Blood-Vessel Mimicking Structures by Stereolithographic Fabrication of Small Porous Tubes Using Cytocompatible Polyacrylate Elastomers, Biofunctionalization and Endothelialization
Abstract
:1. Introduction
2. Results
2.1. Post-Curing Treatments of UV-Cured PA
2.2. Production of Tubular Structures by Stereolithography
2.3. PA Surface Functionalization with Thio-Modified Heparin and RGDC-Peptides
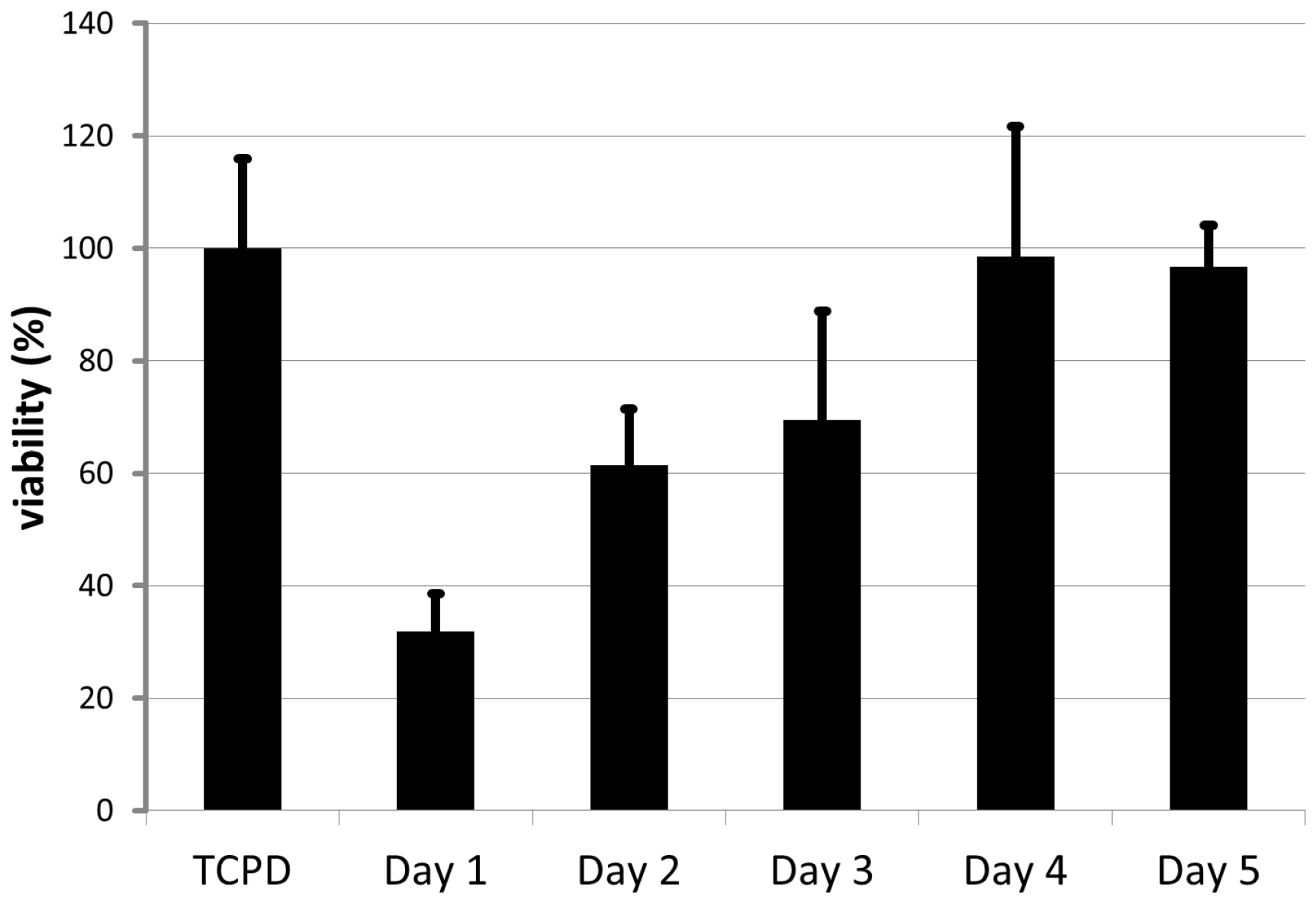
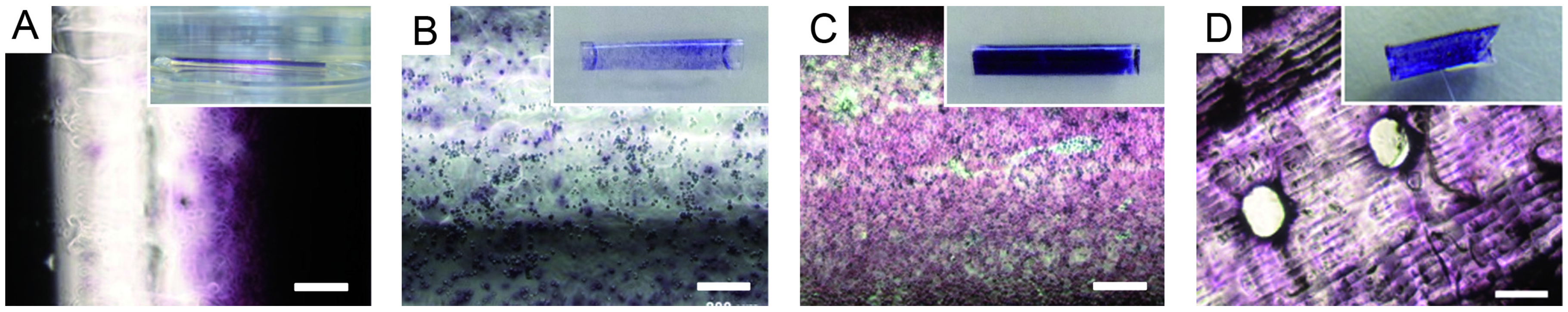
2.4. Endothelial Cell Culture on Planar and Tubular Substrates
2.5. Characterization of Hemolytic Potential and Platelet Adhesion
3. Discussion
3.1. Post-Curing Treatments of UV-Cured PA
3.2. Production of Tubular Structures by Stereolithography
3.3. PA Surface Functionalization with Thio-Modified Heparin and RGDC-Peptides
3.4. Endothelial Cell Culture on Planar and Tubular Substrates
3.5. Characterization of Hemolytic Potential and Platelet Adhesion
4. Experimental Section
4.1. Preparation of (co-BPA-co-IBA-co-IL)-Polyacrylate in Planar and Tubular Geometry
4.1.1. Planar Sample Preparation
4.1.2. Tubular Sample Preparation by Dip Coating
4.1.3. Tubular Sample Preparation by SLA
4.2. Sterilization
4.3. Characterization of Young’s Modulus and Tensile Strength of PA
4.4. Evaluation of the Cytocompatibility
4.5. Synthesis of 3,3-Dithiobis(Propanoic Dihydrazide) (DTPH)
4.6. Synthesis and Characterization of Thio-Modified Heparin
4.7. PA Surface Functionalization with Thio-Modified Heparin and RGDC-Peptides
4.8. Endothelial Cell Culture on Planar and Tubular Substrates
4.8.1. Cell Culture on Planar Substrates
4.8.2. Cell Culture on Tubular Substrates
4.9. Staining of Cells
4.10. Hemolysis and Platelet Adhesion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rose, A.S.; Webster, C.E.; Harrysson, O.L.A.; Formeister, E.J.; Rawal, R.B.; Iseli, C.E. Pre-operative simulation of pediatric mastoid surgery with 3D-printed temporal bone models. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Kurenov, S.N.; Ionita, C.; Sammons, D.; Demmy, T.L. Three-dimensional printing to facilitate anatomic study, device development, simulation, and planning in thoracic surgery. J. Thorac. Cardiovasc. Surg. 2015, 149, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Domingos, M.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef] [Green Version]
