pH-Responsive PLGA Nanoparticle for Controlled Payload Delivery of Diclofenac Sodium
Abstract
:1. Introduction
2. Results and Discussions
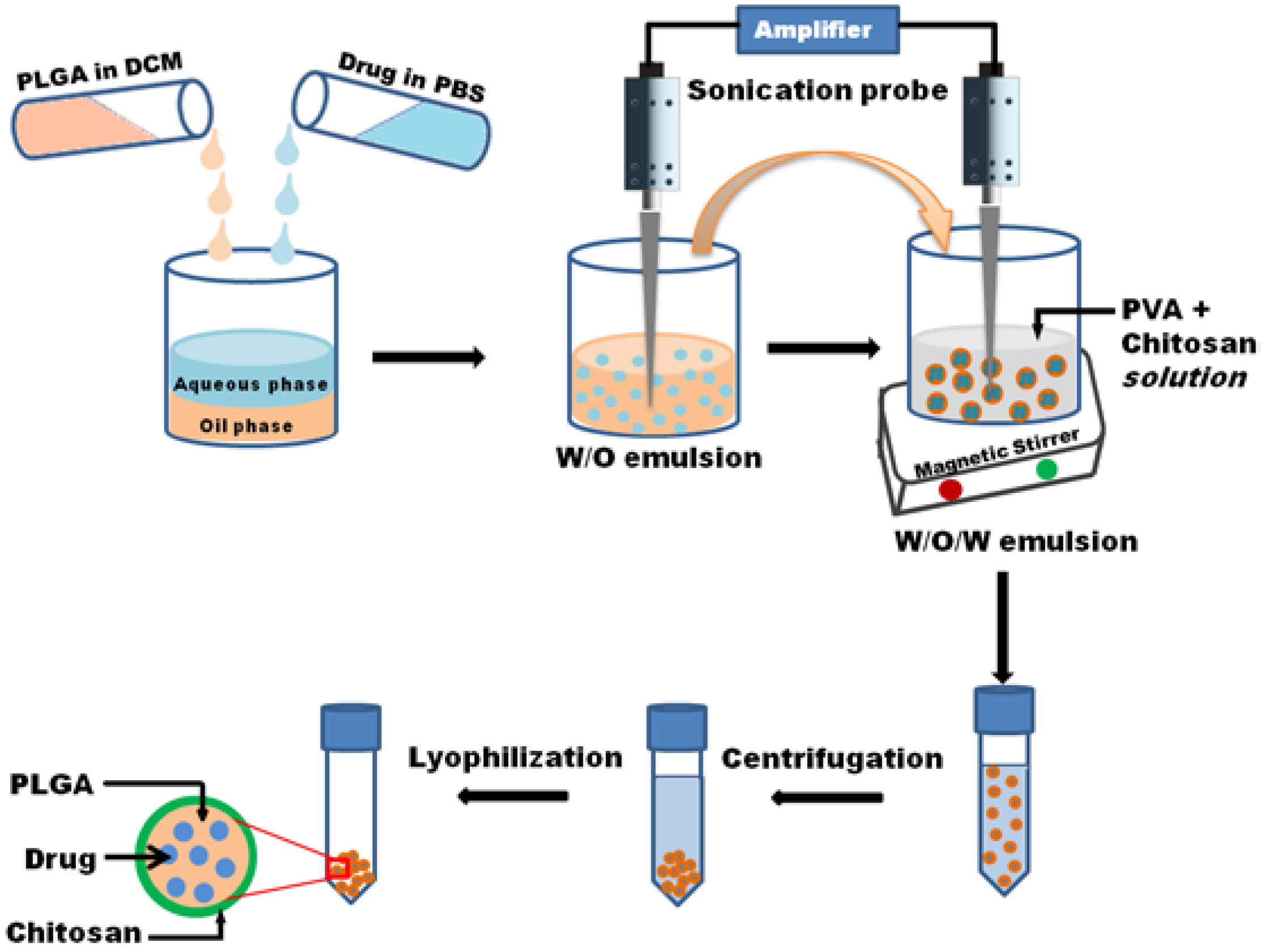
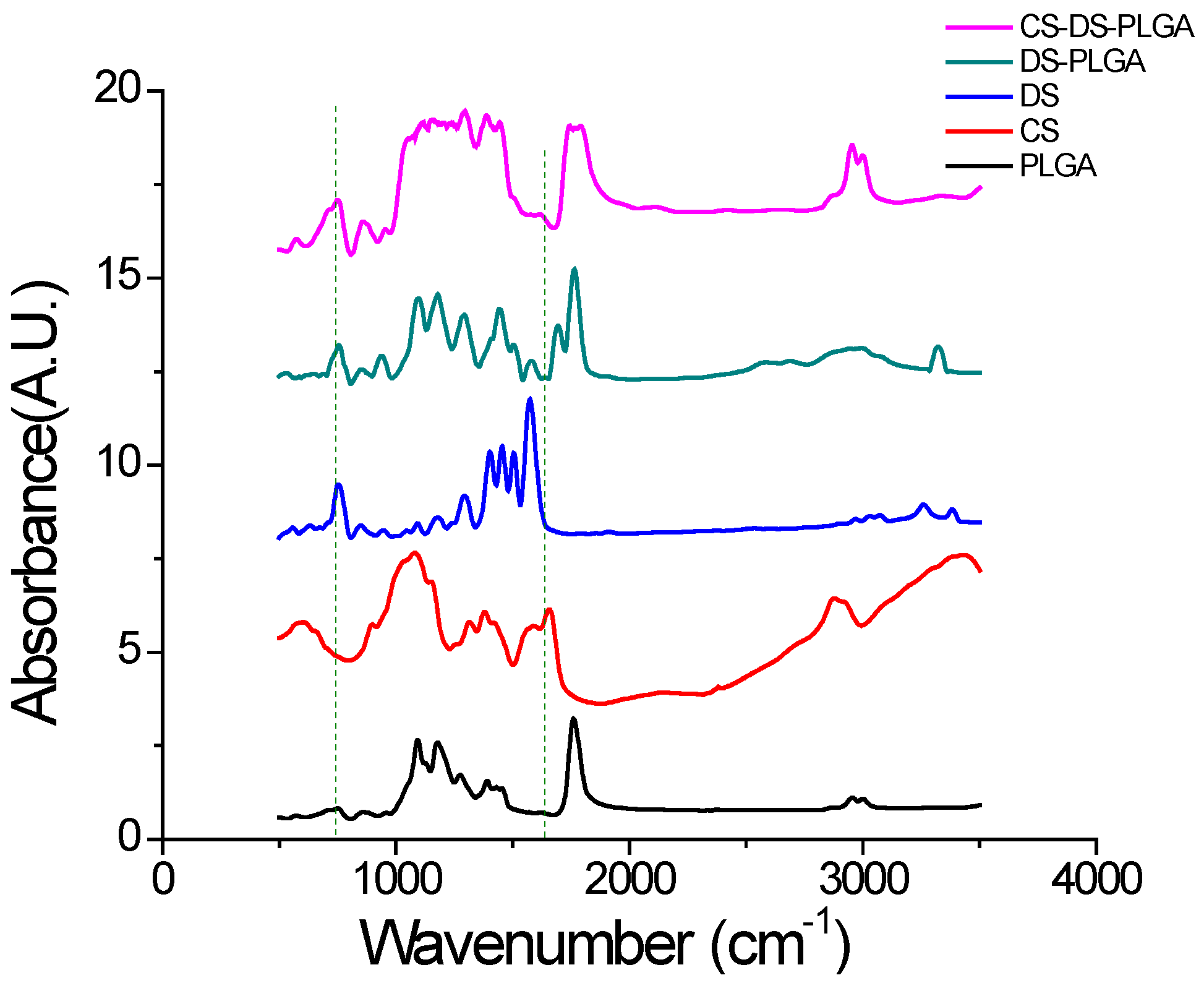
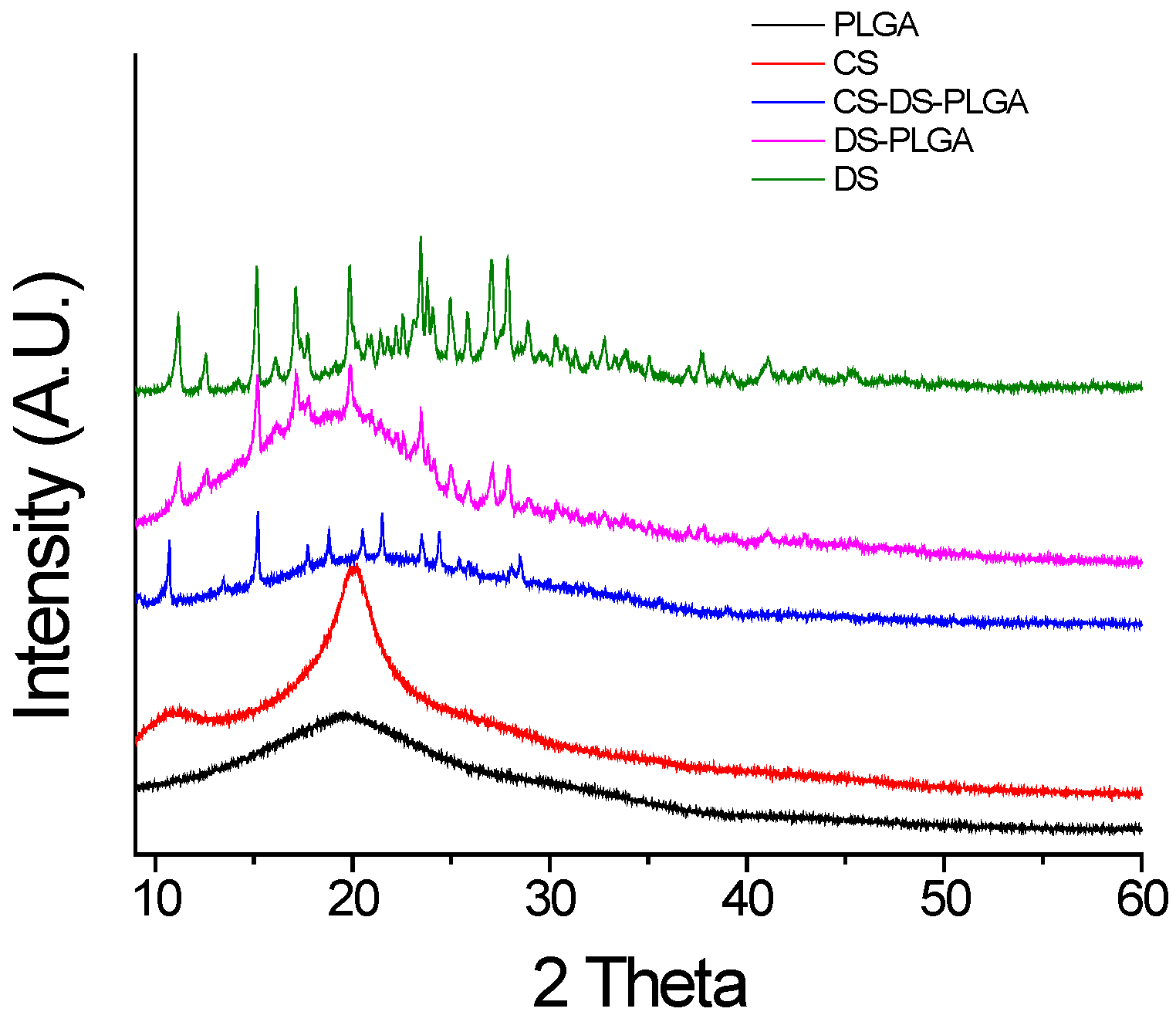
2.1. Design and Characterization of PLGA Nanoparticles
2.2. Effect of Chitosan Concentration
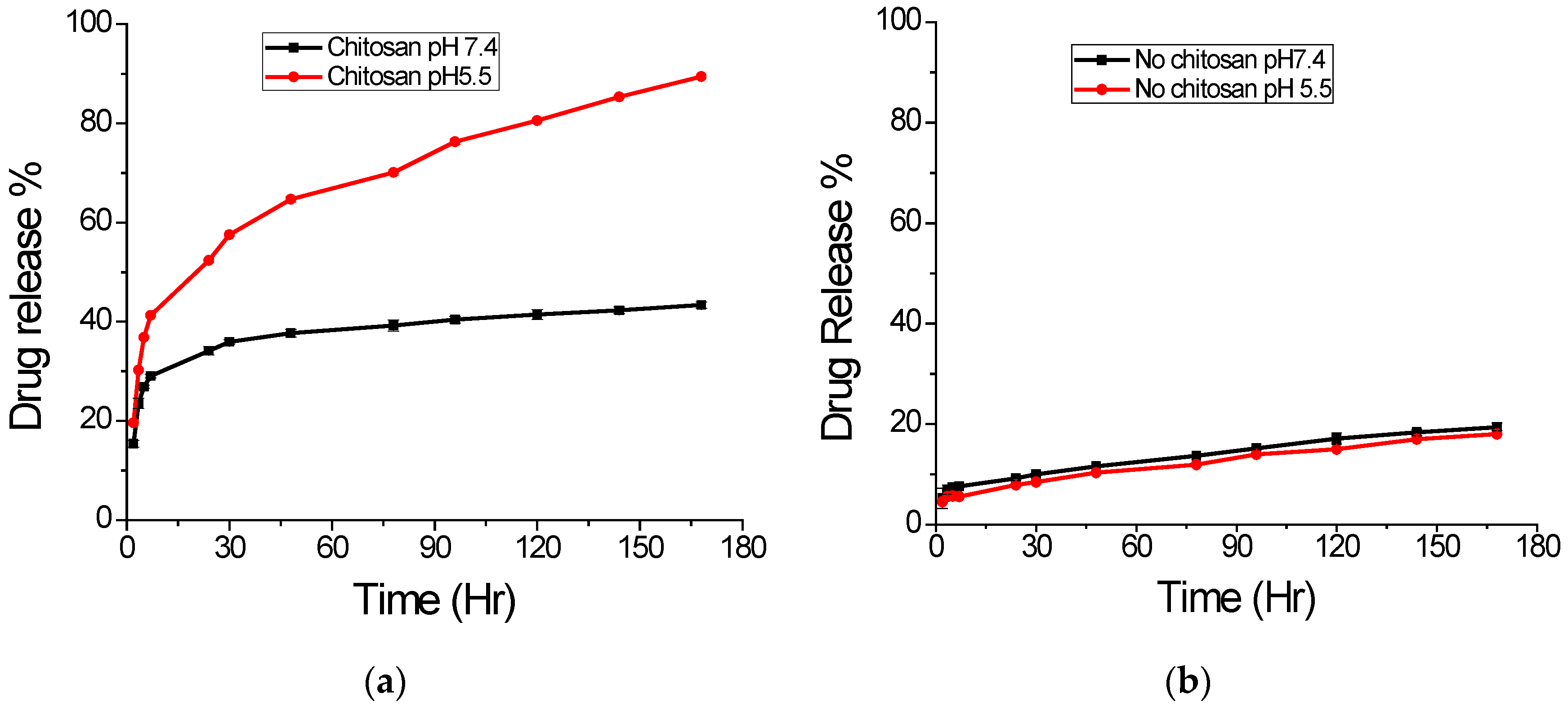
2.3. In Vitro Drug Release
2.4. Effect of pH on Drug Release
3. Materials and Methods
3.1. Materials
3.2. Nanoparticle Preparation
3.3. Morphology Study
3.4. Size Distribution and Zeta Potential
3.5. Fourier Transform Infrared (FTIR) Analysis
3.6. X-Ray Diffraction (XRD) Study
3.7. Entrapment Efficiency and Drug Content
3.8. Study of Effect of Chitosan Concentration on Drug Release
3.9. In Vitro Drug Release Study
3.10. Determination of Effect of pH on Drug Release
3.11. Statistical Analysis
4. Conclusions
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Dev, A.; Binulal, N.S.; Anitha, A.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation of poly(lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohydr. Polym. 2010, 80, 833–838. [Google Scholar] [CrossRef] [Green Version]
- Boakye, M.A.D.; Rijal, N.P.; Adhikari, U.; Bhattarai, N. Fabrication and characterization of electrospun PCL-MgO-Keratin-Based composite nanofibers for biomedical applications. Materials 2015, 8, 4080–4095. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Kong, L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech 2013, 14, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.F.; Wang, J. Delivery systems for siRNA drug development in cancer therapy. Asian J. Pharm. Sci. 2015, 10, 1–12. [Google Scholar] [CrossRef]
