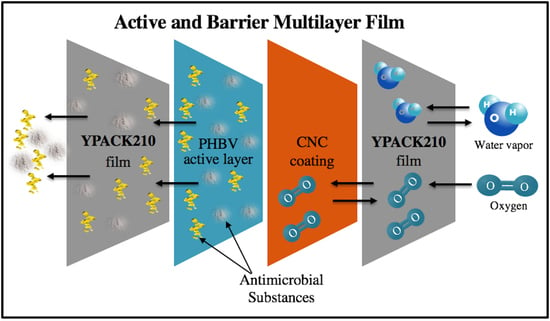
Development of Active Barrier Multilayer Films Based on Electrospun Antimicrobial Hot-Tack Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Cellulose Nanocrystal Interlayers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Food Contact Blown Film
2.3. CNC Coating
2.4. Electrospinning
2.5. Multilayer Assembly
2.6. Characterization of the Multilayers
2.6.1. Film Thickness
2.6.2. Morphology
2.6.3. Transparency
2.6.4. Color
2.6.5. Water Vapor Permeance
2.6.6. Limonene Vapor Permeance
2.6.7. Mechanical Tests
2.6.8. Antimicrobial Tests
2.6.9. Antioxidant Measurements
2.6.10. Migration Tests
2.6.11. Cytotoxicity Assay
CCK-8 Assay
Resazurin Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Morphology
3.2. Transparency and Color
3.3. Barrier Properties
3.4. Mechanical Properties
3.5. Antimicrobial Activity
3.6. Antioxidant Activity
3.7. Migration
3.8. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, G.Q.; Wu, Q.; Jung, Y.K.; Lee, S.Y. 3.21—PHA/PHB. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, VT, USA, 2011; pp. 217–227. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, T.W.; Kim, M.K.; Lee, S.Y.; Lim, S.C. Advanced bacterial polyhydroxyalkanoates: Towards a versatile and sustainable platform for unnatural tailor-made polyesters. Biotechnol. Adv. 2012, 30, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Chapter 8—Polyhydroxyalkanoates in the Food Packaging Industry. In Nanotechnology Applications in Food; Oprea, A.E., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 153–177. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Biodegradability and disintegration of multilayer starch films with electrospun PCL fibres encapsulating carvacrol. Polym. Degrad. Stab. 2020, 173, 109100. [Google Scholar] [CrossRef]
- Fang, J.M.; Fowler, P.A.; Escrig, C.; Gonzalez, R.; Costa, J.A.; Chamudis, L. Development of biodegradable laminate films derived from naturally occurring carbohydrate polymers. Carbohydr. Polym. 2005, 60, 39–42. [Google Scholar] [CrossRef]
- Heidemann, H.M.; Dotto, M.E.R.; Laurindo, J.B.; Carciofi, B.A.M.; Costa, C. Cold plasma treatment to improve the adhesion of cassava starch films onto PCL and PLA surface. Colloids Surf. A:Physicochem. Eng. Asp. 2019, 580, 123739. [Google Scholar] [CrossRef]
- Fabra, M.J.; Sánchez, G.; López-Rubio, A.; Lagaron, J.M. Microbiological and ageing performance of polyhydroxyalkanoate-based multilayer structures of interest in food packaging. LWT Food Sci. Technol. 2014, 59, 760–767. [Google Scholar] [CrossRef]
- Benetto, E.; Jury, C.; Igos, E.; Carton, J.; Hild, P.; Vergne, C.; Di Martino, J. Using atmospheric plasma to design multilayer film from polylactic acid and thermoplastic starch: A screening Life Cycle Assessment. J. Clean. Prod. 2015, 87, 953–960. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation behavior of three-layer sheets based on gelatin and poly (lactic acid) buried under indoor soil conditions. Polym. Degrad. Stab. 2015, 116, 36–44. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Castro-Mayorga, J.L.; Andrade-Mahecha, M.M.; Cabedo, L.; Lagaron, J.M. Antibacterial and Barrier Properties of Gelatin Coated by Electrospun Polycaprolactone Ultrathin Fibers Containing Black Pepper Oleoresin of Interest in Active Food Biopackaging Applications. Nanomaterials 2018, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.; Tang, Y.; Zhu, X.; Zhou, Y. Polylactic acid based biocomposite films reinforced with silanized nanocrystalline cellulose. Int. J. Biol. Macromol. 