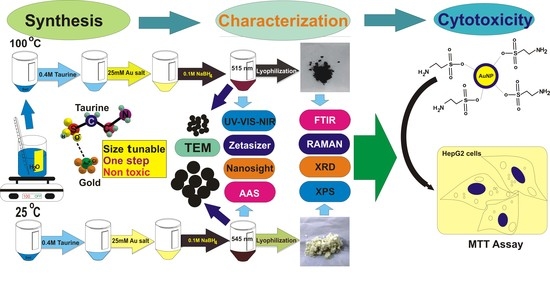
A Novel Approach towards Synthesis and Characterization of Non-Cytotoxic Gold Nanoparticles Using Taurine as Capping Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methodology
2.1.1. Optical Spectroscopy of Taurine-Capped Gold Nanospheres
2.1.2. Hydrodynamic Radius and Surface Charge Measurements Using Nano ZetaSizer
2.1.3. Nanoparticle Tracking Analysis (NTA) Using NanoSight
2.1.4. Transmission Electron Microscopy
2.1.5. Atomic Absorption Spectroscopy (AAS)
2.1.6. FTIR and Raman Spectroscopy
2.1.7. XRD and XPS Analysis
2.2. Cell Culture
Cell Viability Assay
2.3. Nanoparticle Synthesis
3. Results and Discussion
3.1. UV-VIS-NIR Measurements
Stability of Gold Nanoparticles (with/without Taurine) in Phosphate Buffer Saline (PBS)
3.2. ZetaSizer and NanoSight Characterization
3.3. Electron Microscopy Imaging
3.4. Atomic Absorption Spectroscopy
3.5. FTIR and Raman Analysis
3.6. XRD and XPS Analysis
3.7. XPS Analysis
3.8. Cell Viability Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guerrero-Martínez, A.; Alonso-Gómez, J.L.; Auguié, B.; Cid, M.M.; Liz-Marzán, L.M. From individual to collective chirality in metal nanoparticles. Nano Today 2011, 6, 381–400. [Google Scholar] [CrossRef]
- Burrows, N.D.; Lin, W.; Hinman, J.G.; Dennison, J.M.; Vartanian, A.M.; Abadeer, N.S.; Murphy, C.J. Surface chemistry of gold nanorods. Langmuir 2016, 32, 9905–9921. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Wang, Y.; Randrianalisoa, J.; Raeesi, V.; Chan, W.C.; Lipiński, W.; Bischof, J.C. Quantitative comparison of photothermal heat generation between gold nanospheres and nanorods. Sci. Rep. 2016, 6, 29836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholl, J.A.; Koh, A.L.; Dionne, J.A. Quantum plasmon resonances of individual metallic nanoparticles. Nature 2012, 483, 421. [Google Scholar] [CrossRef] [PubMed]
- Prodan, E.; Nordlander, P.; Halas, N.J. Electronic structure and optical properties of gold nanoshells. Nano Lett. 2003, 3, 1411–1415. [Google Scholar] [CrossRef]
- Huang, F.; Baumberg, J.J. Actively tuned plasmons on elastomerically driven Au nanoparticle dimers. Nano Lett. 2010, 10, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.G.; Pelaz, B.; Parak, W.J.; Del Pino, P. Phase transfer and polymer coating methods toward improving the stability of metallic nanoparticles for biological applications. Chem. Mater. 2015, 27, 990–997. [Google Scholar] [CrossRef] [Green Version]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.; Lee, J.H.; Jeong, Y.Y.; Jon, S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J. Am. Chem. Soc. 2007, 129, 7661–7665. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Sau, T.K.; Pal, A.; Jana, N.R.; Wang, Z.L.; Pal, T. Size controlled synthesis of gold nanoparticles using photochemically prepared seed particles. J. Nanopart. Res. 2001, 3, 257–261. [Google Scholar] [CrossRef]
- Wang, A.; Ng, H.P.; Xu, Y.; Li, Y.; Zheng, Y.; Yu, J.; Fu, L. Gold nanoparticles: Synthesis, stability test, and application for the rice growth. J. Nanomater. 2014, 2014, 3. [Google Scholar] [CrossRef]
- Fraga, S.; Faria, H.; Soares, M.E.; Duarte, J.A.; Soares, L.; Pereira, E.; Costa-Pereira, C.; Teixeira, J.P.; de Lourdes Bastos, M.; Carmo, H. Influence of the surface coating on the cytotoxicity, genotoxicity and uptake of gold nanoparticles in human HepG2 cells. J. Appl. Toxicol. 2013, 33, 1111–1119. [Google Scholar] [CrossRef]
