The Importance of Evaluating the Lot-to-Lot Batch Consistency of Commercial Multi-Walled Carbon Nanotube Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanomaterials, Chemicals, and Solutions
2.2. CHN/O Analyses
2.3. Preparation of BSA-MWNT Suspensions
2.4. Characterization of MWNT Suspensions
2.5. Cell Culture
2.6. Crystal Violet Cell Proliferation Assay
2.7. Accumulation of pMWNTs by Cells
2.8. Quantitation of pMWNTs Extracted from Cell Lysates by SDS-PAGE
2.9. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
2.10. Transmission Electron Microscopy (TEM)
2.11. Thermogravimetric Analyses (TGA)
2.12. Microprobe Raman Spectroscopy
2.13. Brunauer-Emmett-Teller (BET) Surface Area Measurements
2.14. X-ray Diffraction (XRD)
2.15. X-ray Photoelectron Spectroscopy (XPS)
2.16. Fourier Transform Infrared (FTIR) Spectroscopy
3. Results
3.1. CHN/O Analyses of MWNTs
3.2. Characterization of BSA-MWNT Suspensions
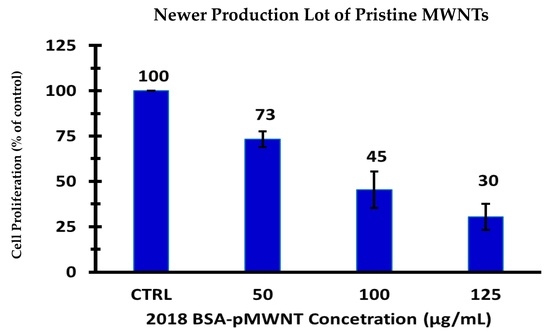
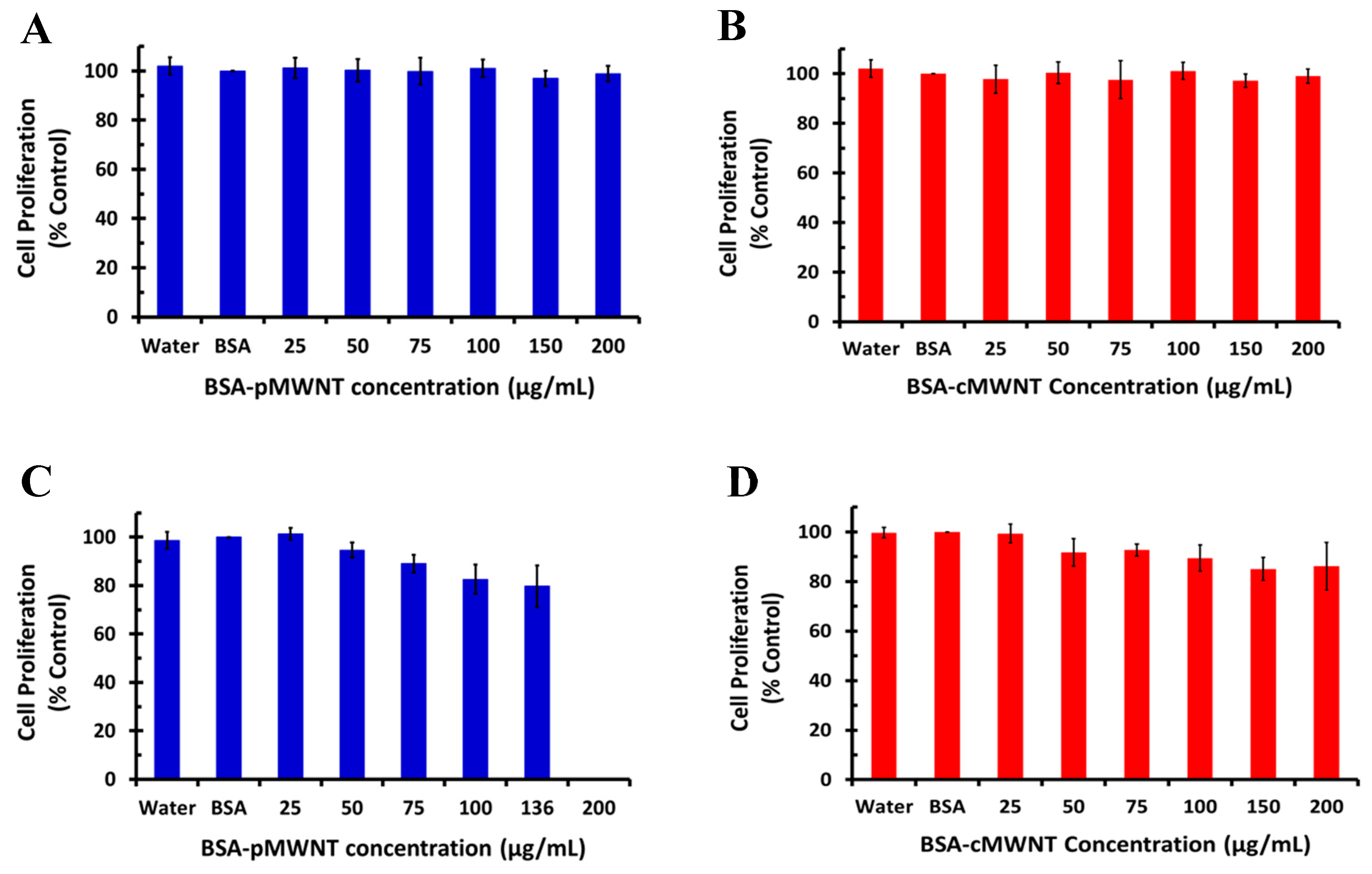
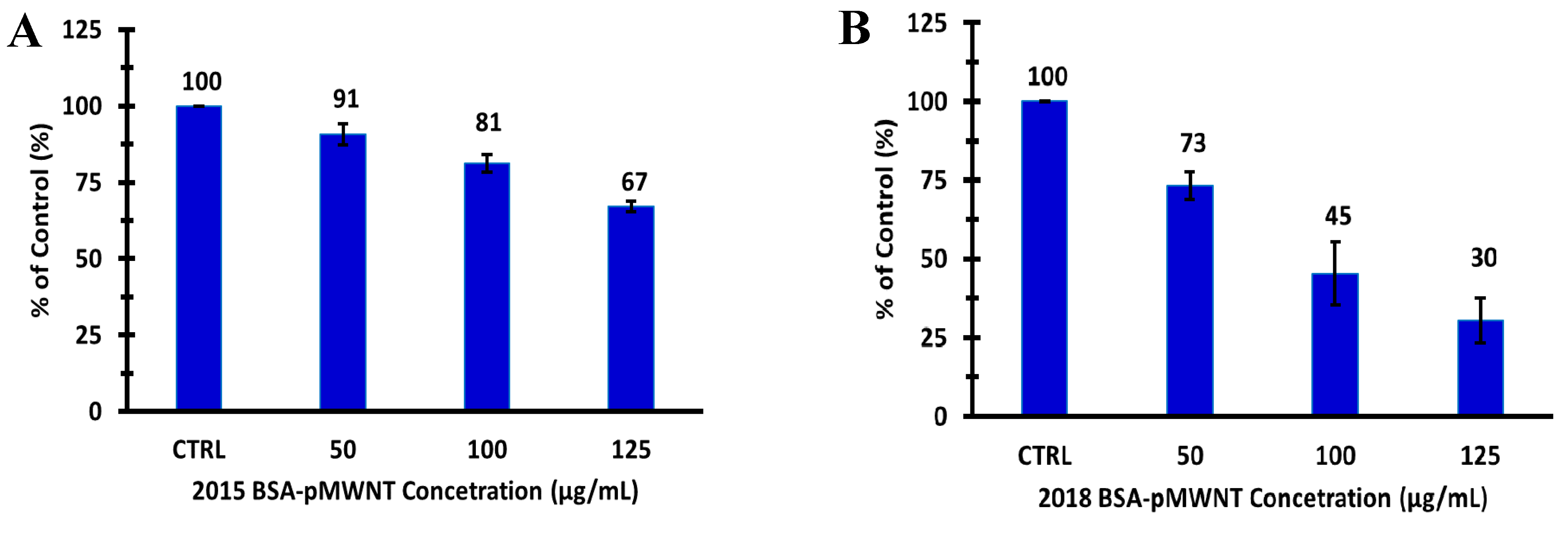
3.3. Macrophage Proliferation Assays
3.4. Accumulation of pMWNTs by Macrophages
3.5. ICP-MS of MWNTs
3.6. TEM and HR-TEM Imaging of MWNTs
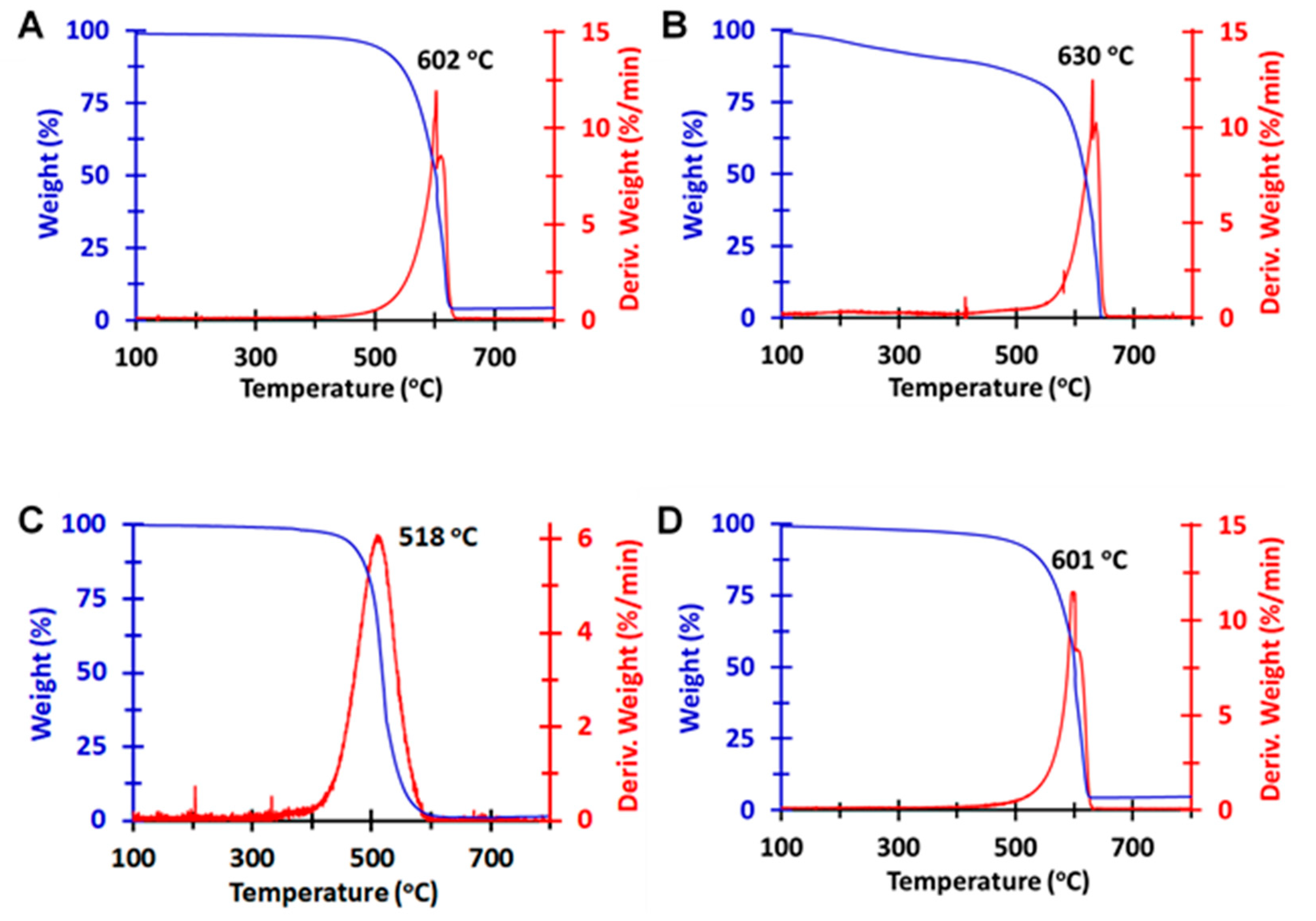
3.7. TGA of MWNTs
3.8. Raman Spectroscopy of MWNTs
3.9. XRD Analyses of MWNTs
3.10. BET Surface Area Measurements of MWNTs
3.11. XPS Analyses of MWNTs
3.12. FTIR Spectroscopy of MWNTs
4. Discussion
4.1. Physicochemical Properties of 2018-pMWNTs that Correlate with Reduced Cell Proliferation
4.2. The Unsuitability of the 2018-pMWNTs as a Replacement for the 2015-pMWNTs
4.3. The Suitability of the 2018-cMWNTs as a Replacement for the 2015-cMWNTs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shen, H.; Liu, T.; Qin, D.; Bo, X.; Wang, L.; Wang, F.; Yuan, Q.; Wagberg, T.; Hu, G.; Zhou, M. Wearable carbon nanotube devices for sensing. In Industrial Applications of Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 179–199. [Google Scholar]
- Rashid, M.; Ralph, S.F. Carbon nanotube membranes: Synthesis, properties, and future filtration applications. Nanomaterials 2017, 7, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, S. Engineering applications of carbon nanotubes. In Carbon Nanotube-Reinforced Polymers; Elsevier: Amsterdam, The Netherlands, 2018; pp. 25–40. [Google Scholar]
- Jayaraman, T.; Murthy, A.P.; Elakkiya, V.; Chandrasekaran, S.; Nithyadharseni, P.; Khan, Z.; Senthil, R.A.; Shanker, R.; Raghavender, M.; Kuppusami, P. Recent development on carbon based heterostructures for their applications in energy and environment: A review. J. Ind. Eng. Chem. 2018, 64, 16–59. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Kumar, P.; Kim, K.-H.; Ok, Y.S. Designer carbon nanotubes for contaminant removal in water and wastewater: A critical review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xie, L.; Hameed, S.; Wang, C.; Ying, Y. Mechanisms and applications of carbon nanotubes in terahertz devices: A review. Carbon 2018, 132, 42–58. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2018, 119, 599–663. [Google Scholar] [CrossRef]
