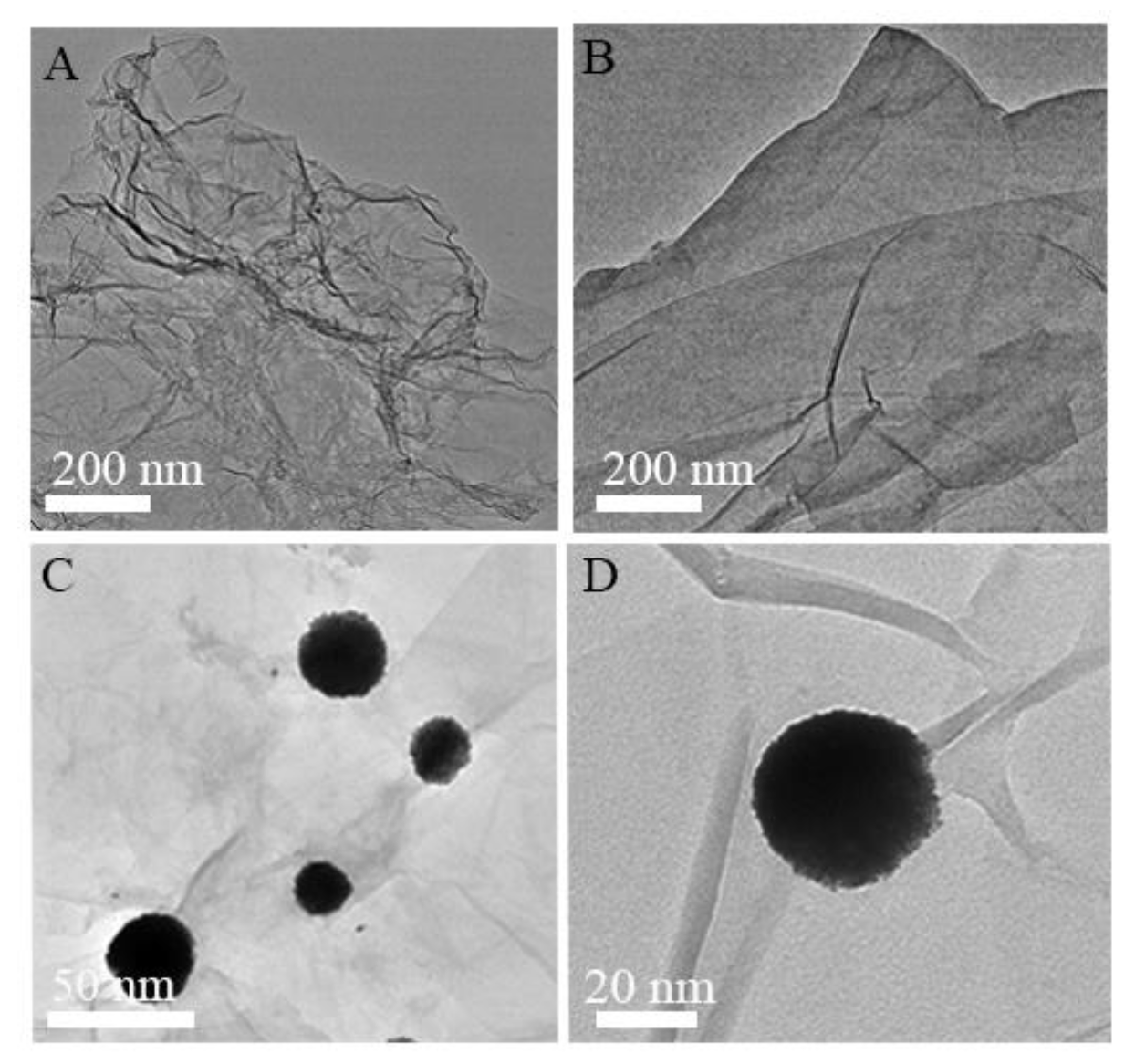
3.1. Characterization of Au@Pt-PEI-RGO Nanocomposites
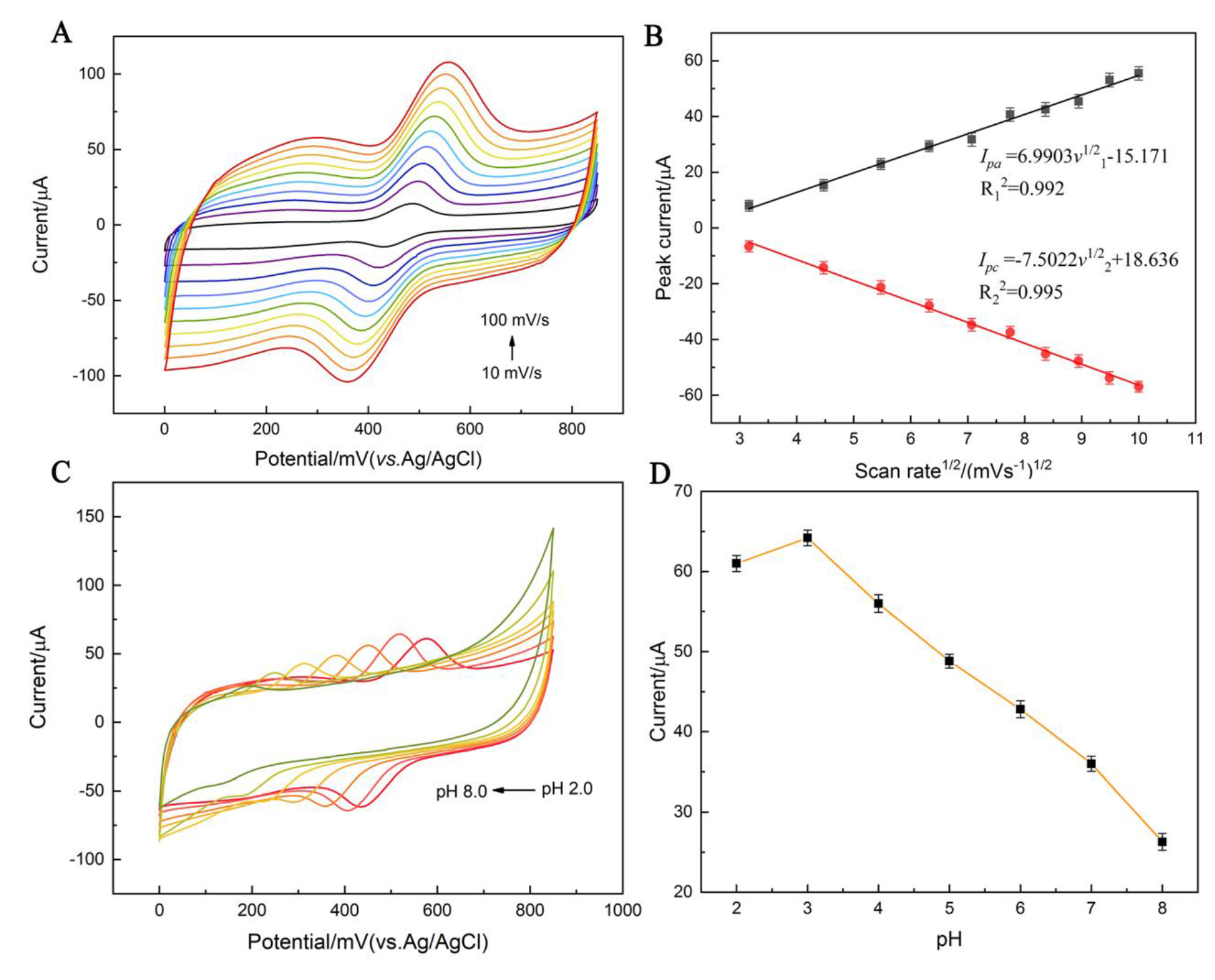
3.2. Electrochemical Behavior of Obtained Materials
3.3. Electrochemical Response of Chicoric Acid at Au@Pt-PEI-RGO/GCE
3.4. Performance of the Proposed Sensor
3.4.1. Optimization of the Experimental Variables
3.4.2. Electrochemical Determination of Chicoric Acid
3.4.3. Interference Immunity, Repeatability, and Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Health Observatory: Geneva: World Health Organization, 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Van Cutsem, E.; Kang, Y.; Chung, H.; Shen, L.; Sawaki, A.; Lordick, F.; Hill, J.; Lehle, M.; Feyereislova, A.; Bang, Y. Efficacy results from the ToGA trial: A phase III study of trastuzumab added to standard chemotherapy in first-line HER2-positive advanced gastric cancer. J. Clin. Oncol. 2009, 27, LBA4509. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mata, R.; Nakkala, J.R.; Sadras, S.R. Polyphenol stabilized colloidal gold nanoparticles from Abutilon indicum leaf extract induce apoptosis in HT-29 colon cancer cells. Colloids Surf. B Biointerfaces 2016, 143, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Turan, I.; Demir, S.; Kilinc, K.; Burnaz, N.A.; Yaman, S.O.; Akbulut, K.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Antiproliferative and apoptotic effect of Morus nigra extract on human prostate cancer cells. Saudi Pharm. J. 2017, 25, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croft, K.D.; Yamashita, Y.; O’Donoghue, H.; Shirasaya, D.; Ward, N.C.; Ashida, H. Screening plant derived dietary phenolic compounds for bioactivity related to cardiovascular disease. Fitoterapia 2018, 126, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Bahramsoltani, R.; Abdolghaffari, A.H.; Sodagari, H.R.; Esfahani, S.A.; Rezaei, N. A mechanistic review on plant-derived natural compounds as dietary supplements for prevention of inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Scarpati, M.; Oriente, G. Chicoric acid (dicaffeyltartic acid): Its isolation from chicory (Chicorium intybus) and synthesis. Tetrahedron 1958, 4, 43–48. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef] [Green Version]
