Thermal Stability of Octadecyltrichlorosilane and Perfluorooctyltriethoxysilane Monolayers on SiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Octadecyltrichlorosilane(OTS) and PTES on SiO2
2.2. Characterizations
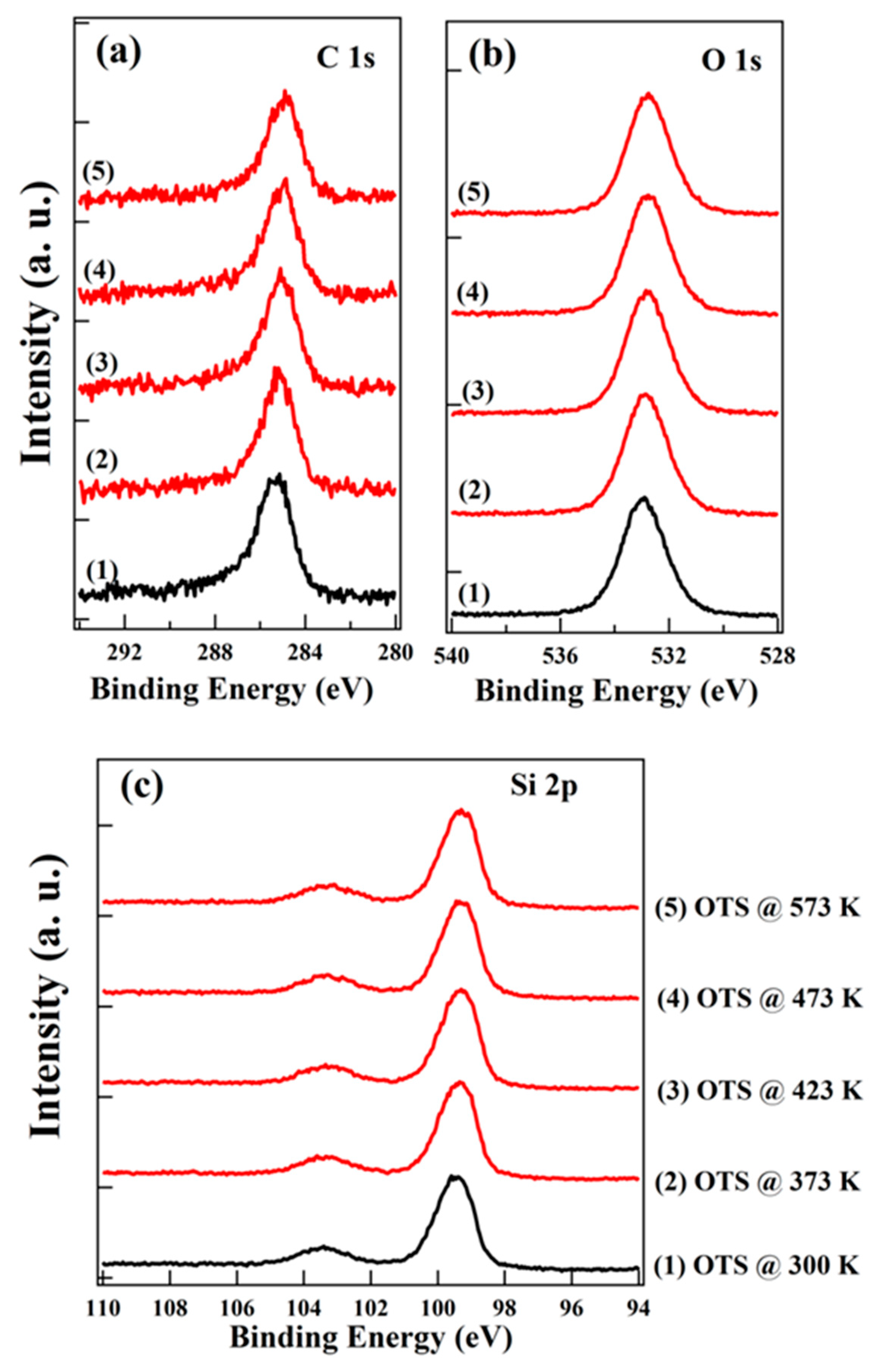
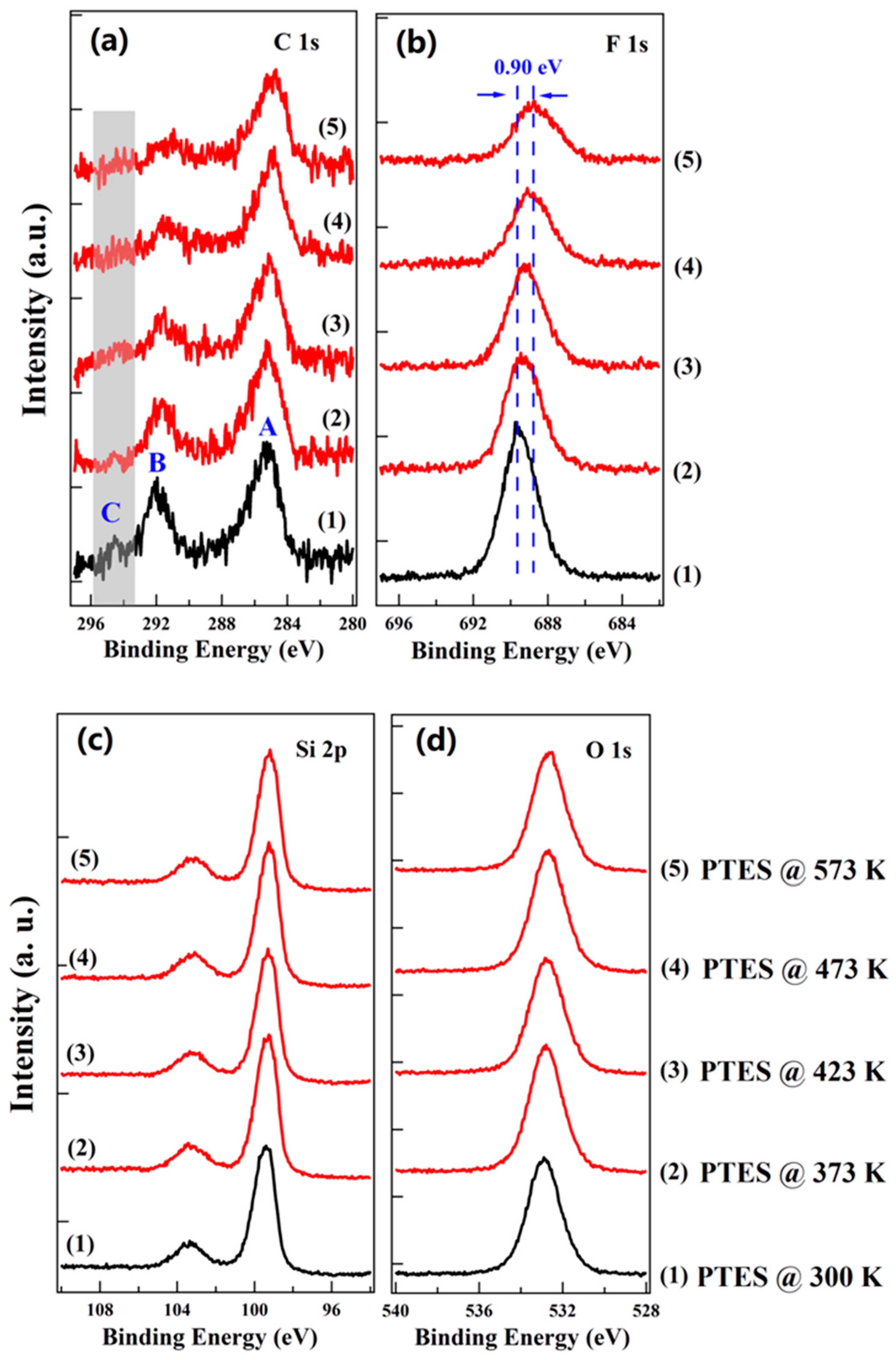
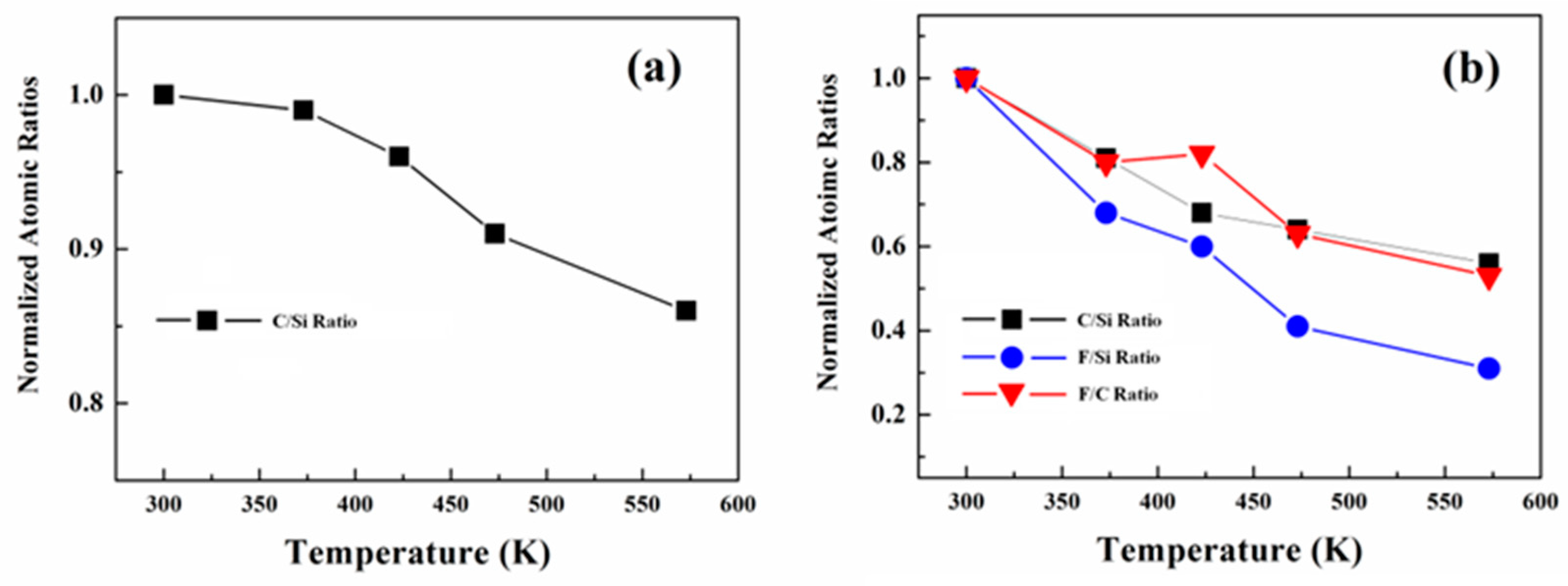
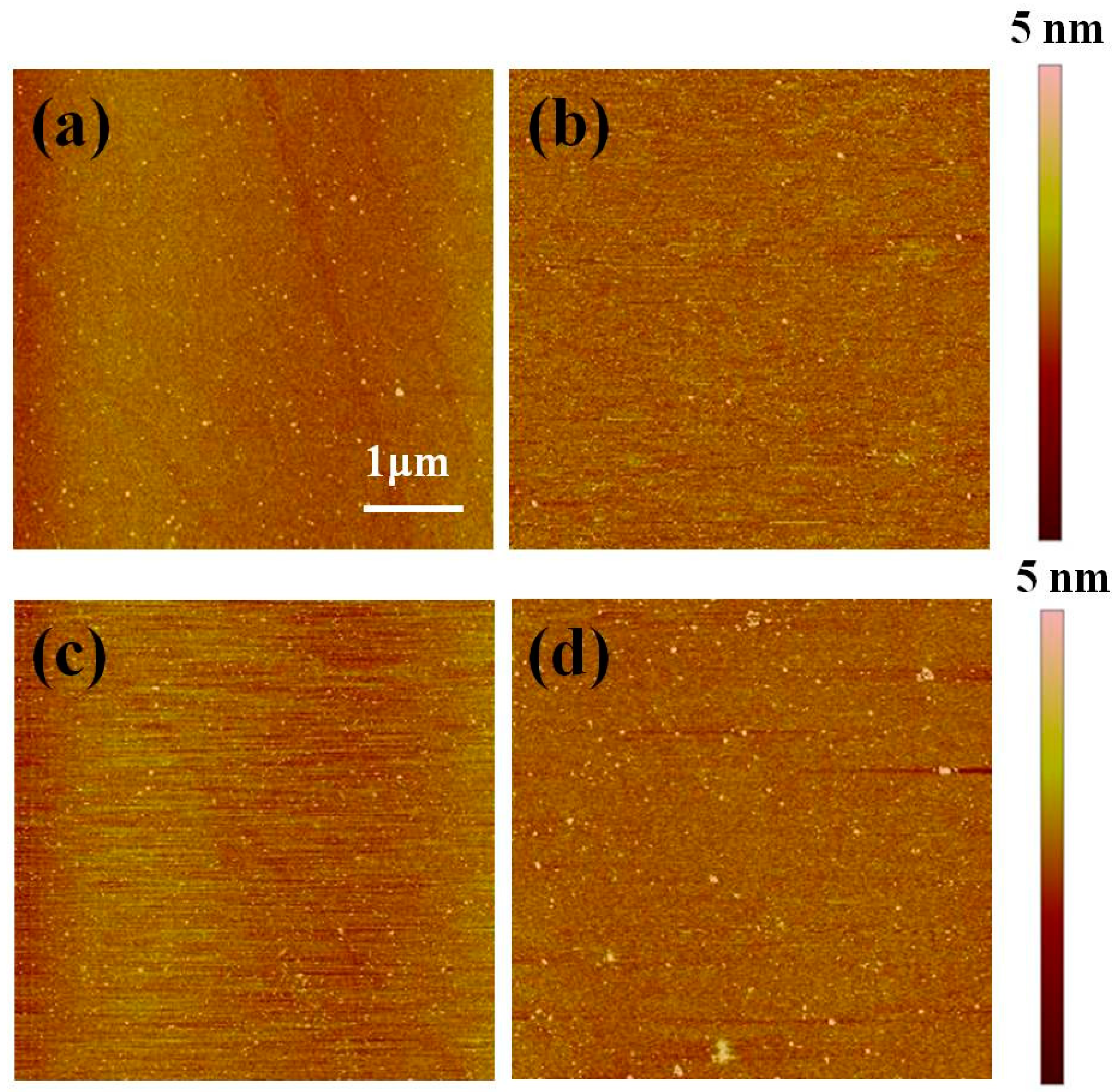
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, U.; Houston, M.R.; Howe, R.T.; Maboudian, R. Alkyltrichlorosiane-based self-assembled monolayer films for stiction reduction in silicon micromachines. J. Microelectromech. Syst. 1998, 7, 252. [Google Scholar] [CrossRef]
- Liu, Z.M.; Yasseri, A.A.; Lindsey, J.S.; Bocian, D.F. Molecular memories that survive silicon device processing and real-world operation. Science 2003, 302, 2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.T.; Yang, X.D.; Dou, W.D.; Wang, P.; Ye, Q.L.; Yang, X.X.; Li, B.X.; Mao, H.Y. Impact of grapheme work function on the electronic structures at the interface between graphene and organic molecules. Nanomaterials 2019, 9, 1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, G.; Sethuraman, M.G. Corrosion protection ability of self-seembled monolayer of 3-amino-5mercapto-1,2,3-triazole on copper electrode. Thin Solid Films 2014, 562, 32. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533. [Google Scholar] [CrossRef]
