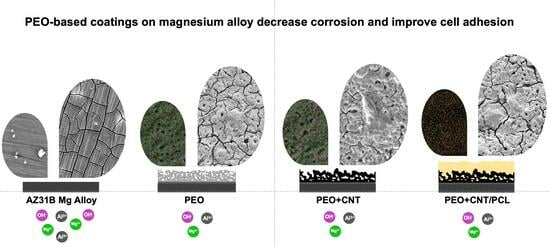
Biological Performance of Duplex PEO + CNT/PCL Coating on AZ31B Mg Alloy for Orthopedic and Dental Applications
Abstract
:1. Introduction
2. Materials and Methods
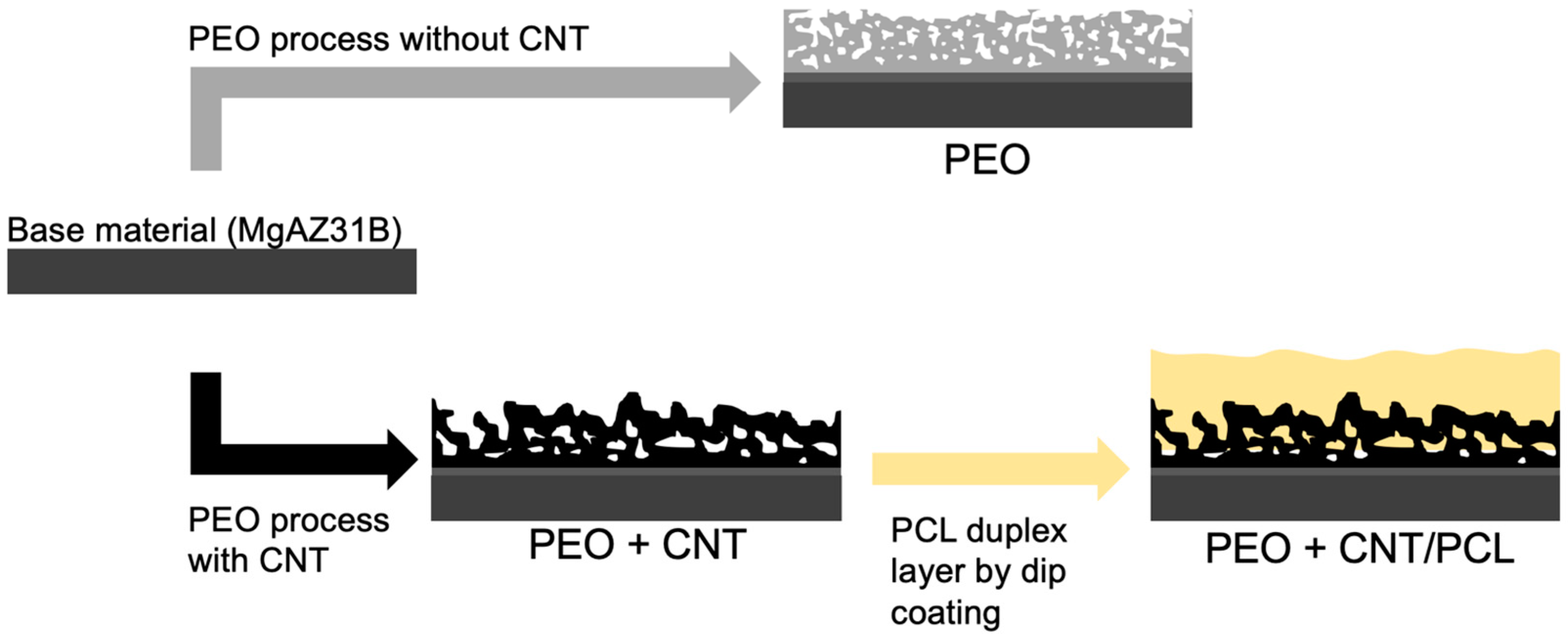
2.1. Sample Preparation
2.2. Plasma Electrolytic Oxidation (PEO) Process and PCL Deposition
2.3. Characterization of Base Material and Coated Surfaces
