Sustainable Production of Hydrogen by Steam Reforming of Ethanol Using Cobalt Supported on Nanoporous Zeolitic Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Characterization Techniques
2.3. Catalytic Study
3. Results and Discussion
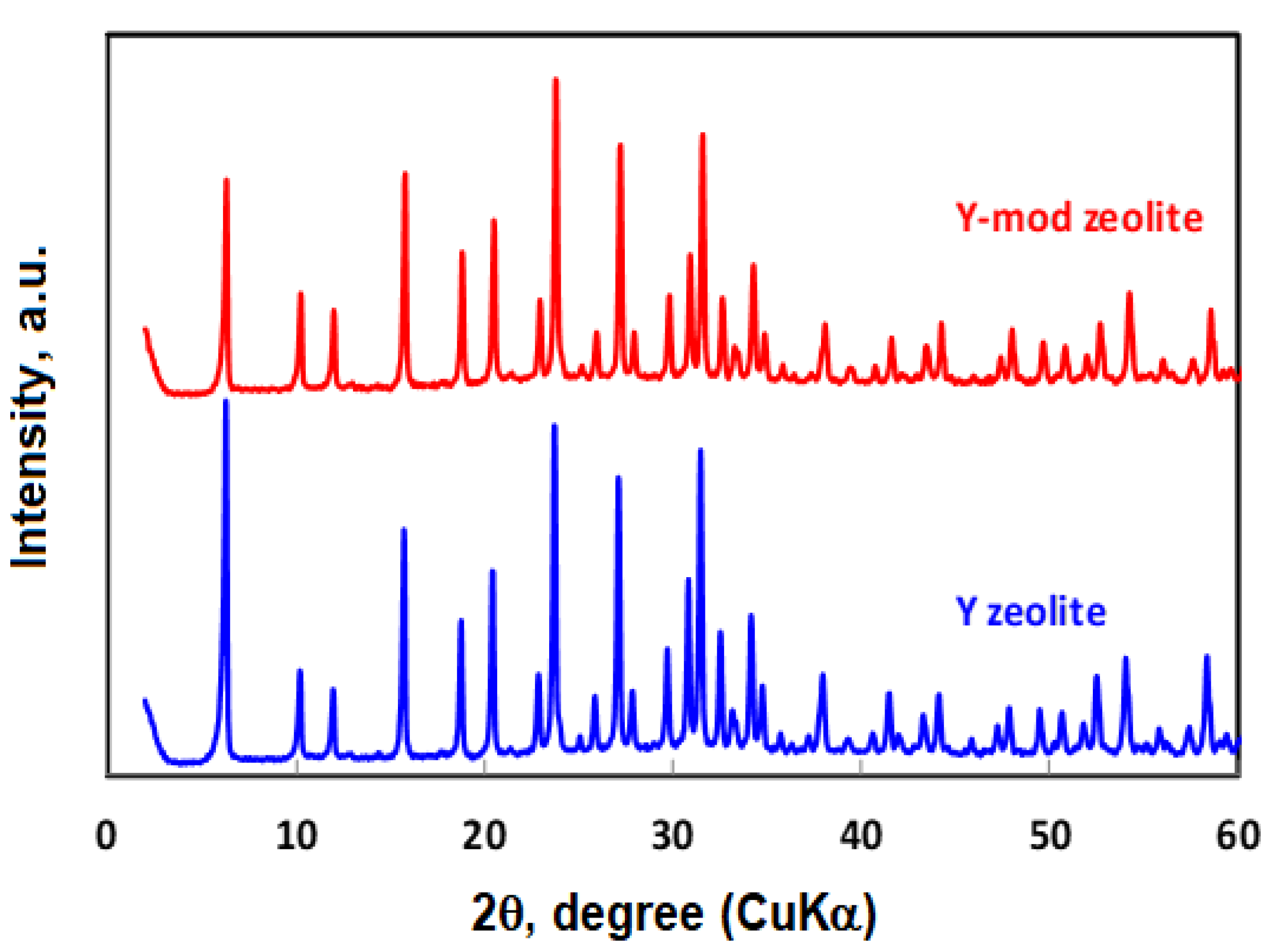
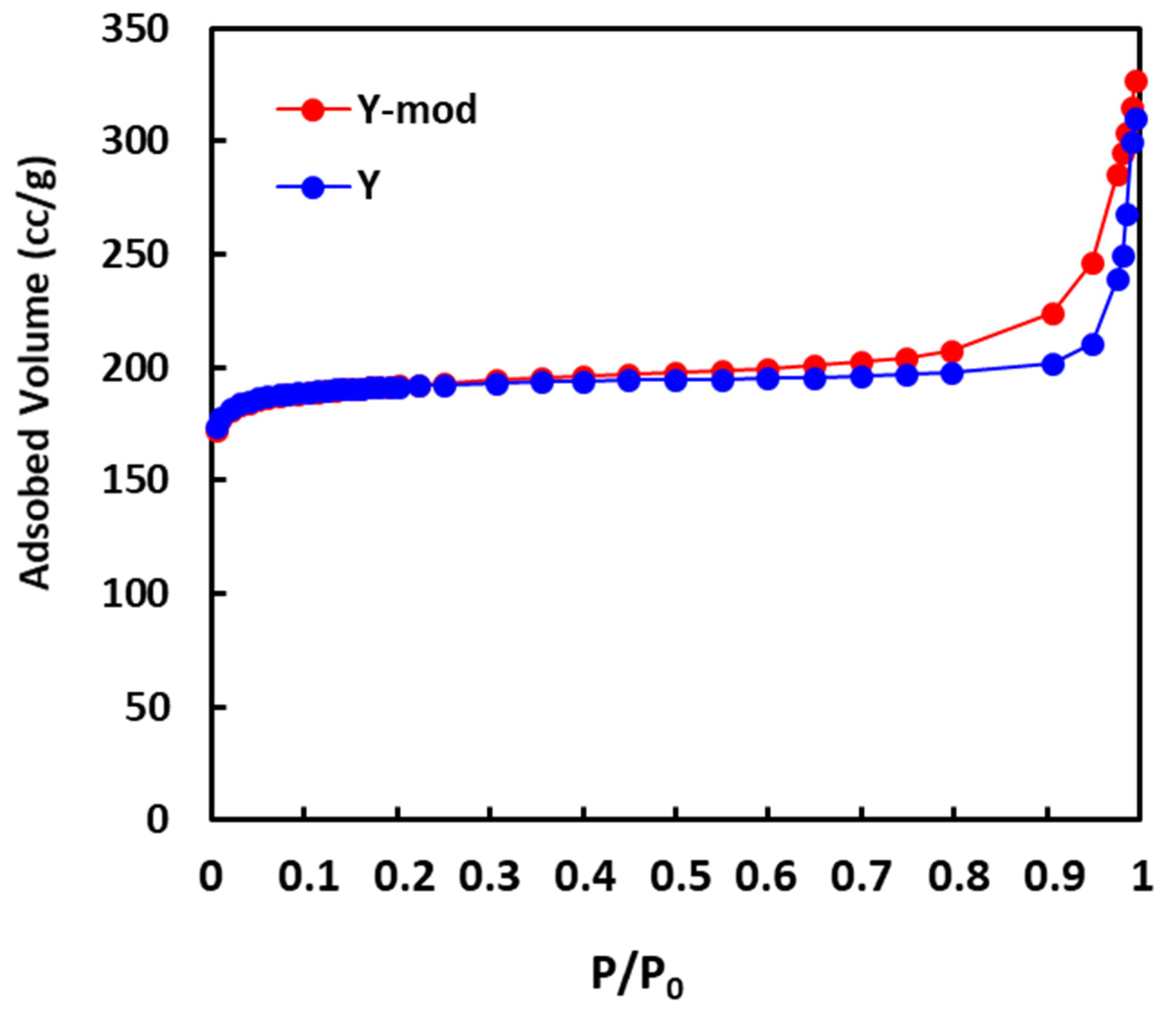
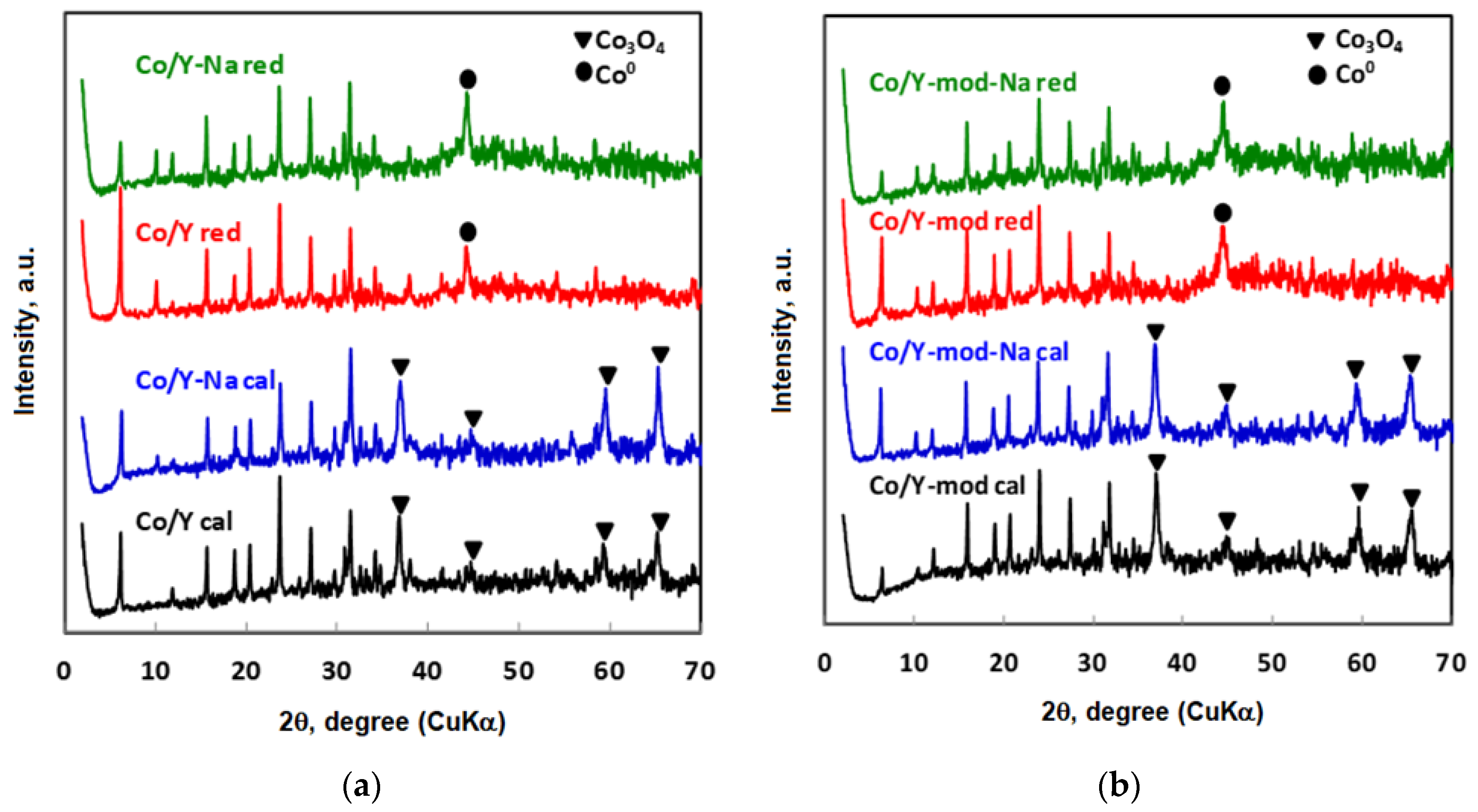
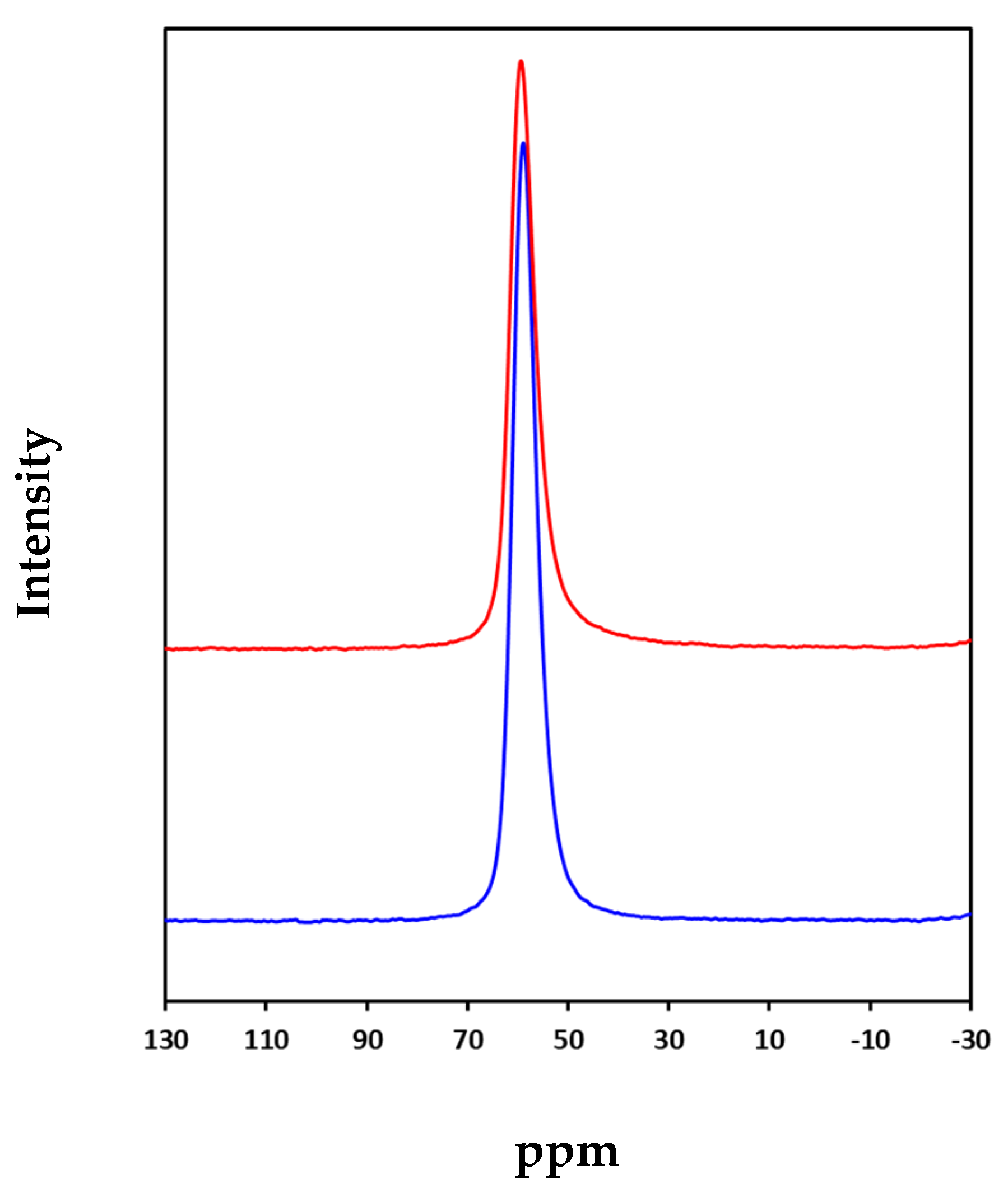
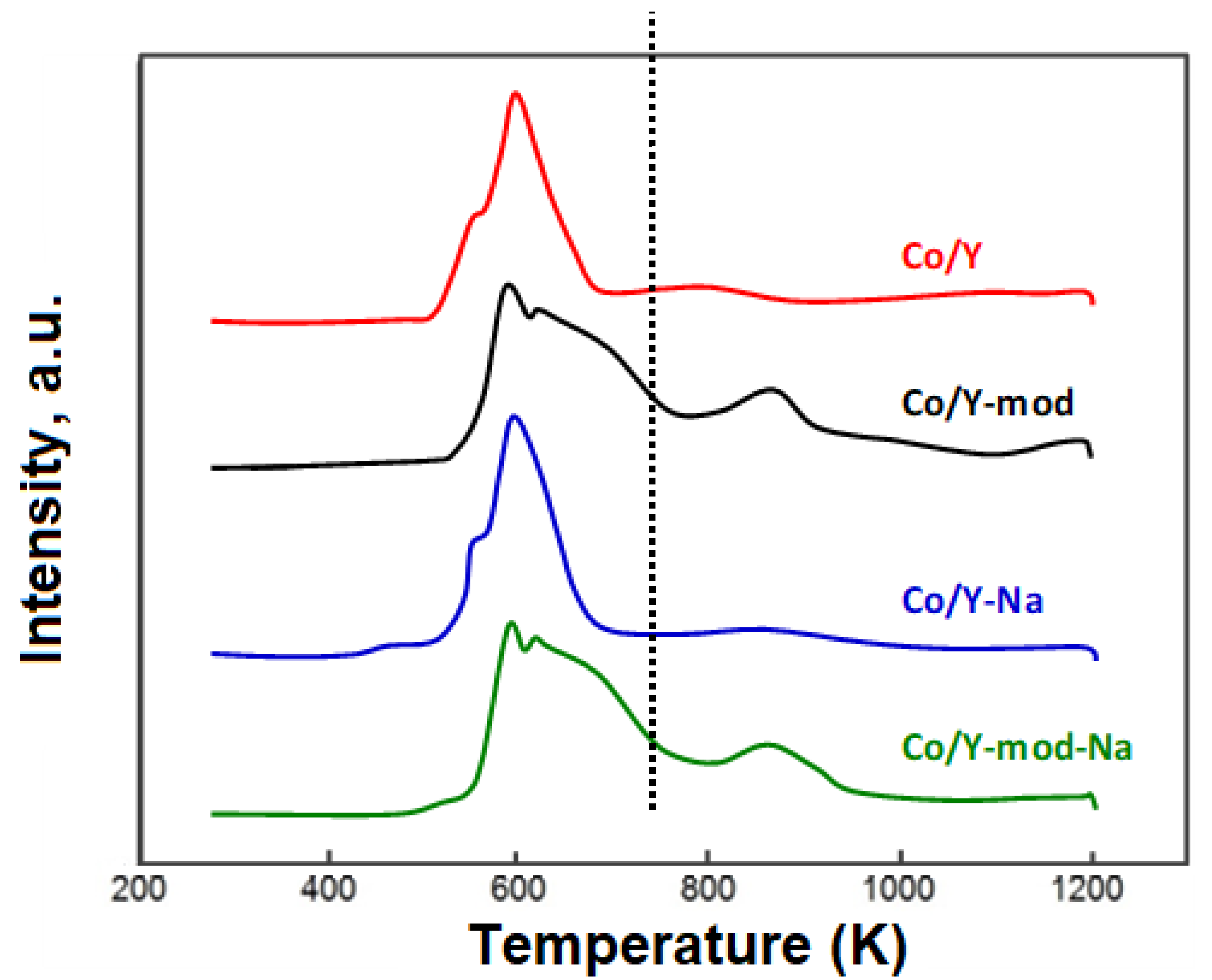
3.1. Characterization
3.2. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zambrano-Monserrate, M.A.; Ruano, M.A.; Sanchez-Alcalde, L. Indirect effects of COVID-19 on the environment. Sci. Total Environ. 2020, 728, 138813. [Google Scholar] [CrossRef] [PubMed]
- Olmos, F.; Hennessy, B.P.; Manousiouthakis, I.V.; Somiari, I.; Manousiouthakis, V.I. Thermodynamic feasibility analysis of a water-splitting thermochemical cycle based on sodium carbonate decomposition. Int. J. Hydrogen Energy 2019, 44, 4041–4061. [Google Scholar] [CrossRef]
- Cloete, S.; Khan, M.N.; Amini, S. Economic assessment of membrane-assisted autothermal reforming for cost effective hydrogen production with CO2 capture. Int. J. Hydrogen Energy 2019, 44, 3492–3510. [Google Scholar] [CrossRef]
- Liguras, D.K.; Konarides, D.I.; Verykios, X.E. Production of hydrogen for fuel cells by steam reforming of ethanol over supported noble metal catalysts. Appl. Catal. B Environ. 2003, 43, 345–354. [Google Scholar] [CrossRef]
- Maggio, G.; Freni, S.; Cavallaro, S. Light alcohols/methane fuelled molten carbonate fuel cells: A comparative study. J. Power Sources 1998, 74, 17–23. [Google Scholar] [CrossRef]
- Brown, L.F. A comparative study of fuels for on-board hydrogen production for fuel-cell-powered automobiles. Int. J. Hydrogen Energy 2001, 26, 381–397. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.; Leung, M.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Šantek, M.I.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Kaiwen, L.; Bin, Y.; Tao, Z. Economic analysis of hydrogen production from steam reforming process: A literature review. Energy Sources Part B Econ. Plan. Policy 2018, 13, 109–115. [Google Scholar] [CrossRef]