- Pluymers, W.; Neamati, N.; Pannecouque, C.; Fikkert, V.; Marchand, C.; Burke, T.R.; Pommier, Y.; Schols, D.; De Clercq, E.; Debyser, Z. Viral entry as the primary target for the anti-HIV activity of chicoric acid and its tetra-acetyl esters. Mol. Pharmacol. 2000, 58, 641–648. [Google Scholar] [CrossRef]
- Park, C.M.; Jin, K.-S.; Lee, Y.-W.; Song, Y.S. Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-κB translocation in LPS stimulated RAW 264.7 cells. Eur. J. Pharmacol. 2011, 660, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, M.; Kalavagunta, P.K.; Li, J.; He, Q.; Zhang, Y.; Ahmad, O.; Yin, H.; Wang, T.; Shang, J. Protective effects of cichoric acid on H2O2-induced oxidative injury in hepatocytes and larval zebrafish models. Biomed. Pharmacother. 2018, 104, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, X.; Zhai, H.; Zhang, D.; Ma, S. Chicoric acid (CA) induces autophagy in gastric cancer through promoting endoplasmic reticulum (ER) stress regulated by AMPK. Biomed. Pharmacother. 2019, 118, 109144. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-L.; Chiu, C.-C.; Chen, J.Y.-F.; Chan, K.-C.; Lin, S.-D. Cytotoxic effects of Echinacea purpurea flower extracts and cichoric acid on human colon cancer cells through induction of apoptosis. J. Ethnopharmacol. 2012, 143, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, C.; Carnat, A.; Fraisse, D.; Lamaison, J.L.; Rock, E.; Michel, H.; Amouroux, P.; Remesy, C. Characterisation and variation of antioxidant micronutrients in lettuce (Lactuca sativa folium). J. Sci. Food Agric. 2004, 84, 2061–2069. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Baur, S.; Klaiber, R.G.; Koblo, A.; Carle, R. Effect of different washing procedures on phenolic metabolism of shredded, packaged iceberg lettuce during storage. J. Agric. Food Chem. 2004, 52, 7017–7025. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Zduńczyk, Z.; Żary-Sikorska, E.; Król, B.; Milala, J.; Jurgoński, A. Effect of the dietary polyphenolic fraction of chicory root, peel, seed and leaf extracts on caecal fermentation and blood parameters in rats fed diets containing prebiotic fructans. Br. J. Nutr. 2011, 105, 710–720. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Xu, H.; Gu, Z.; Wang, C.; Du, Y. Sensitive detection of caffeic acid with trifurcate PtCu nanocrystals modified glassy carbon electrode. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 27–31. [Google Scholar] [CrossRef]
