Fabrication of Hybrid Silver Microstructures from Vermiculite Templates as SERS Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
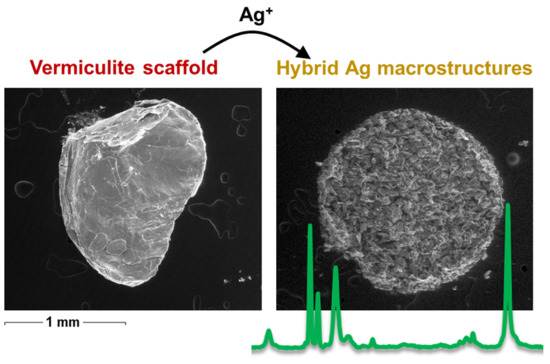
2.2. Sample Preparation
2.3. SERS Characterization
2.4. Instrumentation
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Krishnaswamy, S.; Zivanovic, N.; Sharma, R.; Pe’er, D.; Bodenmiller, B. Learning time-varying information flow from single-cell epithelial to mesenchymal transition data. PLoS ONE 2018, 13, e0203389. [Google Scholar] [CrossRef]
- Karacosta, L.G.; Anchang, B.; Ignatiadis, N.; Kimmey, S.C.; Benson, J.A.; Shrager, J.B.; Tibshirani, R.; Bendall, S.C.; Plevritis, S.K. Mapping lung cancer epithelial-mesenchymal transition states and trajectories with single-cell resolution. Nat. Commun. 2019, 10, 5587. [Google Scholar] [CrossRef] [Green Version]
- Mir-Simon, B.; Morla-Folch, J.; Gisbert-Quilis, P.; Pazos-Perez, N.; Xie, H.-N.; Bastús, N.G.; Puntes, V.; Alvarez-Puebla, R.A.; Guerrini, L. SERS efficiencies of micrometric polystyrene beads coated with gold and silver nanoparticles: The effect of nanoparticle size. J. Opt. 2015, 17, 114012. [Google Scholar] [CrossRef] [Green Version]
- Mariño-Lopez, A.; Sousa-Castillo, A.; Blanco-Formoso, M.; Furini, L.N.; Rodríguez-Lorenzo, L.; Pazos-Perez, N.; Guerrini, L.; Pérez-Lorenzo, M.; Correa-Duarte, M.A.; Alvarez-Puebla, R.A. Microporous plasmonic capsules as stable molecular sieves for direct SERS quantification of small pollutants in natural waters. ChemNanoMat 2019, 5, 46–50. [Google Scholar] [CrossRef]
- Petronella, F.; Curri, M.L.; Striccoli, M.; Fanizza, E.; Mateo-Mateo, C.; Alvarez-Puebla, R.A.; Sibillano, T.; Giannini, C.; Correa-Duarte, M.A.; Comparelli, R. Direct growth of shape controlled TiO2 nanocrystals onto SWCNTs for highly active photocatalytic materials in the visible. Appl. Catal. B Environ. 2015, 178, 91–99. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, Y.H.; Koh, C.S.L.; Gia, C.P.Q.; Han, X.M.; Lay, C.L.; Sim, H.Y.F.; Kao, Y.C.; An, Q.; Ling, X.Y. Designing surface-enhanced Raman scattering (SERS) platforms beyond hotspot engineering: Emerging opportunities in analyte manipulations and hybrid materials. Chem. Soc. Rev. 2019, 48, 731–756. [Google Scholar] [CrossRef]
- Hong, F.; Zhang, F.; Liu, Y.; Yan, H. DNA origami: Scaffolds for creating higher order structures. Chem. Rev. 2017, 117, 12584–12640. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hussein, G.; Brza, M.A.J.; Mohammed, S.T.; Abdulwahid, R.; Raza Saeed, S.; Hassanzadeh, A. Fabrication of interconnected plasmonic spherical silver nanoparticles with enhanced Localized Surface Plasmon Resonance (LSPR) peaks using quince leaf extract solution. Nanomaterials 2019, 9, 1557. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.-P.; Chen, S.; Huang, P.; Wang, X.; Jiang, W.; Pandoli, O.; Cui, D. Bacteria-template synthesized silver microspheres with hollow and porous structures as excellent SERS substrate. Green Chem. 2010, 12, 2038–2042. [Google Scholar] [CrossRef]
- Sun, L.L.; Cai, J.; Sun, Y.M.; Zhang, D.Y. Three-dimensional assembly of silver nanoparticles spatially confined by cellular structure of Spirulina, from nanospheres to nanosheets. Nanotechnology 2019, 30, 10. [Google Scholar] [CrossRef]