- Nanda, S.; Rana, R.; Zheng, Y.; Kozinski, J.; Dalai, A.K. Insights on pathways for hydrogen generation from ethanol. Sustain. Energy Fuels 2017, 1, 1232–1245. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Kumar, A.; Prasad, R.; Upadhyay, S.N. Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renew. Sustain. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Sidhu, T.P.K.; Roy, S. Optimal design of washcoated monolith catalyst for compact, heat -integrated ethanol reformers. Int. J. Hydrogen Energy 2019, 44, 11472–11487. [Google Scholar] [CrossRef]
- Liberatori, J.; Ribeiro, R.; Zanchet, D.; Noronha, F.; Bueno, J.M. Steam reforming of ethanol on supported nickel catalysts. Appl. Catal. A Gen. 2007, 327, 197–204. [Google Scholar] [CrossRef]
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.; Leung, M. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Llorca, J.; Homs, N.; Sales, J.; De La Piscina, P.R. Efficient Production of Hydrogen over Supported Cobalt Catalysts from Ethanol Steam Reforming. J. Catal. 2002, 209, 306–317. [Google Scholar] [CrossRef]
- Muroyama, H.; Nakase, R.; Matsui, T.; Eguchi, K. Ethanol steam reforming over Ni-based spinel oxide. Int. J. Hydrogen Energy 2010, 35, 1575–1581. [Google Scholar] [CrossRef]
- Lindo, M.; Vizcaíno, A.; Calles, J.; Carrero, A. Ethanol steam reforming on Ni/Al-SBA-15 catalysts: Effect of the aluminium content. Int. J. Hydrogen Energy 2010, 35, 5895–5901. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Lee, C.-C.; Wang, C.-H.; Yeh, C.-T.; Wang, C.-B. Application of nickel–lanthanum composite oxide on the steam reforming of ethanol to produce hydrogen. Int. J. Hydrogen Energy 2010, 35, 4069–4075. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y. LaFeyNi1−yO3 supported nickel catalysts used for steam reforming of ethanol. Int. J. Hydrogen Energy 2009, 34, 4735–4746. [Google Scholar] [CrossRef]