- Cardenas, J.A.; Andrews, J.B.; Noyce, S.G.; Franklin, A.D. Carbon nanotube electronics for IoT sensors. Nano Futures 2020, 4, 012001. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Carbon nanotubes: A novel material for multifaceted applications in human healthcare. Chem. Soc. Rev. 2017, 46, 158–196. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Golbabaie, A.; Kasaeian, A. Carbon nanotubes: A review of novel strategies for cancer diagnosis and treatment. Mater. Sci. Eng. C 2017, 76, 1289–1304. [Google Scholar] [CrossRef]
- Sireesha, M.; Jagadeesh Babu, V.; Kranthi Kiran, A.S.; Ramakrishna, S. A review on carbon nanotubes in biosensor devices and their applications in medicine. Nanocomposites 2018, 4, 36–57. [Google Scholar] [CrossRef]
- Raphey, V.; Henna, T.; Nivitha, K.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef]
- Lorite, G.S.; Pitkänen, O.; Mohl, M.; Kordas, K.; Koivisto, J.T.; Kellomäki, M.; Mendonça, M.C.P.; de Jesus, M.B. Carbon nanotube-based matrices for tissue engineering. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 323–353. [Google Scholar]
- Kaur, J.; Gill, G.S.; Jeet, K. Applications of carbon nanotubes in drug delivery: A comprehensive review. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–135. [Google Scholar]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube—A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Liné, C.; Larue, C.; Flahaut, E. Carbon nanotubes: Impacts and behaviour in the terrestrial ecosystem-A review. Carbon 2017, 123, 767–785. [Google Scholar] [CrossRef] [Green Version]
- Narei, H.; Ghasempour, R.; Akhavan, O. Toxicity and safety issues of carbon nanotubes. In Carbon Nanotube-Reinforced Polymers; Elsevier: Amsterdam, The Netherlands, 2018; pp. 145–171. [Google Scholar]
- Chen, M.; Zhou, S.; Zhu, Y.; Sun, Y.; Zeng, G.; Yang, C.; Xu, P.; Yan, M.; Liu, Z.; Zhang, W. Toxicity of carbon nanomaterials to plants, animals and microbes: Recent progress from 2015-present. Chemosphere 2018, 206, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.B.; Hurt, R.H.; Gao, H. The asbestos-carbon nanotube analogy: An update. Toxicol. Appl. Pharmacol. 2018, 361, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon nanotubes: Evaluation of toxicity at biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Malaiya, A.; Kesharwani, P.; Soni, D.; Jain, A. Biomedical applications and toxicities of carbon nanotubes. Drug Chem. Toxicol. 2020. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Just, J.; Hartman, K.B.; Laoudi, Y.; Boudjemaa, S.; Alloyeau, D.; Szwarc, H.; Wilson, L.J.; Moussa, F. Anthropogenic carbon nanotubes found in the airways of Parisian children. EBioMedicine 2015, 2, 1697–1704. [Google Scholar] [CrossRef] [Green Version]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Pettitt, M.E.; Lead, J.R. Minimum physicochemical characterisation requirements for nanomaterial regulation. Environ. Int. 2013, 52, 41–50. [Google Scholar] [CrossRef]
- Birch, M.E.; Ruda-Eberenz, T.A.; Chai, M.; Andrews, R.; Hatfield, R.L. Properties that influence the specific surface areas of carbon nanotubes and nanofibers. Ann. Occup. Hyg. 2013, 57, 1148–1166. [Google Scholar] [PubMed] [Green Version]
- Rashad, A.; Noaman, R.; Mohammed, S.; Yousif, E. Synthesis of carbon nanotube: A review. J. Nanosci. Technol. 2016, 2, 155–162. [Google Scholar]