- Salacinski, H.J.; Goldner, S.; Giudiceandrea, A.; Hamilton, G.; Seifalian, A.M.; Edwards, A.; Carson, R.J. The mechanical behavior of vascular grafts: A review. J. Biomater. Appl. 2001, 15, 241–278. [Google Scholar] [CrossRef] [PubMed]
- Baiguera, S.; Ribatti, D. Endothelialization approaches for viable engineered tissues. Angiogenesis 2013, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, K.; Mei, J.; Li, C.; Zhang, J.; Zheng, W.; An, D.; Xiao, N.; Zhao, Q.; Kong, D.; et al. Fabrication of highly interconnected porous silk fibroin scaffolds for potential use as vascular grafts. Acta Biomater. 2014, 10, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- De Valence, S.; Tille, J.C.; Giliberto, J.P.; Mrowczynski, W.; Gurny, R.; Walpoth, B.H.; Möller, M. Advantages of bilayered vascular grafts for surgical applicability and tissue regeneration. Acta Biomater. 2012, 8, 3914–3920. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; von Recum, H.A. Electro spinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Y.; Ozbolat, I.T. Direct bioprinting of vessel-like tubular microfluidic channels. J. Nanotechnol. Eng. Med. 2013, 4, 020902–020910. [Google Scholar] [CrossRef]
- Luo, Y.; Lode, A.; Gelinsky, M. Direct plotting of three-dimensional hollow fiber scaffolds based on concentrated alginate pastes for tissue engineering. Adv. Healthc. Mater. 2013, 2, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Cyr, K.M.; Matsumoto, A.; Langer, R.; Borenstein, J.T.; Kaplan, D.L. Silk fibroin microfluidic devices. Adv. Mater. 2009, 19, 2847–2850. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Weinberg, E.J.; Kulig, K.M.; Vacanti, J.P.; Wang, Y.; Borenstein, J.T.; Langer, R. Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer. Adv. Mater. 2005, 18, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Fidkowski, C.; Kaazempur-Mofrad, M.R.; Borenstein, J.; Vacanti, J.P.; Langer, R.; Wang, Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005, 11, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, J.T.; Tupper, M.M.; Mack, P.J.; Weinberg, E.J.; Khalil, A.S.; Hsiao, J.; García-Cardeña, G. Functional endothelialized microvascular networks with circular cross-sections in a tissue culture substrate. Biomed. Microdevices 2010, 12, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Tovar, G.E.; Borchers, K. Bioprinting of artificial blood vessels: Current approaches towards a demanding goal. Eur. J. Cardiothorac Surg. 2014, 46, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Bellan, L.M.; Pearsall, M.; Cropek, D.M.; Langer, R. A 3D interconnected microchannel network formed in gelatin by sacrificial shellac microfibers. Adv. Mater. 