- Hernández, I.; Gochi-Ponce, Y.; Larios, J.C.; Fernández, A. Steam reforming of ethanol over nickel-tungsten catalyst. Int. J. Hydrogen Energy 2010, 35, 12098–12104. [Google Scholar] [CrossRef]
- Koh, A.C.W.; Chen, L.; Leong, W.K.; Yang, S.; Johnson, B.F.; Khimyak, T.; Lin, J. Ethanol steam reforming over supported ruthenium and ruthenium–platinum catalysts: Comparison of organometallic clusters and inorganic salts as catalyst precursors. Int. J. Hydrogen Energy 2009, 34, 5691–5703. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, M.; Navarro, R.; Fierro, J. Ethanol steam reforming over Ni/MxOyNi/MxOy–Al2O3Al2O3 (M=CeM=Ce, La, Zr and Mg) catalysts: Influence of support on the hydrogen production. Int. J. Hydrogen Energy 2007, 32, 1462–1471. [Google Scholar] [CrossRef]
- Augusto, B.L.; Ribeiro, M.C.; Aires, F.J.C.S.; Da Silva, V.T.; Noronha, F.B. Hydrogen production by the steam reforming of ethanol over cobalt catalysts supported on different carbon nanostructures. Catal. Today 2020, 344, 66–74. [Google Scholar] [CrossRef]
- Bepari, S.; Basu, S.; Pradhan, N.C.; Dalai, A.K. Steam reforming of ethanol over cerium-promoted Ni-Mg-Al hydrotalcite catalysts. Catal. Today 2017, 291, 47–57. [Google Scholar] [CrossRef]
- Pinton, N.; Vidal, M.; Signoretto, M.; Martínez-Arias, A.; Corberán, V.C. Ethanol steam reforming on nanostructured catalysts of Ni, Co and CeO2: Influence of synthesis method on activity, deactivation and regenerability. Catal. Today 2017, 296, 135–143. [Google Scholar] [CrossRef]
- Da Costa-Serra, J.; Chica, A. Bioethanol steam reforming on Co/ITQ-18 catalyst: Effect of the crystalline structure of the delaminated zeolite ITQ-18. Int. J. Hydrogen Energy 2011, 36, 3862–3869. [Google Scholar] [CrossRef]
- Da Costa-Serra, J.; Guil-Lopez, R.; Chica, A. Co/ZnO and Ni/ZnO catalysts for hydrogen production by bioethanol steam reforming. Influence of ZnO support morphology on the catalytic properties of Co and Ni active phases. Int. J. Hydrogen Energy 2010, 35, 6709–6716. [Google Scholar] [CrossRef]