- Malikov, E.Y.; Muradov, M.B.; Akperov, O.H.; Eyvazova, G.M.; Puskás, R.; Madarász, D.; Nagy, L.; Kukovecz, Á.; Kónya, Z. Synthesis and characterization of polyvinyl alcohol based multiwalled carbon nanotube nanocomposites. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 61, 129–134. [Google Scholar] [CrossRef]
- Boverhof, D.R.; David, R.M. Nanomaterial characterization: Considerations and needs for hazard assessment and safety evaluation. Anal. Bioanal. Chem. 2010, 396, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Podila, R.; Brown, J.M. Toxicity of engineered nanomaterials: A physicochemical perspective. J. Biochem. Mol. Toxicol. 2013, 27, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadeel, B.; Fornara, A.; Toprak, M.S.; Bhattacharya, K. Keeping it real: The importance of material characterization in nanotoxicology. Biochem. Biophys. Res. Commun. 2015, 468, 498–503. [Google Scholar] [CrossRef]
- Gunsolus, I.L.; Haynes, C.L. Analytical aspects of nanotoxicology. Anal. Chem. 2016, 88, 451–479. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc. 2017, 12, 355–371. [Google Scholar] [CrossRef]
- Krug, H.F. The uncertainty with nanosafety: Validity and reliability of published data. Colloids Surf. B Biointerfaces 2018, 172, 113–117. [Google Scholar] [CrossRef]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Kabir, M.; Sood, A. On the cytotoxicity of carbon nanotubes. Curr. Sci. 2009, 96, 664–673. [Google Scholar]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Aschberger, K.; Stone, V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology 2010, 4, 207–246. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Rizwan, M.; Sheikh, A.M.; Hasnain, M.S.; Anwer, K.; Kohli, K. Advancement in carbon nanotubes: Basics, biomedical applications and toxicity. J. Pharm. Pharmacol. 2011, 63, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.-P.; Roesslein, M.; Buerki-Thurnherr, T.; Wick, P. Carbon nanotubes-curse or blessing. Curr. Med. Chem. 2011, 18, 2115–2128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the toxicity of carbon nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Salamon, A.W. The current world of nanomaterial characterization: Discussion of analytical instruments for nanomaterial characterization. Environ. Eng. Sci. 2013, 30, 101–108. [Google Scholar] [CrossRef]
- Tessonnier, J.-P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; Su, D.S. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon 2009, 47, 1779–1798. [Google Scholar] [CrossRef] [Green Version]
- Rausch, J.; Zhuang, R.-C.; Mäder, E. Surfactant assisted dispersion of functionalized multi-walled carbon nanotubes in aqueous media. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1038–1046. [Google Scholar] [CrossRef]
- Levine, K.E.; Han, L.; McWilliams, A.C.; Essader, A.S.; Amato, K.E.; Fernando, R.A.; Browning, D.B.; Greene, L.C.; Ensor, D.S.; Walker, N.J. Characterization of an assortment of commercially available multiwalled carbon nanotubes. Microchim. Acta 2014, 181, 171–179. [Google Scholar] [CrossRef]
- White, C.M.; Banks, R.; Hamerton, I.; Watts, J.F. Characterisation of commercially CVD grown multi-walled carbon nanotubes for paint applications. Prog. Org. Coat. 2016, 90, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Richman, E.K.; Hutchison, J.E. The nanomaterial characterization bottleneck. ACS Nano 2009, 3, 2441–2446. [Google Scholar] [CrossRef] [PubMed]