2020, 162, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, D.; Tam, K.C.; Pan, K.; Zheng, Z. Effect of surface modification of cellulose nanocrystal on nonisothermal crystallization of poly(β-hydroxybutyrate) composites. Carbohydr. Polym. 2017, 157, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, K.C.C.; Montoro, S.R.; Cioffi, M.O.H.; Voorwald, H.J.C. Chapter 13—Polyhydroxyalkanoates and Their Nanobiocomposites With Cellulose Nanocrystals. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; William Andrew Publishing: Boston, MA, USA, 2016; pp. 261–285. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Bhat, A.H.; Ahmad, F. Polymer blend of PLA/PHBV based bionanocomposites reinforced with nanocrystalline cellulose for potential application as packaging material. Carbohydr. Polym. 2017, 157, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Bin Johan, M.R.; Fen, L.B. Comprehensive review on nanocellulose: Recent developments, challenges and future prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884. [Google Scholar] [CrossRef]
- Bao, C.; Chen, X.; Liu, C.; Liao, Y.; Huang, Y.; Hao, L.; Yan, H.; Lin, Q. Extraction of cellulose nanocrystals from microcrystalline cellulose for the stabilization of cetyltrimethylammonium bromide-enhanced Pickering emulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 608, 125442. [Google Scholar] [CrossRef]
- da Rocha Neto, A.C.; Beaudry, R.; Maraschin, M.; Di Piero, R.M.; Almenar, E. Double-bottom antimicrobial packaging for apple shelf-life extension. Food Chem. 2019, 279, 379–388. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; Coimbra, J.S.d.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and Antioxidant Performance of Various Essential Oils and Natural Extracts and Their Incorporation into Biowaste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Layers Made from Electrospun Ultrathin Fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Lopez, K.J.; Enescu, D.; Torres-Giner, S.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.; Fuciños, P.; Lagaron, J.M. Development of electrospun active films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by the incorporation of cyclodextrin inclusion complexes containing oregano essential oil. Food Hydrocoll. 2020, 108, 106013. [Google Scholar] [CrossRef]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185, 1–22. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Torres-Giner, S.; Enescu, D.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.M.; Lagaron, J.M. Electrospun Active Biopapers of Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with Short-Term and Long-Term Antimicrobial Performance. Nanomaterials 2020, 10, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, Z.; Lin, J.; Zhang, X.; Fan, Y.; Xu, X.; Xue, Y.; Liu, D.; Li, J.; Hu, L.; Tang, C. Morphology controlled synthesis zinc oxide and reinforcement in polyhydroxyalkanoates composites. Polym. Compos. 2014, 35, 1701–1706. [Google Scholar] [CrossRef]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M.A.; Hosseini, M. A new active nanocomposite film based on PLA/ZnO nanoparticle/essential oils for the preservation of refrigerated Otolithes ruber fillets. Food Packag. Shelf Life 2019, 19, 94–103. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra, M.J.; Pourrahimi, A.M.; Olsson, R.T.; Lagaron, J.M. The impact of zinc oxide particle morphology as an antimicrobial and when incorporated in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for food packaging and food contact surfaces applications. Food Bioprod. Process. 2017, 101, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Lopez, K.J.; Cabedo, L.; Lagaron, J.M.; Torres-Giner, S. Development of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Monolayers Containing Eugenol and Their Application in Multilayer Antimicrobial Food Packaging. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef]