2012, 24, 5187–5191. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, V.; Polio, S.; Keegan, P.; Lee, J.H.; Fischer, K.; Park, J.K.; Yoo, S.S. On-demand three-dimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnol. Bioeng. 2010, 105, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chai, W.; Huang, Y.; Markwald, R.R. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol. Bioeng. 2012, 109, 3152–3160. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Nishiyama, Y.; Henmi, C.; Iwanaga, S.; Nakagawa, H. Ink jet three-dimensional digital fabrication for biological tissue manufacturing: Analysis of alginate microgel beads produced by ink jet droplets for three dimensional tissue fabrication. J. Imaging Sci. Technol. 2008, 52, 1–16. [Google Scholar] [CrossRef]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.; Melchels, F.P.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2013, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiong, Z.; Wang, X.; Yan, Y.; Liu, H.; Zhang, J. Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J. Bioact. Compat. Polym. 2009, 24, 249–265. [Google Scholar]
- Meyer, W.; Engelhardt, S.; Novosel, E.; Elling, B.; Wegener, M.; Krüger, H. Soft polymers for building up small and smallest blood supplying systems by stereolithography. J. Funct. Biomater. 2012, 3, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Baudis, S.; Nehl, F.; Ligon, S.C.; Nigisch, A.; Bergmeister, H.; Bernhard, D.; Stampfl, J.; Liska, R. Elastomeric degradable biomaterials by photopolymerization-based cad-cam for vascular tissue engineering. Biomed. Mater. 2011, 6, 055003–055008. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Hoch, E.; Borchers, K.; Meyer, W.; Krüger, H.; Tovar, G.E.; Gillner, A. Fabrication of 2D protein microstructures and 3D polymer–protein hybrid microstructures by two-photon polymerization. Biofabrication 2011, 3, 025003. [Google Scholar] [CrossRef] [PubMed]
- Novosel, E.C.; Meyer, W.; Klechowitz, N.; Krüger, H.; Wegener, M.; Walles, H.; Tovar, G.E.; Hirth, T.; Kluger, P.J. Evaluation of cell-material interactions on newly designed, printable polymers for tissue engineering applications. Adv. Eng. Mater. 2011, 13, B467–B475. [Google Scholar] [CrossRef]
- ISO 10993-5:2009(en)–Biological Evaluation of Medical Devices; ISO: Geneva, Switzerland, 2009.