- Bhairamadgi, N.S.; Pujari, S.P.; Trovela, F.G.; Debrassi, A.; Khamis, A.A.; Alonso, J.M.; Zahrani, A.A.A.; Wennekes, T.; Al-Turaif, H.A.; Rijn, C.V.; et al. Hydrolytic and thermal stability of organic monolayers on various inorganic substrates. Langmuir 2014, 30, 5829–5839. [Google Scholar] [CrossRef]
- Zhong, J.; Chinn, J.; Roberts, C.B.; Ashurst, W.R. Vapor-phase deposited chlorosilane-based self-assembled monolayers on various substrates for thermal stability analysis. Ind. Eng. Chem. Res. 2017, 56, 5239. [Google Scholar] [CrossRef]
- Haruyama, Y.; Matsui, S. Electronic structure of fluorinated self-assembled monolayer investigated by photoelectron spectroscopy in the valence band region. Jpn. J. Appl. Phys. 2015, 54, 075202. [Google Scholar] [CrossRef]
- Zhuang, Y.X.; Hansen, O.; Knieling, T.; Wang, C.; Rombach, P.; Lang, W.; Benecke, W.; Kehlenbeck, M.; Koblitz, J. Thermal stability of vapor phase deposited self-assembled monolayers for MEMS anti-stiction. J. Micromech. Microeng 2006, 16, 2259. [Google Scholar] [CrossRef]