- Da Costa-Serra, J.; Navarro, M.; Rey, F.; Chica, A. Bioethanol steam reforming on Ni-based modified mordenite. Effect of mesoporosity, acid sites and alkaline metals. Int. J. Hydrogen Energy 2012, 37, 7101–7108. [Google Scholar] [CrossRef]
- Cortés, A.; Feijoo, G.; Chica, A.; Da Costa-Serra, J.; Moreira, M.T. Environmental implications of biohydrogen based energy production from steam reforming of alcoholic waste. Ind. Crop. Prod. 2019, 138, 111465. [Google Scholar] [CrossRef]
- Fuertes, A.; Da Costa-Serra, J.; Chica, A. New Catalysts based on Ni-Birnessite and Ni-Todorokite for the Efficient Production of Hydrogen by Bioethanol Steam Reforming. Energy Procedia 2012, 29, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Da Costa-Serra, J.; Chica, A. Catalysts based on Co-Birnessite and Co-Todorokite for the efficient production of hydrogen by ethanol steam reforming. Int. J. Hydrogen Energy 2018, 43, 16859–16865. [Google Scholar] [CrossRef]
- Cerdá-Moreno, C.; Da Costa-Serra, J.; Chica, A. Co and La supported on Zn-Hydrotalcite-derived material as efficient catalyst for ethanol steam reforming. Int. J. Hydrogen Energy 2019, 44, 12685–12692. [Google Scholar] [CrossRef]
- Hernández-Soto, M.; Da Costa-Serra, J.; Carratalá, J.; Beneito, R.; Chica, A. Valorization of alcoholic wastes from the vinery industry to produce H2. Int. J. Hydrogen Energy 2019, 44, 9763–9770. [Google Scholar] [CrossRef]
- Llorca, J.; de la Piscina, P.R.; Sales, J.; Homs, N. Direct production of hydrogen from ethanolic aqueous solutions over oxide catalystsDedicated to Professor Rafael Usón on the occasion of his 75th birthday. Chem. Commun. 2001, 641–642. [Google Scholar] [CrossRef]
- Chica, A.; Sayas, S. Effective and stable bioethanol steam reforming catalyst based on Ni and Co supported on all-silica delaminated ITQ-2 zeolite. Catal. Today 2009, 146, 37–43. [Google Scholar] [CrossRef]