- Di Carlo, G.; Curulli, A.; Toro, R.G.; Bianchini, C.; De Caro, T.; Padeletti, G.; Zane, D.; Ingo, G.M. Green synthesis of gold–chitosan nanocomposites for caffeic acid sensing. Langmuir 2012, 28, 5471–5479. [Google Scholar] [CrossRef]
- Hayat, A.; Rhouati, A.; Mishra, R.K.; Alonso, G.A.; Nasir, M.; Istamboulie, G.; Marty, J.L. An electrochemical sensor based on TiO2/activated carbon nanocomposite modified screen printed electrode and its performance for phenolic compounds detection in water samples. Int. J. Environ. Anal. Chem. 2016, 96, 237–246. [Google Scholar] [CrossRef]
- Bharath, G.; Alhseinat, E.; Madhu, R.; Mugo, S.M.; Alwasel, S.; Harrath, A.H. Facile synthesis of Au@ α-Fe2O3@ RGO ternary nanocomposites for enhanced electrochemical sensing of caffeic acid toward biomedical applications. J. Alloy. Compd. 2018, 750, 819–827. [Google Scholar] [CrossRef]
- Dai, H.; Chen, D.; Cao, P.; Li, Y.; Wang, N.; Sun, S.; Chen, T.; Ma, H.; Lin, M. Molybdenum sulfide/nitrogen-doped carbon nanowire-based electrochemical sensor for hydrogen peroxide in living cells. Sens. Actuators B Chem. 2018, 276, 65–71. [Google Scholar] [CrossRef]
- McGhee, C.E.; Loh, K.Y.; Lu, Y. DNAzyme sensors for detection of metal ions in the environment and imaging them in living cells. Curr. Opin. Biotechnol. 2017, 45, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Manibalan, K.; Mani, V.; Chang, P.-C.; Huang, C.-H.; Huang, S.-T.; Marchlewicz, K.; Neethirajan, S. Electrochemical latent redox ratiometric probes for real-time tracking and quantification of endogenous hydrogen sulfide production in living cells. Biosens. Bioelectron. 2017, 96, 233–238. [Google Scholar] [CrossRef]
- Webb, J.A.; Bardhan, R. Emerging advances in nanomedicine with engineered gold nanostructures. Nanoscale 2014, 6, 2502–2530. [Google Scholar] [CrossRef]