- Stolarczyk, E.U.; Sidoryk, K.; Cybulski, M.; Kubiszewski, M.K.; Stolarczyk, K. Design of therapeutic self-assembled monolayers of thiolatedabiraterone. Nanomaterials 2018, 8, 1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Sheadel, D.A.; Xue, W. Surface treatment of polymers for the fabrication of all-polymer MEMS devices. Sen. Actua. A-Phys. 2012, 187, 43. [Google Scholar] [CrossRef]
- Sung, M.M.; Kluth, G.J.; Yauw, O.W.; Maboudian, R. Thermal behavior of alkyl monolayers on silicon surfaces. Langmuir 1997, 13, 6164. [Google Scholar] [CrossRef]
- Fréchette, J.; Maboudian, R.; Carraro, C. Thermal behavior of perfluoroalkylsiloxane monolayers on the oxidized Si(100) surface. Langmuir 2006, 22, 2726. [Google Scholar] [CrossRef] [PubMed]
- Kluth, G.J.; Sung, M.M.; Maboudian, R. Thermal behavior of alkylsiloxane self-assembled monolayers on the oxidized Si(100) surface. Langmuir 1997, 13, 3775. [Google Scholar] [CrossRef]
- Kluth, G.J.; Sander, M.; Sung, M.M.; Maboudian, R. Study of the desorption mechanism of aklylsiloane self-assembled monolayers through isotopic labeling and high resolution electron energy-loss spectroscopy experiments. J. Vac. Sci. Technol. A 1998, 16, 932. [Google Scholar] [CrossRef]
- Chandekar, A.; Sengupta, S.K.; Whitten, J.E. Thermal stability of thiol and silane monolayers: A comparative study. Appl. Surf. Sci. 2010, 256, 2742. [Google Scholar] [CrossRef]