- Zeng, Q.; Shao, D.; Ji, W.; Li, J.; Chen, L.; Song, J. The nanotoxicity investigation of optical nanoparticles to cultured cells in vitro. Toxicol. Rep. 2014, 1, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Ge, X.; Ding, H.; Jiang, H.; Christensen, B.M.; Li, J. Role of glutamate decarboxylase-like protein 1 (GADL1) in taurine biosynthesis. J. Biol. Chem. 2012, 287, 40898–40906. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, Y.; Osuka, K.; Seki, Y.; Gupta, R.C.; Hara, M.; Takayasu, M.; Wakabayashi, T. Taurine reduces inflammatory responses after spinal cord injury. J. Neurotrauma 2010, 27, 403–410. [Google Scholar] [CrossRef]
- Schaffer, S.; Kim, H.W. Effects and mechanisms of taurine as a therapeutic agent. Biomol. Ther. 2018, 26, 225. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.H.; Uddin, M.S.; Kim, I.S.; Choi, D.K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.H.; Lee, Y.T.; Hsieh, H.S.; Hwang, D.F. Effect of taurine on toxicity of aluminum in rats. e-SPEN Eur. E-J. Clin. Nutr. Metab. 2009, 4, e187–e192. [Google Scholar] [CrossRef] [Green Version]
- Maiti, N.; Thomas, S.; Debnath, A.; Kapoor, S. Raman and XPS study on the interaction of taurine with silver nanoparticles. RSC Adv. 2016, 6, 56406–56411. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, C.P.; Allott, K.A.; Murphy, B.P.; Yuen, H.P.; Proffitt, T.M.; Papas, A.; Moral, J.; Pham, T.; O’Regan, M.K.; Phassouliotis, C.; et al. Adjunctive Taurine in First-Episode Psychosis: A Phase 2, Double-Blind, Randomized, Placebo-Controlled Study. J. Clin. Psychiatry 2016, 77, e1610–e1617. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rayavarapu, R.G.; Petersen, W.; Hartsuiker, L.; Chin, P.; Janssen, H.; van Leeuwen, F.W.; Otto, C.; Manohar, S.; van Leeuwen, T.G. In vitro toxicity studies of polymer-coated gold nanorods. Nanotechnology 2010, 21, 145101. [Google Scholar] [CrossRef] [Green Version]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Datta, L.P.; Chatterjee, A.; Acharya, K.; De, P.; Das, M. Enzyme responsive nucleotide functionalized silver nanoparticles with effective antimicrobial and anticancer activity. New J. Chem. 2017, 41, 1538–1548. [Google Scholar] [CrossRef]
- Thompson, A.B.; Calhoun, A.K.; Smagghe, B.J.; Stevens, M.D.; Wotkowicz, M.T.; Hatziioannou, V.M.; Bamdad, C. A gold nanoparticle platform for protein–protein interactions and drug discovery. ACS Appl. Mater. Interfaces 2011, 3, 2979–2987. [Google Scholar] [CrossRef]
- Bhattarai, N.; Khanal, S.; Pudasaini, P.R.; Pahl, S.; Romero-Urbina, D. Citrate stabilized silver nanoparticles: Study of crystallography and surface properties. Int. J. Nanotechnol. Mol. Comput. 2011, 3, 15–28. [Google Scholar] [CrossRef]
- Chen, W.; Deng, H.H.; Hong, L.; Wu, Z.Q.; Wang, S.; Liu, A.L.; Lin, X.H.; Xia, X.H. Bare gold nanoparticles as facile and sensitive colorimetric probe for melamine detection. Analyst 2012, 137, 5382–5386. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Kendall, K.; Toloueinia, P.; Mehrabadi, Y.; Gupta, G.; Newton, J. Aggregation and adhesion of gold nanoparticles in phosphate buffered saline. J. Nanopart. Res. 2012, 14, 758. [Google Scholar] [CrossRef]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- De Temmerman, P.J.; Verleysen, E.; Lammertyn, J.; Mast, J. Size measurement uncertainties of near-monodisperse, near-spherical nanoparticles using transmission electron microscopy and particle-tracking analysis. J. Nanopart. Res. 2014, 16, 2628. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.; Ng, W.B.; Bernt, W.; Cho, N.J. Validation of size estimation of Nanoparticle tracking Analysis on polydisperse Macromolecule Assembly. Sci. Rep. 2019, 9, 2639. [Google Scholar] [CrossRef] [Green Version]