- Cruz, M.V.; Freitas, F.; Paiva, A.; Mano, F.; Dionísio, M.; Ramos, A.M.; Reis, M.A.M. Valorization of fatty acids-containing wastes and byproducts into short- and medium-chain length polyhydroxyalkanoates. New Biotechnol. 2016, 33, 206–215. [Google Scholar] [CrossRef]
- Lanham, A.B.; Ricardo, A.R.; Albuquerque, M.G.E.; Pardelha, F.; Carvalheira, M.; Coma, M.; Fradinho, J.; Carvalho, G.; Oehmen, A.; Reis, M.A.M. Determination of the extraction kinetics for the quantification of polyhydroxyalkanoate monomers in mixed microbial systems. Process. Biochem. 2013, 48, 1626–1634. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Torres, A.; Ferrándiz, M.; Fombuena, V.; Balart, R. Antimicrobial activity of metal cation-exchanged zeolites and their evaluation on injection-molded pieces of bio-based high-density polyethylene. J. Food Saf. 2017, 37, e12348. [Google Scholar] [CrossRef] [Green Version]
- Busolo, M.A.; Lagaron, J.M. Antioxidant polyethylene films based on a resveratrol containing Clay of Interest in Food Packaging Applications. Food Packag. Shelf Life 2015, 6, 30–41. [Google Scholar] [CrossRef]
- European Standard, EN:13130-1:2004. Materials and Articles in Contact with Foodstuffs—Plastics Substances Subject to Limitation—Part 1: Guide to Test Methods for the Specific Migration of Substances from Plastics to Foods and Food Simulants and the Determination of Substances in Plastic; European Committee for Standardization: Brussels, Belgium, 2004. [Google Scholar]
- Chamchoy, K.; Pakotiprapha, D.; Pumirat, P.; Leartsakulpanich, U.; Boonyuen, U. Application of WST-8 based colorimetric NAD(P)H detection for quantitative dehydrogenase assays. BMC Biochem. 2019, 20, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nociari, M.M.; Shalev, A.; Benias, P.; Russo, C. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J. Immunol. Methods 1998, 213, 157–167. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. Bioactive Multilayer Polylactide Films with Controlled Release Capacity of Gallic Acid Accomplished by Incorporating Electrospun Nanostructured Coatings and Interlayers. Appl. Sci. 2019, 9, 533. [Google Scholar] [CrossRef] [Green Version]
- Cerqueira, M.A.; Torres-Giner, S.; Lagaron, J.M. Chapter 6—Nanostructured Multilayer Films. In Nanomaterials for Food Packaging; Cerqueira, M.Â.P.R., Lagaron, J.M., Pastrana Castro, L.M., de Oliveira Soares Vicente, A.A.M., Eds.; Elsevier: Amsterdam, Netherlands, 2018; pp. 147–171. [Google Scholar] [CrossRef]
- Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. High barrier polyhydroxyalcanoate food packaging film by means of nanostructured electrospun interlayers of zein. Food Hydrocoll. 2013, 32, 106–114. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Vartiainen, J.; Peresin, M.S.; Lahtinen, P.; Lagaron, J.M. Improving the water resistance of nanocellulose-based films with polyhydroxyalkanoates processed by the electrospinning coating technique. Cellulose 2018, 25, 1291–1307. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Lorini, L.; Valentino, F.; Sammon, C.; Cabedo, L.; Lagaron, J.M. Valorization of Municipal Biowaste into Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Biopapers for Food Packaging Applications. ACS Appl. Bio Mater. 2020, 3, 6110–6123. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Fabra, M.J.; Castro-Mayorga, J.L.; Bourbon, A.I.; Pastrana, L.M.; Vicente, A.A.; Lagaron, J.M. Use of Electrospinning to Develop Antimicrobial Biodegradable Multilayer Systems: Encapsulation of Cinnamaldehyde and Their Physicochemical Characterization. Food Bioprocess Technol. 2016, 9, 1874–1884. [Google Scholar] [CrossRef] [Green Version]
- Fabra, M.J.; López-Rubio, A.; Ambrosio-Martín, J.; Lagaron, J.M. Improving the barrier properties of thermoplastic corn starch-based films containing bacterial cellulose nanowhiskers by means of PHA electrospun coatings of interest in food packaging. Food Hydrocoll. 2016, 61, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Lopez, K.J.; Andrade-Mahecha, M.M.; Torres-Vargas, O.L. Development of Antimicrobial Biocomposite Films to Preserve the Quality of Bread. Molecules 2018, 23, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun Antimicrobial Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Containing Eugenol Essential Oil Encapsulated in Mesoporous Silica Nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cava, D.; Giménez, E.; Gavara, R.; Lagaron, J.M. Comparative Performance and Barrier Properties of Biodegradable Thermoplastics and Nanobiocomposites versus PET for Food Packaging Applications. J. Plast. Film Sheeting 2006, 22, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. Nanostructured interlayers of zein to improve the barrier properties of high barrier polyhydroxyalkanoates and other polyesters. J. Food Eng. 2014, 127, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Ozturk, S.; Singh, R.K.; Kong, F. Effect of cellulose nanofiber-based coating with chitosan and trans-cinnamaldehyde on the microbiological safety and quality of cantaloupe rind and fresh-cut pulp. Part 1: Microbial safety. LWT 2020, 134, 109972. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Wang, J.; Gardner, D.J.; Tajvidi, M. Cellulose nanofibrils versus cellulose nanocrystals: Comparison of performance in flexible multilayer films for packaging applications. Food Packag. Shelf Life 2020, 23, 100464. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. On the use of different hydrocolloids as electrospun adhesive interlayers to enhance the barrier properties of polyhydroxyalkanoates of interest in fully renewable food packaging concepts. Food Hydrocoll. 2014, 39, 77–84. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Zhang, C.; Feng, F.; Zhang, H. Sequential electrospinning of multilayer ethylcellulose/gelatin/ethylcellulose nanofibrous film for sustained release of curcumin. Food Chem. 2020, 308, 125599. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Andrade-Mahecha, M.M.; Torres-Vargas, O.L. Spice oleoresins containing antimicrobial agents improve the potential use of bio-composite films based on gelatin. Food Packag. Shelf Life 2018, 17, 50–56. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, M.A.; Yoon, C.S.; Na, J.H.; Han, J. Characterization and Preservation Performance of Multilayer Film with Insect Repellent and Antimicrobial Activities for Sliced Wheat Bread Packaging. J. Food Sci. 2019, 84, 3194–3203. [Google Scholar] [CrossRef]