- Ramin, L.; Jabbarzadeh, A. Odd-even effects on the structure, stability, and phase transition of alkanethiol self-assembled monolayers. Langmuir 2011, 27, 9748. [Google Scholar] [CrossRef]
- Sagawa, N.; Uchino, T. Effect of annealing on the visible photoluminescence characteristics of octadecyltrichlorosilane monolayers on silica surfaces. J. Phys. Chem. C 2008, 112, 4581. [Google Scholar] [CrossRef]
- Park, J.; Lee, W.H.; Huh, S.; Sim, S.H.; Kim, S.B.; Cho, K.; Hong, B.H.; Kim, K.S. Work-function engineering of graphene electrodes by self-assembled monolayers for high-performance organic field-effect transistors. J. Phys. Chem. Lett. 2011, 2, 841. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, Z.Z.; Lu, W.; Yao, J.; Zhu, Y.; Tour, J.M. Controlled modulation of electronic properties of grapheme by self-assembled monolayers on SiO2 substrates. ACS Nano 2011, 5, 1535. [Google Scholar] [CrossRef] [PubMed]
- Laibinis, P.E.; Graham, R.L.; Biebuyck, H.A.; Whitesides, G.M. X-ray damage to CF3CO2-terminated organic monolayers on Si/Au: Principal effect of electrons. Science 1991, 254, 981. [Google Scholar] [CrossRef] [PubMed]
- Nansé, G.; Papirer, E.; Fioux, P.; Moguet, F.; Tressaud, A. Fluorination of carbon blacks: An X-ray photoelectron spectroscopy study: I. a literature review of XPS studies of fluorinated carbons. XPS investigation of some reference compounds. Carbon 1997, 35, 175. [Google Scholar] [CrossRef]