- Eaton, P.; Quaresma, P.; Soares, C.; Neves, C.; de Almeida, M.P.; Pereira, E.; West, P. A direct comparison of experimental methods to measure dimensions of synthetic nanoparticles. Ultramicroscopy 2017, 182, 179–190. [Google Scholar] [CrossRef]
- Huang, P.; Li, J.; Liu, X.; Wu, F. Colorimetric determination of aluminum (III) based on the aggregation of Schiff base-functionalized gold nanoparticles. Microchim. Acta 2016, 183, 863–886. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Anderson, J.M.; Johnson, R.L.; Friedel, K.; Trunschke, A.; Schlögl, R.; Schmidt-Rohr, K.; Shanks, B.H. Hydrothermally Stable Heterogeneous Catalysts for Biorenewable-Derived Molecule Conversions to Chemicals. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar]
- Moreira, R.L.; Lobo, R.P.; Dias, A. Infrared dispersion analysis and Raman scattering spectra of taurine single crystals. Spectrochim. Part A Mol. Biomol. Spectrosc. 2018, 188, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Baeck, S.H.; Cuenya, B.R.; McFarland, E.W. Catalytic activity of supported Au nanoparticles deposited from block copolymer micelles. J. Am. Chem. Soc. 2003, 125, 7148–7149. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, J.; Xue, R.; Yang, Y. Spectroscopic characterization of Au3+ biosorption by waste biomass of Saccharomyces cerevisiae. Spectrochim. Part A Mol. Biomol. Spectrosc. 2005, 61, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Iswarya, V.; Manivannan, J.; De, A.; Paul, S.; Roy, R.; Johnson, J.B.; Mukherjee, A. Surface capping and size-dependent toxicity of gold nanoparticles on different trophic levels. Environ. Sci. Pollut. Res. 2016, 23, 4844–4858. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, K.; Rayavarapu, R.G. Chloride ions assisted synthesis of tunable gold nanorods: Seedless synthesis, characterization and in vitro toxicity studies. Vacuum 2019, 166, 377–384. [Google Scholar] [CrossRef]
- John, G.; Schwarz, F.; Becker, J. Taurolidine as an effective and biocompatible additive for plaque-removing techniques on implant surfaces. Clin. Oral Investig. 2015, 19, 1069–1077. [Google Scholar] [CrossRef]








| No. | B Obs. (°2Th) | B Std. (°2Th) | Peak Pos. (°2Th) | B Structure (°2Th) | Crystallite Size (Å) | Crystallite Size (nm) |
|---|---|---|---|---|---|---|
| 1 | 0.076 | 0 | 38.4 | 0.076 | 1107 | 110.7 |
| 2 | 0.102 | 0 | 43.9 | 0.102 | 840 | 84 |
| 3 | 0.307 | 0 | 65.05 | 0.307 | 307 | 30.7 |
| 4 | 0.187 | 0 | 77.6 | 0.187 | 545 | 54.5 |
| No. | B Obs. (°2Th) | B Std. (°2Th) | Peak Pos. (°2Th) | B Structure (°2Th) | Crystallite Size (Å) | Crystallite Size (nm) |
|---|---|---|---|---|---|---|
| 1 | 0.115 | 0 | 38.2 | 0.115 | 731 | 73 |
| 2 | 0.109 | 0 | 44.4 | 0.109 | 787 | 78 |
| 3 | 0.093 | 0 | 64.58 | 0.093 | 1010 | 101 |
| 4 | 0.109 | 0 | 77.61 | 0.109 | 935 | 93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Das, N.; Satija, N.K.; Mandrah, K.; Roy, S.K.; Rayavarapu, R.G. A Novel Approach towards Synthesis and Characterization of Non-Cytotoxic Gold Nanoparticles Using Taurine as Capping Agent. Nanomaterials 2020, 10, 45. https://doi.org/10.3390/nano10010045
Kumar A, Das N, Satija NK, Mandrah K, Roy SK, Rayavarapu RG. A Novel Approach towards Synthesis and Characterization of Non-Cytotoxic Gold Nanoparticles Using Taurine as Capping Agent. Nanomaterials. 2020; 10(1):45. https://doi.org/10.3390/nano10010045
Chicago/Turabian StyleKumar, Akash, Nabojit Das, Neeraj Kumar Satija, Kapil Mandrah, Somendu Kumar Roy, and Raja Gopal Rayavarapu. 2020. "A Novel Approach towards Synthesis and Characterization of Non-Cytotoxic Gold Nanoparticles Using Taurine as Capping Agent" Nanomaterials 10, no. 1: 45. https://doi.org/10.3390/nano10010045