- Fisher, A.B.; Chien, S.; Barakat, A.I.; Nerem, R.M. Endothelial cellular response to altered shear stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L529–L533. [Google Scholar] [PubMed]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Nuttelman, C.R.; Anseth, K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Edit. 2000, 11, 439–457. [Google Scholar] [CrossRef]
- Schmidt, D.; Asmis, L.M.; Odermatt, B.; Kelm, J.; Breymann, C.; Gössi, M.; Genoni, M.; Zund, G.; Hoerstrup, S.P. Engineered living blood vessels: Functional endothelia generated from human umbilical cord-derived progenitors. Ann. Thorac. Surg. 2006, 82, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.; Colombus, S.; Krishnan, V.K.; Krishnan, L.K. Vascular tissue construction on poly(e-caprolactone) scaffolds by dynamic endothelial cell seeding: Effect of pore size. J. Tissue Eng. Regen. Med. 2012, 6, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Narayan, D.; Venkatraman, S.S. Effect of pore size and interpore distance on endothelial cell growth on polymers. J. Biomed. Mater. Res. A 2008, 87, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Von der Mark, K.; Park, J.; Bauer, S.; Schmuki, P. Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell Tissue Res. 2010, 339, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.P.; Moyano, J.V.; Collier, J.H. Multifactorial optimization of endothelial cell growth using modular synthetic extracellular matrices. Integr. Biol. (Camb) 2011, 3, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Madri, J.A.; Yurchenco, P.D. Endothelial-cells interact with the core protein of basement-membrane perlecan through beta-1 and beta-3 integrins-An adhesion modulated by glycosaminoglycan. J. Cell Biol. 1992, 119, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Bos, G.W.; Scharenborg, N.M.; Poot, A.A.; Engbers, G.H.; Terlingen, J.G.; Beugeling, T.; Van Aken, W.G.; Feijen, J. Adherence and proliferation of endothelial cells on surface-immobilized albumin-heparin conjugate. Tissue Eng. 1998, 4, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Salacinski, H.J.; Punshon, G.; Hamilton, G.; Seifalian, A.M. Development of a hybrid cardiovascular graft using a tissue engineering approach. FASEB J. 2002, 16, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.J.; Hou, Y.H.; Zhang, B.B.; Dong, Y.X.; Ding, H.Y. Blood compatibility and interaction with endothelial cells of titanium modified by sequential immobilization of poly (ethylene glycol) and heparin. J. Mater. Chem. B 2014, 2, 892–902. [Google Scholar] [CrossRef]
- Krijgsman, B.; Seifalian, A.M.; Salacinski, H.J.; Tai, N.R.; Punshon, G.; Fuller, B.J.; Hamilton, G. An assessment of covalent grafting of RGD peptides to the surface of a compliant poly(carbonate-urea)urethane vascular conduit versus conventional biological coatings: Its role in enhancing cellular retention. Tissue Eng. 2002, 8, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.B.; García-Echeverría, C.; Asakura, S.; Sun, W.; Mosher, D.F.; Cooper, S.L. Endothelial cell adhesion on polyurethanes containing covalently attached RGD-peptides. Biomaterials 1992, 13, 905–914. [Google Scholar] [CrossRef]
- Walluscheck, K.P.; Steinhoff, G.; Kelm, S.; Haverich, A. Improved endothelial cell attachment on ePTFE vascular grafts pretreated with synthetic RGD containing peptides. Eur. J. Vasc. Endovasc. Surg. 1996, 12, 321–330. [Google Scholar] [CrossRef]