- Crist, R.M.; Grossman, J.H.; Patri, A.K.; Stern, S.T.; Dobrovolskaia, M.A.; Adiseshaiah, P.P.; Clogston, J.D.; McNeil, S.E. Common pitfalls in nanotechnology: Lessons learned from NCI’s nanotechnology characterization laboratory. Integr. Biol. 2013, 5, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.P.; Jurkschat, K.; Crossley, A.; Banks, C.E. Multi-walled carbon nanotube modified basal plane pyrolytic graphite electrodes: Exploring heterogeneity, electro-catalysis and highlighting batch to batch variation. J. Iran. Chem. Soc. 2008, 5, 279–285. [Google Scholar] [CrossRef]
- Braun, E.I.; Pantano, P. The importance of an extensive elemental analysis of single-walled carbon nanotube soot. Carbon 2014, 77, 912–919. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Meredith, A.N.; Lee, M., Jr.; Deutsch, D.; Miadzvedskaya, L.; Braun, E.; Pantano, P.; Harper, S.; Draper, R. Toxicity assessment and bioaccumulation in zebrafish embryos exposed to carbon nanotubes suspended in Pluronic® F-108. Nanotoxicology 2016, 10, 689–698. [Google Scholar] [CrossRef]
- Wang, R.; Lee, M.; Kinghorn, K.; Hughes, T.; Chuckaree, I.; Lohray, R.; Chow, E.; Pantano, P.; Draper, R. Quantitation of cell-associated carbon nanotubes: Selective binding and accumulation of carboxylated carbon nanotubes by macrophages. Nanotoxicology 2018, 12, 677–698. [Google Scholar] [CrossRef]
- Nakata, T. Destruction of challenged endotoxin in a dry heat oven. PDA J. Pharm. Sci. Technol. 1994, 48, 59–63. [Google Scholar]
- Yehia, H.N.; Draper, R.K.; Mikoryak, C.; Walker, E.K.; Bajaj, P.; Musselman, I.H.; Daigrepont, M.C.; Dieckmann, G.R.; Pantano, P. Single-walled carbon nanotube interactions with HeLa cells. J. Nanobiotechnol. 2007, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Hughes, T.; Beck, S.; Vakil, S.; Li, S.; Pantano, P.; Draper, R.K. Generation of toxic degradation products by sonication of Pluronic® dispersants: Implications for nanotoxicity testing. Nanotoxicology 2013, 7, 1272–1281. [Google Scholar] [CrossRef]
- Wang, R.; Mikoryak, C.; Li, S.; Bushdiecker, D., II; Musselman, I.H.; Pantano, P.; Draper, R.K. Cytotoxicity screening of single-walled carbon nanotubes: Detection and removal of cytotoxic contaminants from carboxylated carbon nanotubes. Mol. Pharm. 2011, 8, 1351–1361. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Mikoryak, C.; Chen, E.; Li, S.; Pantano, P.; Draper, R.K. Gel electrophoresis method to measure the concentration of single-walled carbon nanotubes extracted from biological tissue. Anal. Chem. 2009, 81, 2944–2952. [Google Scholar] [CrossRef] [PubMed]
- Braun, E.I.; Huang, A.; Tusa, C.A.; Yukica, M.A.; Pantano, P. Use of Raman spectroscopy to identify carbon nanotube contamination at an analytical balance workstation. J. Occup. Environ. Hyg. 2016, 13, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Occhialini, G.; McCandless, G.T.; Alahakoon, S.B.; Cameron, V.; Nielsen, S.O.; Smaldone, R.A. Computational and experimental studies on the effects of monomer planarity on covalent organic framework formation. J. Am. Chem. Soc. 2017, 139, 10506–10513. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.T.; Thomas, M.C.; Chabal, Y.J.; Balkus, K.J., Jr. Nanocast carbon microsphere flowers from a lanthanum-based template. Mater. Lett. 2019, 234, 224–227. [Google Scholar] [CrossRef]