- Yu, G.; Wu, W.; Pan, X.; Zhao, Q.; Wei, X.; Lu, Q. High sensitive and selective sensing of hydrogen peroxide released from pheochromocytoma cells based on Pt-Au bimetallic nanoparticles electrodeposited on reduced graphene sheets. Sensors 2015, 15, 2709–2722. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Manna, L.; Cabot, A.; Hens, Z.; Talapin, D.V.; Kagan, C.R.; Klimov, V.I.; Rogach, A.L.; Reiss, P.; Milliron, D.J. Prospects of Nanoscience with Nanocrystals; ACS Publications: Washington, DC, USA, 2015. [Google Scholar]
- Jia, Y.; Jiang, Y.; Zhang, J.; Zhang, L.; Chen, Q.; Xie, Z.; Zheng, L. Unique excavated rhombic dodecahedral PtCu3 alloy nanocrystals constructed with ultrathin nanosheets of high-energy {110} facets. J. Am. Chem. Soc. 2014, 136, 3748–3751. [Google Scholar] [CrossRef]
- Huang, K.-J.; Liu, Y.-J.; Liu, Y.-M.; Wang, L.-L. Molybdenum disulfide nanoflower-chitosan-Au nanoparticles composites based electrochemical sensing platform for bisphenol A determination. J. Hazard. Mater. 2014, 276, 207–215. [Google Scholar] [CrossRef]
- Chen, A.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef]
- Liu, W.; Hiekel, K.; Huebner, R.; Sun, H.; Ferancova, A.; Sillanpää, M. Pt and Au bimetallic and monometallic nanostructured amperometric sensors for direct detection of hydrogen peroxide: Influences of bimetallic effect and silica support. Sens. Actuators B Chem. 2018, 255, 1325–1334. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, Y.; Song, Y.; Gu, Y.; Yan, X.; Lu, N.; Liu, H.; Xu, Z.; Xu, H.; Zhang, Z. AuPt/MOF-Graphene: A Synergistic Catalyst with Surprisingly High Peroxidase-Like Activity and Its Application for H2O2 Detection. Anal. Chem. 2019, 91, 10589–10595. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Tripathi, P.P.; Gandhi, S. Graphene nanosheets as an electric mediator for ultrafast sensing of urokinase plasminogen activator receptor-A biomarker of cancer. Biosens. Bioelectron. 2019, 141, 111398. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, J.; Li, C.; Tang, L.; Zhang, Z.; Yang, M. A novel composite film derived from cysteic acid and PDDA-functionalized graphene: Enhanced sensing material for electrochemical determination of metronidazole. Talanta 2013, 104, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.P.; Dutta, K. Progress and Recent Trends in Microbial Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hu, C.; Zhai, X.; Liu, L.; Zhao, Y.; Jiang, L.; Qu, L. Spontaneous reduction and assembly of graphene oxide into three-dimensional graphene network on arbitrary conductive substrates. Sci. Rep. 2013, 3, 2065. [Google Scholar] [CrossRef] [Green Version]