- Hoesli, C.A.; Garnier, A.; Juneau, P.M.; Chevallier, P.; Duchesne, C.; Laroche, G. A fluorophore-tagged RGD peptide to control endothelial cell adhesion to micropatterned surfaces. Biomaterials 2014, 35, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, G.; Magenau, A.; Böcking, T.; Gaus, K.; Gooding, J.J. The relative importance of topography and rgd ligand density for endothelial cell adhesion. PLoS One 2011, 6, e21869. [Google Scholar] [CrossRef] [PubMed]
- Dudash, L.A.; Kligman, F.; Sarett, S.M.; Kottke-Marchant, K.; Marchant, R.E. Endothelial cell attachment and shear response on biomimetic polymer-coated vascular grafts. J. Biomed. Mater. Res. A 2012, 100, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Rémy, M.; Bareille, R.; Rerat, V.; Bourget, C.; Marchand-Brynaert, J.; Bordenave, L. Polyethylene terephthalate membrane grafted with peptidomimetics: Endothelial cell compatibility and retention under shear stress. J. Biomater. Sci. Polym. Edit. 2013, 24, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cooper, S. Adhesion of endothelial cells and endothelial progenitor cells on peptide-linked polymers in shear flow. Tissue Eng. Part A 2013, 19, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Inoue, O.; Suzuki-Inoue, K.; McCarty, O.J.; Moroi, M.; Ruggeri, Z.M.; Kunicki, T.J.; Ozaki, Y.; Watson, S.P. Laminin stimulates spreading of platelets through integrin alpha(6)beta(1)-dependent activation of GPVI. Blood 2006, 107, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Inoue, O.; Suzuki-Inoue, K.; Ozaki, Y. Redundant mechanism of platelet adhesion to laminin and collagen under flow-involvement of von willebrand factor and glycoprotein Ib-IX-V. J. Biol. Chem. 2008, 283, 16279–16282. [Google Scholar] [CrossRef] [PubMed]
- McCarty, O.J.; Zhao, Y.; Andrew, N.; Machesky, L.M.; Staunton, D.; Frampton, J.; Watson, S.P. Evaluation of the role of platelet integrins in fibronectin-dependent spreading and adhesion. J. Thromb. Haemost. 2004, 2, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cortés, J.; Mrksich, M. The platelet integrin αIIbβ3 binds to the RGD and AGD motifs in fibrinogen. Chem. Biol. 2009, 16, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, Z.; Song, L.; Zhao, Q.; Zhang, J.; Li, D.; Wang, S.; Han, J.; Zheng, X.L.; Yang, Z.; et al. Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials 2012, 33, 2880–2891. [Google Scholar] [CrossRef] [PubMed]
- Corum, L.E.; Hlady, V. Screening platelet-surface interactions using negative surface charge gradients. Biomaterials 2010, 31, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Smith, J.W. A redox site involved in integrin activation. J. Biol. Chem. 2000, 275, 39964–39972. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Aboulfatova, K.; Pownall, H.J.; Cook, R.; Dong, J.F. Shear-induced disulfide bond formation regulates adhesion activity of von willebrand factor. J. Biol. Chem. 2007, 282, 35604–35611. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Hu, Y.; Seiler, N.; Riester, D.; Meyer, W.; Krüger, H.; Wehner, M.; Bremus-Köbberling, E.; Gillner, A. 3D-microfabrication of polymer-protein hybrid structures with a q-switched microlaser. J. Laser Micro/Nanoeng. 2011, 6, 54–58. [Google Scholar] [CrossRef]