- Veyan, J.-F.; de Obaldia, E.; Alcantar-Peña, J.J.; Montes-Gutierrez, J.; Arellano-Jimenez, M.J.; Yacaman, M.J.; Auciello, O. Argon atoms insertion in diamond: New insights in the identification of carbon C 1s peak in X-ray photoelectron spectroscopy analysis. Carbon 2018, 134, 29–36. [Google Scholar] [CrossRef]
- Perkins, C.K.; Mansergh, R.H.; Park, D.-H.; Nanayakkara, C.E.; Ramos, J.C.; Decker, S.R.; Huang, Y.; Chabal, Y.J.; Keszler, D.A. Aqueous process to limit hydration of thin-film inorganic oxides. Solid State Sci. 2016, 61, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Allegri, M.; Perivoliotis, D.K.; Bianchi, M.G.; Chiu, M.; Pagliaro, A.; Koklioti, M.A.; Trompeta, A.-F.A.; Bergamaschi, E.; Bussolati, O.; Charitidis, C.A. Toxicity determinants of multi-walled carbon nanotubes: The relationship between functionalization and agglomeration. Toxicol. Rep. 2016, 3, 230–243. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Von Dem Bussche, A.; Buechner, M.; Yan, A.; Kane, A.B.; Hurt, R.H. Adsorption of essential micronutrients by carbon nanotubes and the implications for nanotoxicity testing. Small 2008, 4, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Casey, A.; Herzog, E.; Lyng, F.; Byrne, H.; Chambers, G.; Davoren, M. Single walled carbon nanotubes induce indirect cytotoxicity by medium depletion in A549 lung cells. Toxicol. Lett. 2008, 179, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Catelas, I.; Petit, A.; Zukor, D.J.; Antoniou, J.; Huk, O.L. TNF-α secretion and macrophage mortality induced by cobalt and chromium ions in vitro-Qualitative analysis of apoptosis. Biomaterials 2003, 24, 383–391. [Google Scholar] [CrossRef]
- Kónya, Z.; Zhu, J.; Niesz, K.; Mehn, D.; Kiricsi, I. End morphology of ball milled carbon nanotubes. Carbon 2004, 42, 2001–2008. [Google Scholar] [CrossRef]
- Chen, S.; Hu, S.; Smith, E.F.; Ruenraroengsak, P.; Thorley, A.J.; Menzel, R.; Goode, A.E.; Ryan, M.P.; Tetley, T.D.; Porter, A.E. Aqueous cationic, anionic and non-ionic multi-walled carbon nanotubes, functionalised with minimal framework damage, for biomedical application. Biomaterials 2014, 35, 4729–4738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Fairbrother, D.H. Chemical and structural characterization of carbon nanotube surfaces. Anal. Bioanal. Chem. 2010, 396, 1003–1014. [Google Scholar] [CrossRef]
- Wu, Z.; Hamilton, R.F., Jr.; Wang, Z.; Holian, A.; Mitra, S. Oxidation debris in microwave functionalized carbon nanotubes: Chemical and biological effects. Carbon 2014, 68, 678–686. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Qian, D.; Dickey, E. Purification and structural annealing of multiwalled carbon nanotubes at graphitization temperatures. Carbon 2001, 39, 1681–1687. [Google Scholar] [CrossRef]
- Figarol, A.; Pourchez, J.; Boudard, D.; Forest, V.; Berhanu, S.; Tulliani, J.-M.; Lecompte, J.-P.; Cottier, M.; Bernache-Assollant, D.; Grosseau, P. Thermal annealing of carbon nanotubes reveals a toxicological impact of the structural defects. J. Nanoparticle Res. 2015, 17, 194. [Google Scholar] [CrossRef]