- Dash, S.S.; Majumdar, R.; Sikder, A.K.; Bag, B.G.; Patra, B.K. Saraca indica bark extract mediated green synthesis of polyshaped gold nanoparticles and its application in catalytic reduction. Appl. Nanosci. 2014, 4, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, R.; De Angelis, R.J.; Davis, B.H. Structural studies of Pt-Sn catalysts on high and low surface area alumina supports. Catal. Lett. 1990, 4, 303–308. [Google Scholar] [CrossRef]
- Ray, S.C.; Bhunia, S.K.; Saha, A.; Jana, N.R. Graphene oxide (GO)/reduced-GO and their composite with conducting polymer nanostructure thin films for non-volatile memory device. Microelectron. Eng. 2015, 146, 48–52. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Liu, Y.; Wang, W.; Wang, M.; Zhou, Y.; Wu, S.; Shen, J. Facilitated Utilization of Active Sites with Core-Shell PdPt@ Pt/RGO Nanocluster Structures for Improved Electrocatalytic Ethylene Glycol Oxidation. ChemElectroChem 2018, 5, 2645–2652. [Google Scholar] [CrossRef]
- Sakthivel, R.; Kubendhiran, S.; Chen, S.M.; Ranganathan, P.; Rwei, S.P. Functionalized Carbon Black Nanospheres Hybrid with MoS2 Nanoclusters for the Effective Electrocatalytic Reduction of Chloramphenicol. Electroanalysis 2018, 30, 1828–1836. [Google Scholar] [CrossRef]
- Devi, P.; Sharma, C.; Kumar, P.; Kumar, M.; Bansod, B.K.; Nayak, M.K.; Singla, M.L. Selective electrochemical sensing for arsenite using rGO/Fe3O4 nanocomposites. J. Hazard. Mater. 2017, 322, 85–94. [Google Scholar] [CrossRef]
- Wu, M.; Sun, M.; Zhou, H.; Ma, J.Y.; Ma, T. Carbon Counter Electrodes in Dye-Sensitized and Perovskite Solar Cells. Adv. Funct. Mater. 2019, 30, 1906451. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980; Volume 2. [Google Scholar]
- Ali, M.A.; Kamil Reza, K.; Srivastava, S.; Agrawal, V.V.; John, R.; Malhotra, B.D. Lipid–lipid interactions in aminated reduced graphene oxide interface for biosensing application. Langmuir 2014, 30, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Bukkitgar, S.D.; Shetti, N.P. Electrochemical behavior of an anticancer drug 5-fluorouracil