- Costantino, L.; Gandolfi, F.; Bossy-Nobs, L.; Tosi, G.; Gurny, R.; Rivasi, F.; Angela Vandelli, M.; Forni, F. Nanoparticulate drug carriers based on hybrid poly(d,l-lactide-co-glycolide)-dendron structures. Biomaterials 2006, 27, 4635–4645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, Y.; Zhang, X.; Shi, C.; Wang, X.; Wang, X.; Jin, Z.; Cui, S. Chitosan-coated poly(lactic-co-glycolic) acid nanoparticles as an efficient delivery system for Newcastle disease virus DNA vaccine. Int. J. Nanomed. 2014, 9, 4609–4619. [Google Scholar] [CrossRef] [PubMed]
- Warner, T.D.; Giuliano, F.; Vojnovic, I.; Bukasa, A.; Mitchell, J.A.; Vane, J.R. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc. Natl. Acad. Sci. USA 1999, 96, 7563–7568. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Sena, M.M.; Chaudhry, Z.F.; Collins, C.H.; Poppi, R.J. Direct determination of diclofenac in pharmaceutical formulations containing B vitamins by using UV spectrophotometry and partial least squares regression. J. Pharm. Biomed. Anal. 2004, 36, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Italia, J.L.; Bhatt, D.K.; Bhardwaj, V.; Tikoo, K.; Kumar, M.N. PLGA nanoparticles for oral delivery of cyclosporine: Nephrotoxicity and pharmacokinetic studies in comparison to Sandimmune Neoral. J. Control. Release 2007, 119, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Italia, J.L.; Yahya, M.M.; Singh, D.; Ravi Kumar, M.N. Biodegradable nanoparticles improve oral bioavailability of amphotericin B and show reduced nephrotoxicity compared to