- Satoh, T.; Imanishi, M.; Nishikawa, T.; Mori, T. Properties of interface between organic hole-transporting layer and indium tin oxide anode modified by fluorinated self-assembled monolayer. Jpn. J. Appl. Phys. 2012, 51, 035701. [Google Scholar]
- Kushmerick, J.G.; Hankins, M.G.; de Boer, M.P.; Clews, P.J.; Carpick, R.W.; Bunker, B.C. The influence of coating structure on micromachinestiction. Tribol. Lett. 2001, 10, 103–108. [Google Scholar] [CrossRef]
- Duhm, S.; Heimel, G.; Salzmann, I.; Glowatzki, H.; Johnson, R.L.; Vollmer, A.; Rabe, J.P.; Koch, N. Orientation-dependent ionization energies and interface dipoles in ordered molecular assemblies. Nat. Mater. 2008, 7, 326. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Yamada, K.; Tsutsumi, J.; Sato, N. Complete description of ionization energy and electron affinity in organic solids: Determining contributions from electronic polarization, energy band dispersion, and molecular orientation. Phys. Rev. B 2015, 92, 075145. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Qi, D.C.; Huang, H.; Gao, X.Y.; Wee, A.T.S. Organic-organic heterojunction interfaces: Effect of molecular orientation. Adv. Funct. Mater. 2011, 21, 410. [Google Scholar] [CrossRef]