- Gherardi, R.; Becerril, R.; Nerin, C.; Bosetti, O. Development of a multilayer antimicrobial packaging material for tomato puree using an innovative technology. LWT Food Sci. Technol. 2016, 72, 361–367. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Gavara, R.; Hernández-Muñoz, P. Natural Antimicrobial—Containing EVOH Coatings on PP and PET Films: Functional and Active Property Characterization. Packag. Technol. Sci. 2014, 27, 901–920. [Google Scholar] [CrossRef] [Green Version]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, P.; Incarnato, L.; De Marco, I. Supercritical CO2 impregnation of α-tocopherol into PET/PP films for active packaging applications. J. CO2 Util. 2019, 34, 266–273. [Google Scholar] [CrossRef]
- Lin, Q.-B.; Li, H.; Zhong, H.-N.; Zhao, Q.; Xiao, D.-H.; Wang, Z.-W. Migration of Ti from nano-TiO2-polyethylene composite packaging into food simulants. Food Addit. Contam. Part A 2014, 31, 1284–1290. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on the safety evaluation of the substance zinc oxide, nanoparticles, uncoated and coated with [3-(methacryloxy)propyl] trimethoxysilane, for use in food contact materials. EFSA J. 2015, 13, 4063. [Google Scholar] [CrossRef]
- Ozaki, A.; Kishi, E.; Ooshima, T.; Hase, A.; Kawamura, Y. Contents of Ag and other metals in food-contact plastics with nanosilver or Ag ion and their migration into food simulants. Food Addit. Contam. Part A 2016, 33, 1490–1498. [Google Scholar] [CrossRef]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M.A.; Adun, P.; Ostadrahimid, A. Kinetic release study of zinc from polylactic acid based nanocomposite into food simulants. Polym. Test. 2019, 76, 254–260. [Google Scholar] [CrossRef]
- EC (EU). Amending and correcting Regulation on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2016, 1416, 230. [Google Scholar]
- EC (EU). Commission recommendation on the definition of nanomaterial. Annex II: Restrictions on materials and articles. Off. J. Eur. Union 2011, 696, 38–40. [Google Scholar]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Patel, P.R.; Dhanavade, M.J.; Badiger, M.V.; Marathe, Y.N.; Bohara, R.A. Electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/polyethylene oxide (PEO) microfibers reinforced with ZnO nanocrystals for antibacterial and antibiofilm wound dressing applications. New New J. Chem. 2020, 44, 9754–9766. [Google Scholar] [CrossRef]
- Kang, T.; Guan, R.; Chen, X.; Song, Y.; Jiang, H.; Zhao, J. In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells. Nanoscale Res. Lett. 2013, 8, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apte, G.; Repanas, A.; Willems, C.; Mujtaba, A.; Schmelzer, C.E.H.; Raichur, A.; Syrowatka, F.; Groth, T. Effect of Different Crosslinking Strategies on Physical Properties and Biocompatibility of Freestanding Multilayer Films Made of Alginate and Chitosan. Macromol. Biosci. 2019, 19, 1900181. [Google Scholar] [CrossRef] [PubMed]
- Frígols, B.; Martí, M.; Salesa, B.; Hernández-Oliver, C.; Aarstad, O.; Teialeret Ulset, A.S.; Inger Sætrom, G.; Aachmann, F.L.; Serrano-Aroca, Á. Graphene oxide in zinc alginate films: Antibacterial activity, cytotoxicity, zinc release, water sorption/diffusion, wettability and opacity. PLoS ONE 2019, 14, e0212819. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Darmawan, E.T.; Zhang, M.; Zhang, L.; Bryers, J.D. Development of a poly(ether urethane) system for the controlled release of two novel anti-biofilm agents based on gallium or zinc and its efficacy to prevent bacterial biofilm formation. J. Control. Release Off. J. Control. Release Soc. 2013, 172, 1035–1044. [Google Scholar] [CrossRef] [Green Version]