- Campos-Skrobot, F.C.; Rizzo-Domingues, R.C.; Fernandes, N.R.C.; Cantão, M.P. Novel zeolite-supported rhodium catalysts for ethanol steam reforming. J. Power Sources 2008, 183, 713–716. [Google Scholar] [CrossRef]
- Inokawa, H.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Difference in the catalytic activity of transition metals and their cations loaded in zeolite Y for ethanol steam reforming. Int. J. Hydrogen Energy 2010, 35, 11719–11724. [Google Scholar] [CrossRef]
- Inokawa, H.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Promotion of H2 production from ethanol steam reforming by zeolite basicity. Int. J. Hydrogen Energy 2011, 36, 15195–15202. [Google Scholar] [CrossRef]
- Kwak, B.S.; Lee, J.S.; Choi, B.-H.; Ji, M.J.; Kang, M.; Lee, J.S. Hydrogen-rich gas production from ethanol steam reforming over Ni/Ga/Mg/Zeolite Y catalysts at mild temperature. Appl. Energy 2011, 88, 4366–4375. [Google Scholar] [CrossRef]
- Qin, Z.; Lakiss, L.; Gilson, J.-P.; Thomas, K.; Goupil, J.-M.; Fernandez, C.; Valtchev, V. Chemical Equilibrium Controlled Etching of MFI-Type Zeolite and Its Influence on Zeolite Structure, Acidity, and Catalytic Activity. Chem. Mater. 2013, 25, 2759–2766. [Google Scholar] [CrossRef]
- Ernst, B.; Libs, S.; Chaumette, P.; Kiennemann, A. Preparation and characterization of Fischer–Tropsch active Co/SiO2 catalysts. Appl. Catal. A Gen. 1999, 186, 145–168. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Griboval, A.; Bechara, R.; Villain, F. Pore-Size Control of Cobalt Dispersion and Reducibility in Mesoporous Silicas. J. Phys. Chem. B 2001, 105, 9805–9811. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Griboval, A.; Bechara, R.; Zholobenko, V. Pore Size Effects in Fischer Tropsch Synthesis over Cobalt-Supported Mesoporous Silicas. J. Catal. 2002, 206, 230–241. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, Q.; Wang, P.; Wang, Y.; Wan, H. Characterizations of Cobalt Oxide Nanoparticles within Faujasite Zeolites and the Formation of Metallic Cobalt. Chem. Mater. 2004, 16, 1967–1976. [Google Scholar] [CrossRef]