intravenous Fungizone. Pharm. Res. 2009, 26, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Zilberstein, A.C.; Jager, E.; Campos, M.M.; Morrone, F.B.; Calixto, J.B.; Pohlmann, A.R.; Guterres, S.S.; Battastini, A.M. Effects of indomethacin-loaded nanocapsules in experimental models of inflammation in rats. Br. J. Pharmacol. 2009, 158, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J. Drug Target. 2008, 16, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.; Taetz, S.; Schneider, M.; Schaefer, U.F.; Lehr, C.M. Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: Effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomedicine 2007, 3, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, F.; Ghahremani, M.H.; Ostad, S.N.; Atyabi, F.; Seyedabadi, M.; Malekshahi, M.R.; Amini, M.; Dinarvand, R. Folate-receptor-targeted delivery of docetaxel nanoparticles prepared by PLGA-PEG-folate conjugate. J. Drug Target. 2008, 16, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Rijal, N.P.; Pai, D.; Sankar, J.; Bhattarai, N. Synthesis and characterization of Chitosan-Mg-Based composite scaffolds for bone repair applications. In Proceedings of the ASME 2015 International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13–19 November 2015; American Society of Mechanical Engineers: New York, NY, USA, 2015. [Google Scholar]
- Amoozgar, Z.; Park, J.; Lin, Q.; Yeo, Y. Low molecular-weight chitosan as a pH-sensitive stealth coating for tumor-specific drug delivery. Mol. Pharm. 2012, 9, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Francis Suh, J.K.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef]