| Sample | a* | b* | L* | ΔE | T |
|---|---|---|---|---|---|
| YPACK210 film | 0.74 ± 0.02 a | −0.41 ± 0.01 a | 90.44 ± 0.07 a | - | 6.83 ± 0.12 a |
| Active multilayer with CNC | 0.28 ± 0.01 b | 1.31 ± 0.02 b | 89.81 ± 0.17 b | 1.88 ± 0.08 a | 4.29 ± 0.15 b |
| Active multilayer without CNC | 0.25 ± 0.03 b | 1.42 ± 0.14 b | 89.86 ± 0.22 b | 1.84 ± 0.19 a | 5.94 ± 0.17 c |
| Sample | Thickness (mm) | Permeance | |
|---|---|---|---|
| WVP × 1011 (kg·m−2·Pa−1·s−1) | LP × 1011 (kg·m−2·Pa−1·s−1) | ||
| YPACK210 film 1 layer | 0.060 | 3.22 ± 0.12 a | 3.78 ± 0.37 a |
| YPACK210 1 layer (modeled) | 0.140 | 1.38 b | 1.62 b |
| Active multilayer with CNC * | 0.140 | 0.87 ± 0.92 c | 1.36 ± 0.24 c |
| Active multilayer with CNC ** | 0.140 | 0.89 ± 0.50 c | 1.39 ± 0.77 c |
| Active multilayer without CNC | 0.131 | 1.32 ± 0.17 b | 1.59 ± 0.38 b |
| Sample | Direction Measure | E (MPa) | T (mJ/m3) | ||
|---|---|---|---|---|---|
| YPACK210 film | TD | 2066 ± 284 a | 23.1 ± 1.8 a | 173 ± 26 a | 40.99 ± 8.21 a |
| Active multilayer with CNC | 1491 ± 207 b | 20.0 ± 1.4 b | 59.1 ± 56 b | 11.95 ± 12.3 b | |
| Active multilayer without CNC | 1446 ± 190 b | 19.2 ± 1.2 b | 51.6 ± 45 c | 10.36 ± 9.78 c | |
| YPACK210 film | MD | 2510 ± 98 a | 29.6 ± 1.4 a | 76.3 ± 25 a | 19.17 ± 6.99 a |
| Active multilayer with CNC | 1828 ± 184 b | 23.5 ± 0.7 b | 45.6 ± 34 b | 9.67 ± 7.67 b | |
| Active multilayer without CNC | 1811 ± 79 b | 22.28 ± 1.1 b | 32.3 ± 17 c | 6.87 ± 3.54 c |
| Sample | Bacteria | Day | Control Log (CFU/mL) | Active Log (CFU/mL) | R |
|---|---|---|---|---|---|
| Active multilayer with CNC * | S. aureus | 1 | 6.95 ± 0.14 | 5.76 ± 0.09 | 1.19 ± 0.10 |
| 3 | 6.90 ± 0.07 | 5.68 ± 0.08 | 1.22 ± 0.08 | ||
| 8 | 6.89 ± 0.13 | 5.61 ± 0.15 | 1.28 ± 0.12 | ||
| 15 | 6.91 ± 0.11 | 5.58 ± 0.12 | 1.33 ± 0.11 | ||
| E. coli | 1 | 6.85 ± 0.15 | 5.72 ± 0.17 | 1.13 ± 0.16 | |
| 3 | 6.83 ± 0.08 | 5.64 ± 0.09 | 1.19 ± 0.07 | ||
| 8 | 6.82 ± 0.15 | 5.60 ± 0.14 | 1.22 ± 0.13 | ||
| 15 | 6.83 ± 0.14 | 5.57 ± 0.12 | 1.26 ± 0.11 | ||
| Active multilayer with CNC ** | S. aureus | 1 | 6.95 ± 0.14 | 6.04 ± 0.12 | 0.91 ± 0.10 |
| 3 | 6.90 ± 0.07 | 5.92 ± 0.08 | 0.98 ± 0.09 | ||
| 8 | 6.89 ± 0.13 | 5.86 ± 0.11 | 1.03 ± 0.12 | ||
| 15 | 6.91 ± 0.11 | 5.83 ± 0.09 | 1.08 ± 0.10 | ||
| E. coli | 1 | 6.85 ± 0.15 | 5.99 ± 0.13 | 0.86 ± 0.13 | |
| 3 | 6.83 ± 0.08 | 5.92 ± 0.09 | 0.91 ± 0.08 | ||
| 8 | 6.82 ± 0.15 | 5.83 ± 0.14 | 0.99 ± 0.12 | ||
| 15 | 6.83 ± 0.14 | 5.79 ± 0.11 | 1.04 ± 0.13 | ||
| Active multilayer without CNC | S. aureus | 1 | 6.95 ± 0.14 | 5.74 ± 0.15 | 1.21 ± 0.17 |
| 3 | 6.90 ± 0.07 | 5.66 ± 0.09 | 1.24 ± 0.09 | ||
| 8 | 6.89 ± 0.13 | 5.59 ± 0.12 | 1.30 ± 0.15 | ||
| 15 | 6.91 ± 0.11 | 5.56 ± 0.08 | 1.35 ± 0.10 | ||
| E. coli | 1 | 6.85 ± 0.15 | 5.69 ± 0.12 | 1.16 ± 0.08 | |
| 3 | 6.83 ± 0.08 | 5.63 ± 0.09 | 1.20 ± 0.10 | ||
| 8 | 6.82 ± 0.15 | 5.57 ± 0.13 | 1.25 ± 0.14 | ||
| 15 | 6.83 ± 0.14 | 5.56 ± 0.11 | 1.27 ± 0.12 |
| Sample | Bacteria | Day | Control Log (CFU/mL) | Multilayer Log (CFU/mL) | R |
|---|---|---|---|---|---|
| Active multilayer with CNC * | S. aureus | 1 | 6.95 ± 0.14 | 5.75 ± 0.11 | 1.20 ± 0.09 |
| 3 | 6.90 ± 0.07 | 5.66 ± 0.08 | 1.24 ± 0.07 | ||
| 8 | 6.89 ± 0.13 | 5.59 ± 0.12 | 1.30 ± 0.11 | ||
| 15 | 6.91 ± 0.11 | 5.57 ± 0.08 | 1.34 ± 0.07 | ||