- Enache, D.I.; Rebours, B.; Roy-Auberger, M.; Revel, R. In Situ XRD Study of the Influence of Thermal Treatment on the Characteristics and the Catalytic Properties of Cobalt-Based Fischer–Tropsch Catalysts. J. Catal. 2002, 205, 346–353. [Google Scholar] [CrossRef]
- Liu, X.; Yi, R.; Wang, Y.; Qiu, G.; Zhang, N.; Li, X. Highly Ordered Snowflakelike Metallic Cobalt Microcrystals. J. Phys. Chem. C 2007, 111, 163–167. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Horie, M.; Konno, M.; Rodríguez-González, B.; Liz-Marzán, L.M. Preparation and Properties of Silica-Coated Cobalt Nanoparticles. J. Phys. Chem. B 2003, 107, 7420–7425. [Google Scholar] [CrossRef]
- Zeisberger, M.; Dutz, S.; Muller, R.; Hergt, R.; Matoussevitch, N.; Bönnemann, H. Metallic cobalt nanoparticles for heating applications. J. Magn. Magn. Mater. 2007, 311, 224–227. [Google Scholar] [CrossRef]
- Fu, R.; Baumann, T.F.; Cronin, S.; Dresselhaus, G.; Dresselhaus, M.S.; Satcher, J.H. Formation of Graphitic Structures in Cobalt- and Nickel-Doped Carbon Aerogels. Langmuir 2005, 21, 2647–2651. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction; Addison–Wesley: London, UK, 1878. [Google Scholar]
- Emeis, C. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Esquivel, D.; Jimenez-Sanchidrian, C.; Romero-Salguero, F.J. Metal-Exchanged β Zeolites as Catalysts for the Conversion of Acetone to Hydrocarbons. Materials 2012, 5, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castner, D.G.; Watson, P.R.; Chan, I.Y. X-ray absorption spectroscopy, x-ray photoelectron spectroscopy, and analytical electron microscopy studies of cobalt catalysts. 2. Hydrogen reduction properties. J. Phys. Chem. 1990, 94, 819–828. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Lynch, J.; Bazin, D.; Rebours, B.; Zanier, N.; Moisson, B.; Chaumette, P. Reducibility of Cobalt Species in Silica-Supported Fischer–Tropsch Catalysts. J. Catal. 1997, 168, 16–25. [Google Scholar] [CrossRef]