- Rijal, N.P.; Adhikari, U.; Bhattarai, N. Magnesium incorporated polycaprolactone-based composite nanofibers. In Proceedings of the ASME 2015 International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13–19 November 2015; American Society of Mechanical Engineers: New York, NY, USA, 2015. [Google Scholar]
- Chronopoulou, L.; Massimi, M.; Giardi, M.F.; Cametti, C.; Devirgiliis, L.C.; Dentini, M.; Palocci, C. Chitosan-coated PLGA nanoparticles: A sustained drug release strategy for cell cultures. Colloids Surf. B Biointerfaces 2013, 103, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.A.; Calvo, P.; Alonso, M.J. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv. Drug Deliv. Rev. 2001, 47, 83–97. [Google Scholar] [CrossRef]
- Dodane, V.; Amin Khan, M.; Merwin, J.R. Effect of chitosan on epithelial permeability and structure. Int. J. Pharm. 1999, 182, 21–32. [Google Scholar] [CrossRef]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Controlling the in vitro release profiles for a system of haloperidol-loaded PLGA nanoparticles. Int. J. Pharm. 2008, 346, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, A.; Reddy, G.B.; Venkatesham, M.; Veerabhadram, G.; Kumar, D.A.; Natarajan, S.; Yang, M.Y.; Hu, A.; Singh, S.S. Efficient pH dependent drug delivery to target cancer cells by gold nanoparticles capped with carboxymethyl chitosan. Int. J. Mol. Sci. 2014, 15, 8216–8234. [Google Scholar] [CrossRef] [PubMed]