- Gong, H.; Kim, S.-T.; Lee, J.D.; Yim, S. Simple quantification of surface carboxylic acids on chemically oxidized multi-walled carbon nanotubes. Appl. Surf. Sci. 2013, 266, 219–224. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Bom, D.; Andrews, R.; Jacques, D.; Anthony, J.; Chen, B.; Meier, M.S.; Selegue, J.P. Thermogravimetric analysis of the oxidation of multiwalled carbon nanotubes: Evidence for the role of defect sites in carbon nanotube chemistry. Nano Lett. 2002, 2, 615–619. [Google Scholar] [CrossRef]
- McKee, G.S.; Vecchio, K.S. Thermogravimetric analysis of synthesis variation effects on CVD generated multiwalled carbon nanotubes. J. Phys. Chem. B 2006, 110, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, Z.; Hu, Y.; Li, J.; Fan, X. Multiple functionalization of multi-walled carbon nanotubes with carboxyl and amino groups. Appl. Surf. Sci. 2013, 276, 476–481. [Google Scholar] [CrossRef]
- Jain, S.; Thakare, V.S.; Das, M.; Godugu, C.; Jain, A.K.; Mathur, R.; Chuttani, K.; Mishra, A.K. Toxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization density. Chem. Res. Toxicol. 2011, 24, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Jorio, A.; Pimenta, M.; Souza Filho, A.; Saito, R.; Dresselhaus, G.; Dresselhaus, M. Characterizing carbon nanotube samples with resonance Raman scattering. New J. Phys. 2003, 5, 139. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhang, S.; Wang, X. Surface functional groups and defects on carbon nanotubes affect adsorption–desorption hysteresis of metal cations and oxoanions in water. Environ. Sci. Nano 2014, 1, 488–495. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Wang, J.; Cui, S. Sulfonitric treatment of multiwalled carbon nanotubes and their dispersibility in water. Materials 2018, 11, 2442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy. J. Raman Spectrosc. Int. J. Orig. Work All Asp. Raman Spectrosc. Incl. High. Order Process. Brillouin Rayleigh Scatt. 2007, 38, 728–736. [Google Scholar] [CrossRef]
- Mohanapriya, S.; Sridhar, P.; Pitchumani, S.; Shukla, A. Influence of surface pretreatment of MWNTs support on PEFC performance. ECS Trans. 2010, 28, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Malikov, E.; Akperov, O.; Muradov, M.; Eyvazova, G.; Maharramov, A.; Kukovecz, A.; Konya, Z. Oxidation of multiwalled carbon nanotubes using different oxidation agents like nitric acid and potassium permanganate. News Baku Univ. 2014, 4, 49–59. [Google Scholar]
- Raza, M.A.; Kanwal, Z.; Riaz, S.; Naseem, S. Synthesis, characterization and antibacterial properties of nano-sized cobalt particles. In Proceedings of the 2016 World Congress on Advances in Civil Enviromental, and Materials Research (ACEM16), Jeju Island, Korea, 28 August–1 September 2016; Volume 28. 6p. [Google Scholar]
- Cheng, J.; Zhang, X.; Ye, Y. Synthesis of nickel nanoparticles and carbon encapsulated nickel nanoparticles supported on carbon nanotubes. J. Solid State Chem. 2006, 179, 91–95. [Google Scholar] [CrossRef]
- Hamilton, R.F.; Wu, Z.; Mitra, S.; Holian, A. The effects of varying degree of MWCNT carboxylation on bioactivity in various in vivo and in vitro exposure models. Int. J. Mol. Sci. 2018, 19, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Okpalugo, T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- Pacheco, F.G.; Cotta, A.A.; Gorgulho, H.F.; Santos, A.P.; Macedo, W.A.; Furtado, C.A. Comparative temporal analysis of multiwalled carbon nanotube oxidation reactions: Evaluating chemical modifications on true nanotube surface. Appl. Surf. Sci. 2015, 357, 1015–1023. [Google Scholar] [CrossRef]