- Pal, U.; Lopez, D.N.C.; Carcano-Montiel, M.G.; Lopez-Reyes, L.; Diaz-Nunez, P.; Pena-Rodriguez, O. Nanoparticle-assembled gold microtubes built on fungi templates for SERS-based molecular sensing. ACS Appl. Nano Mater. 2019, 2, 2533–2541. [Google Scholar] [CrossRef]
- Jones, M.R.; Osberg, K.D.; Macfarlane, R.J.; Langille, M.R.; Mirkin, C.A. Templated techniques for the synthesis and assembly of plasmonic nanostructures. Chem. Rev. 2011, 111, 3736–3827. [Google Scholar] [CrossRef]
- Długosz, O.; Banach, M. Kinetic, isotherm and thermodynamic investigations of the adsorption of Ag+ and Cu2+ on vermiculite. J. Mol. Liq. 2018, 258, 295–309. [Google Scholar] [CrossRef]
- Długosz, O.; Banach, M. Sorption of Ag+ and Cu2+ by vermiculite in a fixed-bed column: Design, process optimization and dynamics investigations. Appl. Sci. 2018, 8, 2221. [Google Scholar] [CrossRef] [Green Version]
- Malandrino, M.; Abollino, O.; Giacomino, A.; Aceto, M.; Mentasti, E. Adsorption of heavy metals on vermiculite: Influence of pH and organic ligands. J. Colloid Interface Sci. 2006, 299, 537–546. [Google Scholar] [CrossRef]
- da Fonseca, M.G.; de Oliveira, M.M.; Arakaki, L.N.H.; Espinola, J.G.P.; Airoldi, C. Natural vermiculite as an exchanger support for heavy cations in aqueous solution. J. Colloid Interface Sci. 2005, 285, 50–55. [Google Scholar] [CrossRef]
- Hundáková, M.; Valášková, M.; Tomášek, V.; Pazdziora, E.; Matějová, K. Silver and/or copper vermiculites and their antibacterial effect. Acta Geodyn. Geomater. 2013, 10, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Noack, J.; Fritz, C.; Flügel, C.; Hemmann, F.; Gläsel, H.-J.; Kahle, O.; Dreyer, C.; Bauer, M.; Kemnitz, E. Metal fluoride-based transparent nanocomposites with low refractive indices. Dalton Trans. 2013, 42, 5706–5710. [Google Scholar] [CrossRef]
- Bartkowiak, D.; Merk, V.; Reiter-Scherer, V.; Gernert, U.; Rabe, J.P.; Kneipp, J.; Kemnitz, E. Porous MgF2-over-gold nanoparticles (MON) as plasmonic substrate for analytical applications. RSC Adv. 2016, 6, 71557–71566. [Google Scholar] [CrossRef] [Green Version]
- Stawiński, W.; Węgrzyn, A.; Dańko, T.; Freitas, O.; Figueiredo, S.; Chmielarz, L. Acid-base treated vermiculite as high performance adsorbent: Insights into the mechanism of cationic dyes adsorption, regeneration, recyclability and stability studies. Chemosphere 2017, 173, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Carron, K.T.; Hurley, L.G. Axial and azimuthal angle determination with surface-enhanced raman-spectroscopy—Thiophenol on copper, silver, and gold metal-surfaces. J. Phys. Chem. 1991, 95, 9979–9984. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pazos-Perez, N.; Guerrini, L.; Alvarez-Puebla, R.A. Fabrication of Hybrid Silver Microstructures from Vermiculite Templates as SERS Substrates. Nanomaterials 2020, 10, 481. https://doi.org/10.3390/nano10030481
Pazos-Perez N, Guerrini L, Alvarez-Puebla RA. Fabrication of Hybrid Silver Microstructures from Vermiculite Templates as SERS Substrates. Nanomaterials. 2020; 10(3):481. https://doi.org/10.3390/nano10030481
Chicago/Turabian StylePazos-Perez, Nicolas, Luca Guerrini, and Ramon A. Alvarez-Puebla. 2020. "Fabrication of Hybrid Silver Microstructures from Vermiculite Templates as SERS Substrates" Nanomaterials 10, no. 3: 481. https://doi.org/10.3390/nano10030481