- Riva, R.; Miessner, H.; Vitali, R.; Del Piero, G. Metal–support interaction in Co/SiO2 and Co/TiO2. Appl. Catal. A Gen. 2000, 196, 111–123. [Google Scholar] [CrossRef]
- Sexton, B. An XPS and TPR study of the reduction of promoted cobalt-kieselguhr Fischer-Tropsch catalysts. J. Catal. 1986, 97, 390–406. [Google Scholar] [CrossRef]
- Van Steen, E.; Sewell, G.S.; Makhothe, R.A.; Micklethwaite, C.; Manstein, H.; De Lange, M.; O’Connor, C.T. TPR Study on the Preparation of Impregnated Co/SiO2Catalysts. J. Catal. 1996, 162, 220–229. [Google Scholar] [CrossRef]
- Fierro, V.; Akdim, O.; Provendier, H.; Mirodatos, C. Ethanol oxidative steam reforming over Ni-based catalysts. J. Power Sources 2005, 145, 659–666. [Google Scholar] [CrossRef]
- Llorca, J.; De La Piscina, P.R.; Dalmon, J.-A.; Sales, J.; Homs, N. CO-free hydrogen from steam-reforming of bioethanol over ZnO-supported cobalt catalysts. Appl. Catal. B Environ. 2003, 43, 355–369. [Google Scholar] [CrossRef]

| Catalyst | Si/Al | Na (wt. %) | BET Area (m2/g) | Mesopore Volume (cm3/g) | Micropore Volume (cm3/g) |
|---|---|---|---|---|---|
| Y | 2.4 | 0.21 | 629 | 0.034 | 0.292 |
| Y-mod | 3.9 | 0.11 | 544 | 0.110 | 0.244 |
| Co/Y | 2.3 | 0.15 | 499 | 0.036 | 0.241 |
| Co/Y-mod | 3.8 | 0.06 | 409 | 0.080 | 0.180 |
| Co/Y-Na | 2.4 | 3.13 | 406 | 0.020 | 0.190 |
| Co/Y-mod-Na | 3.9 | 4.21 | 371 | 0.037 | 0.146 |
| Catalyst | Co (wt. %) | Co0, XRD (nm) | Co0 Dispersion (%) a | Reducibility % (873 K) |
|---|---|---|---|---|
| Co/Y | 19.1 | 29 | 3.3 | 95.4 |
| Co/Y-mod | 19.0 | 14 | 6.9 | 88.3 |
| Co/Y-Na | 19.3 | 28 | 3.4 | 94.2 |
| Co/Y-mod-Na | 18.7 | 13 | 7.4 | 89.1 |
| Catalyst | B (mmol py) | L (mmol py) | ||||
|---|---|---|---|---|---|---|
| 523 (K) | 623 (K) | 673 (K) | 523 (K) | 623 (K) | 673 (K) | |
| Y | 0.254 | 0.127 | 0.076 | 0.034 | 0.023 | 0.023 |
| Y-mod | 0.235 | 0.127 | 0.029 | 0.070 | 0.061 | 0.018 |