- Bodmeier, R.; Chen, H.; Paeratakul, O. A Novel Approach to the Oral Delivery of Micro- or Nanoparticles. Pharm. Res. 1989, 6, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Lamosa, M.L.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Design of microencapsulated chitosan microspheres for colonic drug delivery. J. Control. Release 1998, 52, 109–118. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine 2010, 6, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, Z.; Luo, Z.; Yu, J.; Liang, R.; Li, X.; Chen, H. Chitosan grafted methoxy poly(ethylene glycol)-poly(epsilon-caprolactone) nanosuspension for ocular delivery of hydrophobic diclofenac. Sci. Rep. 2015, 5, 11337. [Google Scholar] [CrossRef] [PubMed]
- Pool, H.; Quintanar, D.; Figueroa, J.D.; Mano, C.M.; Bechara, E.J.H.; Godinez, L.A.; Mendoza, S. Antioxidant effects of quercetin and catechin encapsulated into PLGA nanoparticles. J. Nanomater. 2012. [Google Scholar] [CrossRef]
- Silva, G.S.; Oliveira, P.C.; Giordani, D.S.; de Castro, H.F. Chitosan/siloxane hybrid. polymer: Synthesis, characterization and performance as a support. for immobilizing enzyme. J. Braz. Chem. Soc. 2011, 22, 1407–1417. [Google Scholar] [CrossRef]
- Shivakumar, H.N.; Desai, B.G.; Deshmukh, G. Design and optimization of diclofenac sodium controlled release solid dispersions by response surface methodology. Indian J. Pharm. Sci. 2008, 70, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Tunçay, M.; Çaliş, S.; Kaş, H.S.; Ercan, M.T.; Peksoy, İ.; Hincal, A.A. Diclofenac sodium incorporated PLGA (50:50) microspheres: Formulation considerations and in vitro/in vivo evaluation. Int. J. Pharm. 2000, 195, 179–188. [Google Scholar] [CrossRef]