- Yamada, Y.; Yasuda, H.; Murota, K.; Nakamura, M.; Sodesawa, T.; Sato, S. Analysis of heat-treated graphite oxide by X-ray photoelectron spectroscopy. J. Mater. Sci. 2013, 48, 8171–8198. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Mejia, J.; Tichelaar, F.; Saout, C.; Toussaint, O.; Masereel, B.; Mekhalif, Z.; Lucas, S.; Delhalle, J. Effects of the dispersion methods in Pluronic F108 on the size and the surface composition of MWCNTs and their implications in toxicology assessment. J. Nanoparticle Res. 2011, 13, 655–667. [Google Scholar] [CrossRef]
- Nishikiori, H.; Tanigaki, T.; Endo, M.; Fujii, T. Quantitative characterization of acidic groups on acid-treated multi-walled carbon nanotubes using 1-aminopyrene as a fluorescent probe. Carbon 2014, 66, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Trykowski, G.; Biniak, S.; Stobinski, L.; Lesiak, B. Preliminary investigations into the purification and functionalization of multiwall carbon nanotubes. Acta Phys. Pol.-Ser. A Gen. Phys. 2010, 118, 515–518. [Google Scholar] [CrossRef]
- Stéfani, D.; Paula, A.J.; Vaz, B.G.; Silva, R.A.; Andrade, N.F.; Justo, G.Z.; Ferreira, C.V.; Souza Filho, A.G.; Eberlin, M.N.; Alves, O.L. Structural and proactive safety aspects of oxidation debris from multiwalled carbon nanotubes. J. Hazard. Mater. 2011, 189, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kouklin, N.; Tzolov, M.; Straus, D.; Yin, A.; Xu, J. Infrared absorption properties of carbon nanotubes synthesized by chemical vapor deposition. Appl. Phys. Lett. 2004, 85, 4463–4465. [Google Scholar] [CrossRef]
- Fenoglio, I.; Greco, G.; Tomatis, M.; Muller, J.; Raymundo-Pinero, E.; Béguin, F.; Fonseca, A.; Nagy, J.B.; Lison, D.; Fubini, B. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: Physicochemical aspects. Chem. Res. Toxicol. 2008, 21, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.K.; Ye, J.; Han, Q.L.; Tao, R.; Liu, F.; Wang, W. Toxicity and bioactivity of cobalt nanoparticles on the monocytes. Orthop. Surg. 2015, 7, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.-C. Defects in carbon nanotubes. Acc. Chem. Res. 2002, 35, 1063–1069. [Google Scholar] [CrossRef]
- Liu, Y.; Liggio, J.; Li, S.-M.; Breznan, D.; Vincent, R.; Thomson, E.M.; Kumarathasan, P.; Das, D.; Abbatt, J.; Antinñolo, M.; et al. Chemical and toxicological evolution of carbon nanotubes during atmospherically relevant aging processes. Environ. Sci. Technol. 2015, 49, 2806–2814. [Google Scholar] [CrossRef]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forró, L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef]
- Firme, C.P., III; Bandaru, P.R. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 245–256. [Google Scholar] [CrossRef]
- Bai, W.; Raghavendra, A.; Podila, R.; Brown, J.M. Defect density in multiwalled carbon nanotubes influences ovalbumin adsorption and promotes macrophage activation and cD4+ T-cell proliferation. Int. J. Nanomed. 2016, 11, 4357–4371. [Google Scholar]
- Jiang, W.; Wang, Q.; Qu, X.; Wang, L.; Wei, X.; Zhu, D.; Yang, K. Effects of charge and surface defects of multi-walled carbon nanotubes on the disruption of model cell membranes. Sci. Total Environ. 2017, 574, 771–780. [Google Scholar] [CrossRef]