| Co/Y | 0.010 | 0.005 | 0.004 | 0.777 | 0.312 | 0.140 |
| Co/Y-mod | 0.103 | 0.025 | 0.019 | 0.861 | 0.385 | 0.249 |
| Co/Y-Na | - | - | - | 0.532 | 0.276 | 0.127 |
| Co/Y-mod-Na | - | - | - | 0.671 | 0.302 | 0.177 |
| Catalyst | T (K) | Ethanol Conv. % mol | Selectivity % mol | |||||
|---|---|---|---|---|---|---|---|---|
| CH4 | CO | CO2 | H2 | C2H4 | C2H4O | |||
| Co-Y | 673 | 98.5 | 1.1 | 4.3 | 4.7 | 17.5 | 71.6 | 0.7 |
| 773 | 99.8 | 1.7 | 3.4 | 21.4 | 39.0 | 32.7 | 1.7 | |
| 873 | 99.9 | 3.4 | 11.2 | 22.2 | 43.5 | 18.7 | 1.0 | |
| Co-Y mod | 673 | 99.9 | 0.0 | 0.0 | 0.3 | 0.2 | 95.8 | 3.6 |
| 773 | 100 | 0.6 | 1.0 | 0.9 | 13.9 | 78.7 | 4.6 | |
| 873 | 99.8 | 2.2 | 5.0 | 2.3 | 25.7 | 59.6 | 4.9 | |
| Co-Y Na | 673 | 94.7 | 7.0 | 7.0 | 14.2 | 68.9 | 0.6 | 2.2 |
| 773 | 95.9 | 3.7 | 5.1 | 18.1 | 70.3 | 0.8 | 1.0 | |
| 873 | 99.9 | 4.6 | 11.6 | 12.0 | 69.7 | 0.8 | 1.2 | |
| Co-Y mod Na | 673 | 99.8 | 4.9 | 6.0 | 15.2 | 73.3 | 0.2 | 0.4 |
| 773 | 99.9 | 3.0 | 2.6 | 20.3 | 74.0 | 0.1 | 0.0 | |
| 873 | 99.9 | 2.0 | 4.3 | 19.5 | 74.1 | 0.0 | 0.0 | |
| Catalyst | Carbon (wt. %) |
|---|---|
| Co/Y | 31.3 |
| Co/Y-mod | 34.7 |
| Co/Y-Na | 24.8 |
| Co/Y-mod-Na | 19.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa-Serra, J.F.; Navarro, M.T.; Rey, F.; Chica, A. Sustainable Production of Hydrogen by Steam Reforming of Ethanol Using Cobalt Supported on Nanoporous Zeolitic Material. Nanomaterials 2020, 10, 1934. https://doi.org/10.3390/nano10101934
da Costa-Serra JF, Navarro MT, Rey F, Chica A. Sustainable Production of Hydrogen by Steam Reforming of Ethanol Using Cobalt Supported on Nanoporous Zeolitic Material. Nanomaterials. 2020; 10(10):1934. https://doi.org/10.3390/nano10101934
Chicago/Turabian Styleda Costa-Serra, Javier Francisco, Maria Teresa Navarro, Fernando Rey, and Antonio Chica. 2020. "Sustainable Production of Hydrogen by Steam Reforming of Ethanol Using Cobalt Supported on Nanoporous Zeolitic Material" Nanomaterials 10, no. 10: 1934. https://doi.org/10.3390/nano10101934