- Morlock, M.; Kissel, T.; Li, Y.X.; Koll, H.; Winter, G. Erythropoietin loaded microspheres prepared from biodegradable LPLG–PEO–LPLG triblock copolymers: Protein stabilization and in vitro release properties. J. Control. Release 1998, 56, 105–115. [Google Scholar] [CrossRef]
- McCall, R.L.; Sirianni, R.W. PLGA nanoparticles formed by single- or double-emulsion with vitamin E-TPGS. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef] [PubMed]




| Sample | Size (nm) (Mean ± S.D) | Zeta Potential (mV) (Mean ± S.D) | Drug Contain (%) |
|---|---|---|---|
| DS-PLGA | 415 ± 6 | −14 ± 0.4 | 3.92 |
| CS-DS-PLGA | 420 ± 6 | 27 ± 0.6 | 3.13 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanal, S.; Adhikari, U.; Rijal, N.P.; Bhattarai, S.R.; Sankar, J.; Bhattarai, N. pH-Responsive PLGA Nanoparticle for Controlled Payload Delivery of Diclofenac Sodium. J. Funct. Biomater. 2016, 7, 21. https://doi.org/10.3390/jfb7030021
Khanal S, Adhikari U, Rijal NP, Bhattarai SR, Sankar J, Bhattarai N. pH-Responsive PLGA Nanoparticle for Controlled Payload Delivery of Diclofenac Sodium. Journal of Functional Biomaterials. 2016; 7(3):21. https://doi.org/10.3390/jfb7030021
Chicago/Turabian StyleKhanal, Shalil, Udhab Adhikari, Nava P. Rijal, Shanta R. Bhattarai, Jagannathan Sankar, and Narayan Bhattarai. 2016. "pH-Responsive PLGA Nanoparticle for Controlled Payload Delivery of Diclofenac Sodium" Journal of Functional Biomaterials 7, no. 3: 21. https://doi.org/10.3390/jfb7030021
APA StyleKhanal, S., Adhikari, U., Rijal, N. P., Bhattarai, S. R., Sankar, J., & Bhattarai, N. (2016). pH-Responsive PLGA Nanoparticle for Controlled Payload Delivery of Diclofenac Sodium. Journal of Functional Biomaterials, 7(3), 21. https://doi.org/10.3390/jfb7030021