at methylene blue modified carbon paste electrode. Mater. Sci. Eng. C 2016, 65, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Mølgaard, P.; Johnsen, S.; Christensen, P.; Cornett, C. HPLC method validated for the simultaneous analysis of cichoric acid and alkamides in Echinacea purpurea plants and products. J. Agric. Food Chem. 2003, 51, 6922–6933. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, G.; Liu, Q.; Duan, X.; Liu, Z.; Liu, X. Pharmacokinetics, tissue distribution, and plasma protein binding study of chicoric acid by HPLC-MS/MS. J. Chromatogr. B 2016, 1031, 139–145. [Google Scholar] [CrossRef]
- Manček, B.; Kreft, S. Determination of cichoric acid content in dried press juice of purple coneflower (Echinacea purpurea) with capillary electrophoresis. Talanta 2005, 66, 1094–1097. [Google Scholar] [CrossRef]




| Methods | Detection Range | Limit of Detection | Reference |
|---|---|---|---|
| High-performance liquid chromatography (HPLC) | 0.25 M–1.25 M | 11 mM | [50] |
| Liquid chromatography coupled with tandem mass spectrometry (HPLC–MS/MS) | 0.05 mM–2 mM | 2.23 μM | [51] |
| Capillary electrophoresis (CE) | 74.1 mM–592.8 mM | 1.1 mM | [52] |
| Electrochemical sensor based on Au@Pt-PEI-RGO nanohybrids | 0.3 μM–0.03 mM | 4.8 nM | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, J.; Pan, M.; Liu, X.; Liu, J.; Li, B.; Chen, Q. An Ultrasensitive Non-Enzymatic Sensor for Quantitation of Anti-Cancer Substance Chicoric Acid Based on Bimetallic Nanoalloy with Polyetherimide-Capped Reduced Graphene Oxide. Nanomaterials 2020, 10, 499. https://doi.org/10.3390/nano10030499
Jiao J, Pan M, Liu X, Liu J, Li B, Chen Q. An Ultrasensitive Non-Enzymatic Sensor for Quantitation of Anti-Cancer Substance Chicoric Acid Based on Bimetallic Nanoalloy with Polyetherimide-Capped Reduced Graphene Oxide. Nanomaterials. 2020; 10(3):499. https://doi.org/10.3390/nano10030499
Chicago/Turabian StyleJiao, Jun, Meixin Pan, Xinran Liu, Jian Liu, Binshuai Li, and Qiang Chen. 2020. "An Ultrasensitive Non-Enzymatic Sensor for Quantitation of Anti-Cancer Substance Chicoric Acid Based on Bimetallic Nanoalloy with Polyetherimide-Capped Reduced Graphene Oxide" Nanomaterials 10, no. 3: 499. https://doi.org/10.3390/nano10030499