- Fukagawa, H.; Kera, S.; Kataoka, T.; Hosoumi, S.; Watanabe, Y.; Kudo, K.; Ueno, N. The role of the ionization potential in vacuum-level alignment at organic semiconductor interfaces. Adv. Mater. 2007, 19, 665. [Google Scholar] [CrossRef]
- Kobayshi, S.; Nishikawa, T.; Takenobu, T.; Mori, S.; Shimoda, T.; Mitani, T.; Shimotani, H.; Yoshimoto, N.; Ogawa, S.; Iwasa, Y. Control of carrier density by self-assembled monolayers in organic field-effect transistors. Nat. Mater. 2004, 3, 317. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, H.; Wang, P.; Yang, X.; Mao, H. Thermal Stability of Octadecyltrichlorosilane and Perfluorooctyltriethoxysilane Monolayers on SiO2. Nanomaterials 2020, 10, 210. https://doi.org/10.3390/nano10020210
Yang X, Wang H, Wang P, Yang X, Mao H. Thermal Stability of Octadecyltrichlorosilane and Perfluorooctyltriethoxysilane Monolayers on SiO2. Nanomaterials. 2020; 10(2):210. https://doi.org/10.3390/nano10020210
Chicago/Turabian StyleYang, Xiangdong, Haitao Wang, Peng Wang, Xuxin Yang, and Hongying Mao. 2020. "Thermal Stability of Octadecyltrichlorosilane and Perfluorooctyltriethoxysilane Monolayers on SiO2" Nanomaterials 10, no. 2: 210. https://doi.org/10.3390/nano10020210