- Li, Y.; Cao, J. The impact of multi-walled carbon nanotubes (MWCNTs) on macrophages: Contribution of MWCNT characteristics. Sci. China Life Sci. 2018, 61, 1333–1351. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Huaux, F.; Fonseca, A.; Nagy, J.B.; Moreau, N.; Delos, M.; Raymundo-Pinero, E.; Béguin, F.; Kirsch-Volders, M.; Fenoglio, I. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: Toxicological aspects. Chem. Res. Toxicol. 2008, 21, 1698–1705. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Brown, J.M.; Chen, R.; Ke, P.C.; Lai, X.; Mitra, S.; Witzmann, F.A. Comparison of nanotube–protein corona composition in cell culture media. Small 2013, 9, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health concerns of various nanoparticles: A review of their in vitro and in vivo toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Element | 2015-pMWNTs (%) | 2015-cMWNTs (%) | 2018-pMWNTs (%) | 2018-cMWNTs (%) |
|---|---|---|---|---|
| C | 96.49 | 94.30 | 97.29 | 94.19 |
| H | 1.06 | 1.08 | 0.06 | 0.11 |
| N | 0.00 | 0.00 | 0.01 | 0.20 |
| O | 1.97 | 2.80 | 2.90 | 5.60 |
| Total | 99.52 | 98.18 | 100.26 | 100.11 |
| BSA–MWNT Suspension 1 | Relative MWNT Concentration (μg/mL) 2 | Dynamic Light Scattering 3 | Zeta Potential (mV) 6 | |
|---|---|---|---|---|
| HDD (nm) 4 | PDI 5 | |||
| 2015-pMWNTs | 417 ± 19 | 81.4 ± 5.4 | 0.21 | −31.8 ± 1.9 |
| 2015-cMWNTs | 496 ± 34 | 85.7 ± 6.8 | 0.20 | −33.8 ± 1.6 |
| 2018-pMWNTs | 275 ± 18 | 81.3 ± 2.1 | 0.20 | −29.1 ± 1.8 |
| 2018-cMWNTs | 456 ± 15 | 84.1 ± 1.6 | 0.21 | −32.8 ± 1.5 |
| Element | 2015-pMWNTs | 2018-pMWNTs |
|---|---|---|
| Fe (ppm) | 1689.8 | 475.4 |
| Ni (ppm) | 5591.6 | 8.8 |
| Co (ppm) | 24.6 | 1241.8 |
| MWNT Product | Outer Diameter (nm) | Inner Diameter (nm) |
|---|---|---|
| 2015-pMWNTs | 18 ± 3 | 5.6 ± 1.3 |
| 2015-cMWNTs | 19 ± 5 | 5.7 ± 1.7 |
| 2018-pMWNTs | 21 ± 4 | 5.3 ± 0.6 |
| 2018-cMWNTs | 21 ± 4 | 5.6 ± 2.1 |
| MWNT Powder | % Carbon 1 | % Oxygen 2 | Subtotal 3 | O/C 4 |
|---|---|---|---|---|
| 2015-pMWNTs | 96.4 | 3.6 | 100.0 | 0.04 |
| 2015-cMWNTs | 94.4 | 5.6 | 100.0 | 0.06 |
| 2018-pMWNTs | 99.1 | 0.9 | 100.0 | 0.01 |
| 2018-cMWNTs | 95.6 | 4.4 | 100.0 | 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, M.T.; Veyan, J.F.; Pham, H.; Rahman, R.; Yousuf, S.; Brown, A.; Lin, J.; Balkus, K.J., Jr.; Diwakara, S.D.; Smaldone, R.A.; et al. The Importance of Evaluating the Lot-to-Lot Batch Consistency of Commercial Multi-Walled Carbon Nanotube Products. Nanomaterials 2020, 10, 1930. https://doi.org/10.3390/nano10101930
Huynh MT, Veyan JF, Pham H, Rahman R, Yousuf S, Brown A, Lin J, Balkus KJ Jr., Diwakara SD, Smaldone RA, et al. The Importance of Evaluating the Lot-to-Lot Batch Consistency of Commercial Multi-Walled Carbon Nanotube Products. Nanomaterials. 2020; 10(10):1930. https://doi.org/10.3390/nano10101930
Chicago/Turabian StyleHuynh, Mai T., Jean Francois Veyan, Hong Pham, Raina Rahman, Samad Yousuf, Alexander Brown, Jason Lin, Kenneth J. Balkus, Jr., Shashini D. Diwakara, Ronald A. Smaldone, and et al. 2020. "The Importance of Evaluating the Lot-to-Lot Batch Consistency of Commercial Multi-Walled Carbon Nanotube Products" Nanomaterials 10, no. 10: 1930. https://doi.org/10.3390/nano10101930