- Jacobs, P.F. Rapid Prototyping & Manufacturing: Fundamentals of Stereolithography; Society Manufacturing Engineering: Dearborn, MI, USA, 1992. [Google Scholar]
- Vercruysse, K.P.; Marecak, D.M.; Marecek, J.F.; Prestwich, G.D. Synthesis and in vitro degradation of new polyvalent hydrazide cross-linked hydrogels of hyaluronic acid. Bioconjug Chem. 1997, 8, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Liu, Y.; Luo, Y.; Roberts, M.C.; Prestwich, G.D. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules 2002, 3, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Kirihara, M.; Asai, Y.; Ogawa, S.; Noguchi, T.; Hatanov, A.; Hirai, Y. A mild and environmentally benign oxidation of thiols to disulfides. Synthesis 2004, 2007, 3286–3289. [Google Scholar] [CrossRef]
- Motlagh, D.; Allen, J.; Hoshi, R.; Yang, J.; Lui, K.; Ameer, G. Hemocompatibility evaluation of poly(diol citrate) in vitro for vascular tissue engineering. J. Biomed. Mater. Res. A 2007, 82, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.H.; Nollert, M.U. Platelet-derived NO slows thrombus growth on a collagen type III surface. Thromb. J. 2004, 2, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| PA Material | Young’s Modulus (MPa) | Tensile Strength (MPa) |
|---|---|---|
| UV (4 min) | 18.8 ± 1.7 | 2.9 ± 0.4 |
| UV (4min) + EtOH (70%) treatment and dried in vacuum | 18.3 ± 0.4 | 1.7 ± 0.1 |
| UV (4 min) + γ-sterilization | 21.3 ± 2.1 | 3.2 ± 0.5 |
| UV (4 min) + PBS buffer | 24.6 ± 2.3 | 3.5 ± 0.1 |
| Title | Untreated PA | RGDC | Thio-Modified Heparin | Thio-Modified Heparin/RGD |
|---|---|---|---|---|
| Mean average/at.% | ||||
| C1 (284, 6 eV) | 49.4 | 50.3 | 46.6 | 45.0 |
| S1 (168.2 eV to 168.4 eV) | 0.0 | 0.0 | 0.2 | 0.2 |
| S2 (163.4 eV) | 0.0 | 0.0 | 0.1 | 0.2 |
| N total | 0.0 | 0.0 | 0.6 | 1.1 |
| Standard deviation/at.% | ||||
| C1 (284, 6 eV) | 2.5 | 0.1 | 0.1 | 0.5 |
| S1 (168.2 eV to 168.4 eV) | 0.0 | 0.0 | 0.0 | 0.0 |
| S2 (163.4 eV) | 0.0 | 0.0 | 0.0 | 0.1 |
| N total | 0.0 | 0.0 | 0.1 | 0.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huber, B.; Engelhardt, S.; Meyer, W.; Krüger, H.; Wenz, A.; Schönhaar, V.; Tovar, G.E.M.; Kluger, P.J.; Borchers, K. Blood-Vessel Mimicking Structures by Stereolithographic Fabrication of Small Porous Tubes Using Cytocompatible Polyacrylate Elastomers, Biofunctionalization and Endothelialization. J. Funct. Biomater. 2016, 7, 11. https://doi.org/10.3390/jfb7020011
Huber B, Engelhardt S, Meyer W, Krüger H, Wenz A, Schönhaar V, Tovar GEM, Kluger PJ, Borchers K. Blood-Vessel Mimicking Structures by Stereolithographic Fabrication of Small Porous Tubes Using Cytocompatible Polyacrylate Elastomers, Biofunctionalization and Endothelialization. Journal of Functional Biomaterials. 2016; 7(2):11. https://doi.org/10.3390/jfb7020011
Chicago/Turabian StyleHuber, Birgit, Sascha Engelhardt, Wolfdietrich Meyer, Hartmut Krüger, Annika Wenz, Veronika Schönhaar, Günter E. M. Tovar, Petra J. Kluger, and Kirsten Borchers. 2016. "Blood-Vessel Mimicking Structures by Stereolithographic Fabrication of Small Porous Tubes Using Cytocompatible Polyacrylate Elastomers, Biofunctionalization and Endothelialization" Journal of Functional Biomaterials 7, no. 2: 11. https://doi.org/10.3390/jfb7020011