| E. coli | 1 | 6.85 ± 0.15 | 5.70 ± 0.14 | 1.15 ± 0.13 | |
| 3 | 6.83 ± 0.08 | 5.62 ± 0.09 | 1.21 ± 0.08 | ||
| 8 | 6.82 ± 0.15 | 5.58 ± 0.14 | 1.24 ± 0.11 | ||
| 15 | 6.83 ± 0.14 | 5.54 ± 0.11 | 1.29 ± 0.09 | ||
| Active multilayer with CNC ** | S. aureus | 1 | 6.95 ± 0.14 | 6.02 ± 0.16 | 0.93 ± 0.15 |
| 3 | 6.90 ± 0.07 | 5.90 ± 0.07 | 1.00 ± 0.08 | ||
| 8 | 6.89 ± 0.13 | 5.83 ± 0.18 | 1.06 ± 0.16 | ||
| 15 | 6.91 ± 0.11 | 5.81 ± 0.09 | 1.10 ± 0.09 | ||
| E. coli | 1 | 6.85 ± 0.15 | 5.96 ± 0.13 | 0.89 ± 0.12 | |
| 3 | 6.83 ± 0.08 | 5.90 ± 0.09 | 0.93 ± 0.11 | ||
| 8 | 6.82 ± 0.15 | 5.81 ± 0.12 | 1.01 ± 0.14 | ||
| 15 | 6.83 ± 0.14 | 5.77 ± 0.15 | 1.06 ± 0.12 | ||
| Active multilayer without CNC | S. aureus | 1 | 6.95 ± 0.14 | 5.72 ± 0.09 | 1.23 ± 0.10 |
| 3 | 6.90 ± 0.07 | 5.64 ± 0.08 | 1.26 ± 0.07 | ||
| 8 | 6.89 ± 0.13 | 5.57 ± 0.11 | 1.32 ± 0.11 | ||
| 15 | 6.91 ± 0.11 | 5.54 ± 0.10 | 1.37 ± 0.09 | ||
| E. coli | 1 | 6.85 ± 0.15 | 5.67 ± 0.16 | 1.18 ± 0.18 | |
| 3 | 6.83 ± 0.08 | 5.61 ± 0.07 | 1.22 ± 0.08 | ||
| 8 | 6.82 ± 0.15 | 5.54 ± 0.14 | 1.28 ± 0.14 | ||
| 15 | 6.83 ± 0.14 | 5.53 ± 0.19 | 1.30 ± 0.17 |
| Sample | Day | Open System | Closed System |
|---|---|---|---|
| (μg eq. Trolox/g Sample) | (μg eq. Trolox/g Sample) | ||
| Active monolayer | 1 | 17.44 ± 0.14 a | --- |
| 3 | 15.94 ± 0.10 b,A | 15.98 ± 0.19 a,A | |
| 8 | 14.28 ± 0.17 c,B | 14.42 ± 0.02 b,B | |
| 15 | 12.49 ± 0.08 d,C | 13.76 ± 0.12 c,D | |
| Active multilayer with CNC | 1 | 9.11 ± 0.09 e | --- |
| 3 | 7.74 ± 0.03 f,E | 8.14 ± 0.02 d,F | |
| 8 | 7.42 ± 0.01 f,G | 7.44 ± 0.01 e,G | |
| 15 | 6.25 ± 0.02 g,H | 7.04 ± 0.12 e,I | |
| Active multilayer without CNC | 1 | 12.66 ± 0.12 h | --- |
| 3 | 10.90 ± 0.02 i,J | 11.01 ± 0.06 f,J | |
| 8 | 9.67 ± 0.03 j,K | 10.07 ± 0.03 g,L | |
| 15 | 9.00 ± 0.09 k,M | 9.29 ± 0.04 h,M |
| Sample | Food Simulants | |||||
|---|---|---|---|---|---|---|
| 10% (vol/vol) Aqueous Ethanol | 3% (wt/vol) Aqueous Acetic Acid | Olive Oil | ||||
| Zn (mg/L) | Zn (mg/dm2) | Zn (mg/L) | Zn (mg/dm2) | Zn (mg/L) | Zn (mg/dm2) | |
| Active multilayer with CNC | 0.051 ± 0.019 | 0.00089 ± 0.00021 | 0.185 ± 0.401 | 0.0032 ± 0.0051 | 26.662 ± 11.303 | 0.426 ± 0.142 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa-Lopez, K.J.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Escuin, J.M.; Bourbon, A.I.; Cabedo, L.; Nevo, Y.; Cerqueira, M.A.; Lagaron, J.M. Development of Active Barrier Multilayer Films Based on Electrospun Antimicrobial Hot-Tack Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Cellulose Nanocrystal Interlayers. Nanomaterials 2020, 10, 2356. https://doi.org/10.3390/nano10122356
Figueroa-Lopez KJ, Torres-Giner S, Angulo I, Pardo-Figuerez M, Escuin JM, Bourbon AI, Cabedo L, Nevo Y, Cerqueira MA, Lagaron JM. Development of Active Barrier Multilayer Films Based on Electrospun Antimicrobial Hot-Tack Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Cellulose Nanocrystal Interlayers. Nanomaterials. 2020; 10(12):2356. https://doi.org/10.3390/nano10122356
Chicago/Turabian StyleFigueroa-Lopez, Kelly J., Sergio Torres-Giner, Inmaculada Angulo, Maria Pardo-Figuerez, Jose Manuel Escuin, Ana Isabel Bourbon, Luis Cabedo, Yuval Nevo, Miguel A. Cerqueira, and Jose M. Lagaron. 2020. "Development of Active Barrier Multilayer Films Based on Electrospun Antimicrobial Hot-Tack Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Cellulose Nanocrystal Interlayers" Nanomaterials 10, no. 12: 2356. https://doi.org/10.3390/nano10122356