2.4. Biological Assays: Effects on Cell Metabolism and Cell Adhesion
2.5. Statistical Analysis
3. Results and Discussion
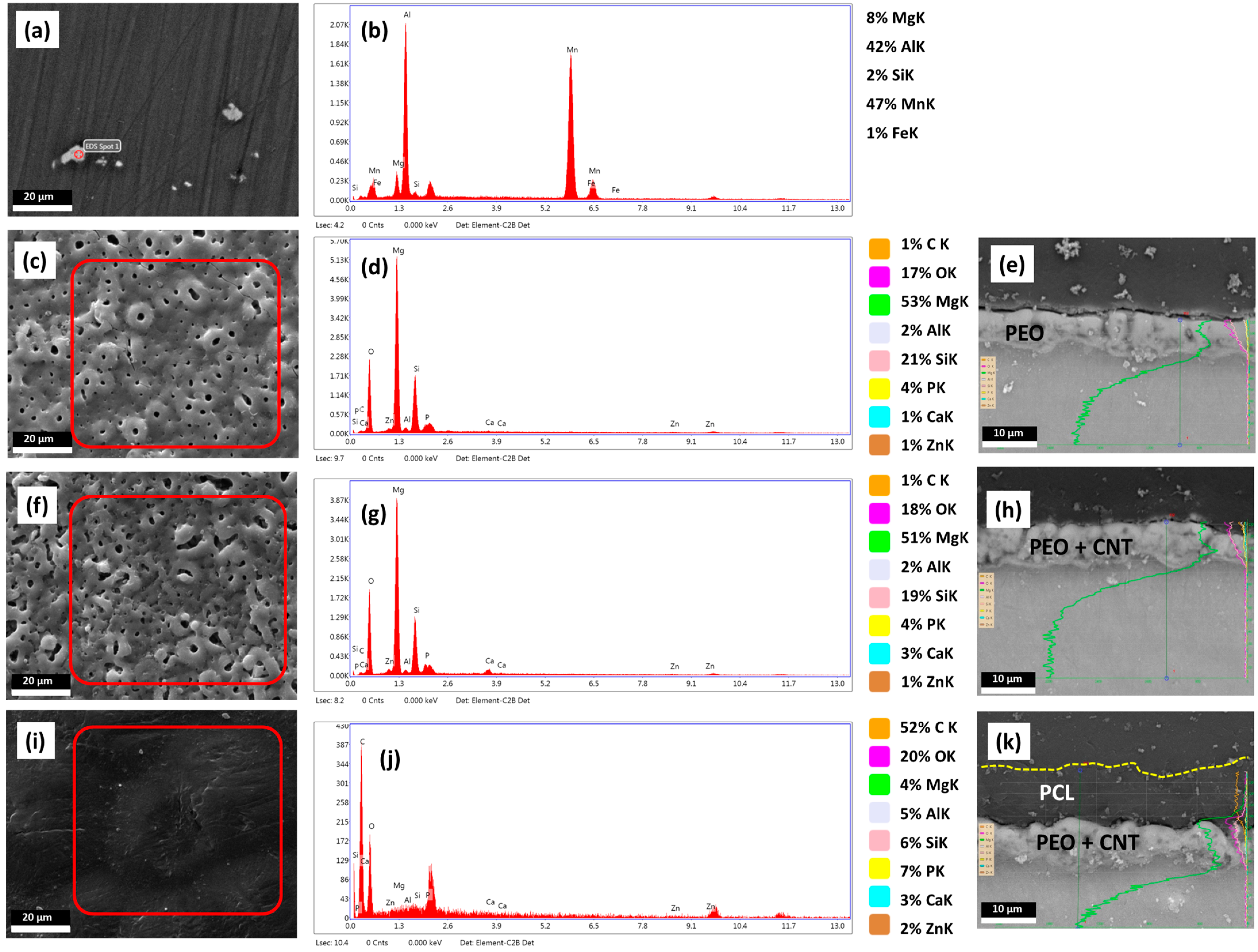
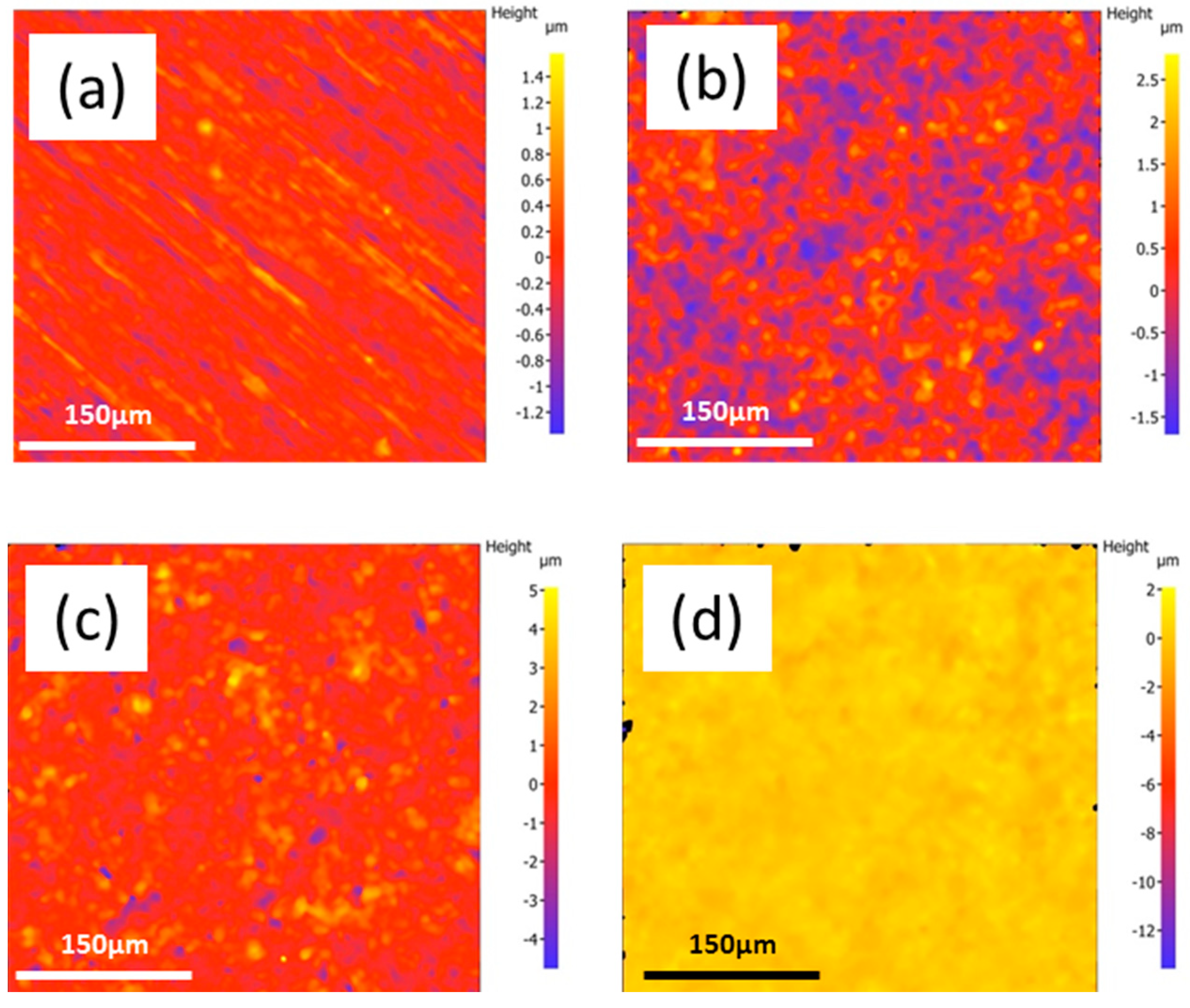
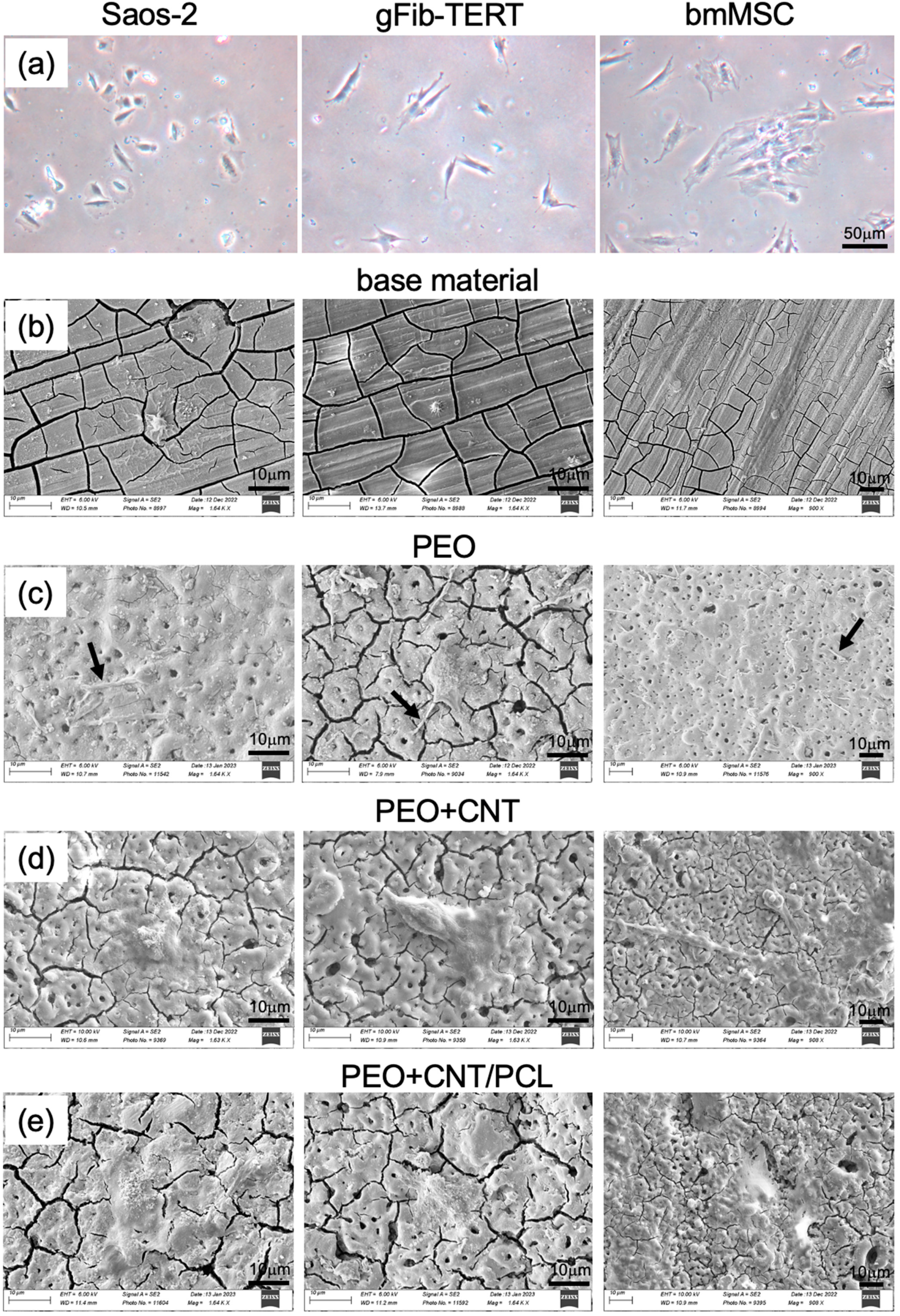
3.1. Characterisation of the Mg Alloy Surfaces
3.2. Wettability
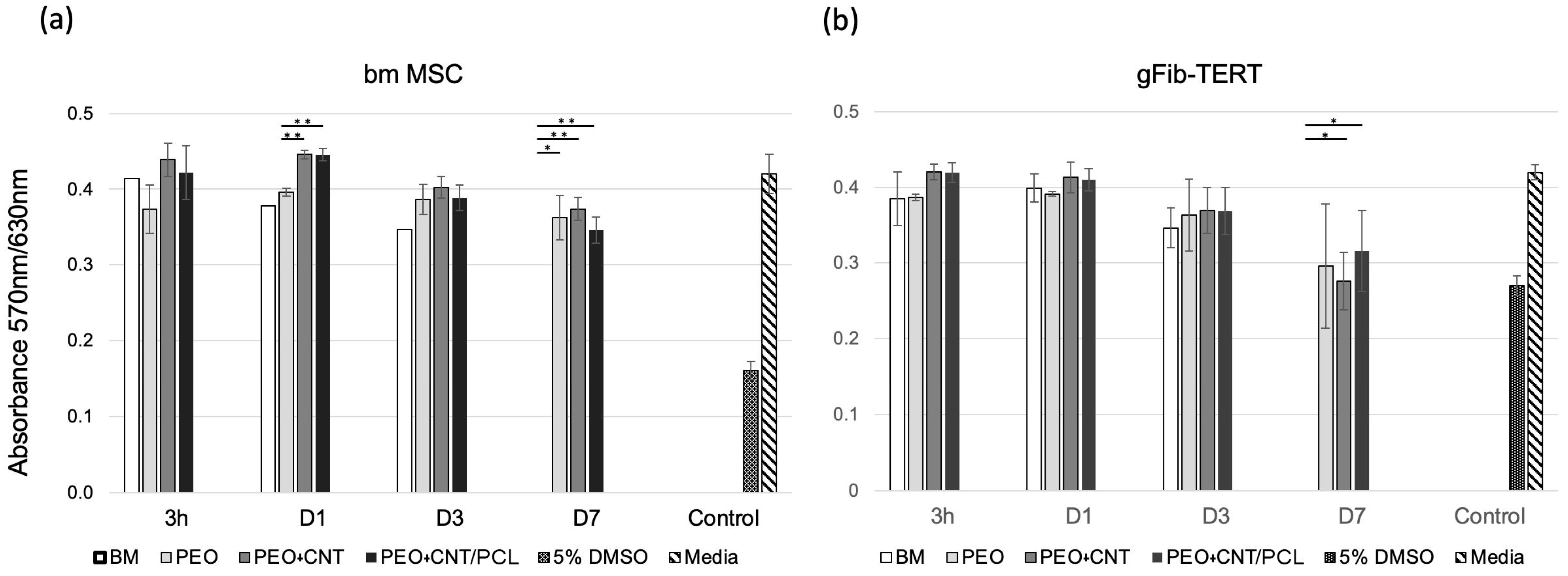
3.3. Cell Metabolism and Cell Adhesion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of New Metallic Alloys for Biomedical Applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Sumner, D.R. Long-Term Implant Fixation and Stress-Shielding in Total Hip Replacement. J. Biomech. 2015, 48, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Shi, Q.; Wang, J.; Chen, X.; Hao, Y.; Zhang, Y.; Wang, X. The Unfavorable Role of Titanium Particles Released from Dental Implants. Nanotheranostics 2021, 5, 321. [Google Scholar] [CrossRef] [PubMed]
- Kamrani, S.; Fleck, C. Biodegradable Magnesium Alloys as Temporary Orthopaedic Implants: A Review. Biometals 2019, 32, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Hopkins, C.; Chow, D.H.; Qin, L. Biodegradable Magnesium-based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current Status on Clinical Applications of Magnesium-Based Orthopaedic Implants: A Review from Clinical Translational Perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Lee, J.-W.; Han, H.-S.; Han, K.-J.; Park, J.; Jeon, H.; Ok, M.-R.; Seok, H.-K.; Ahn, J.-P.; Lee, K.E.; Lee, D.-H. Long-Term Clinical Study and Multiscale Analysis of in Vivo Biodegradation Mechanism of Mg Alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, H.; Sun, J. Long-Term in Vivo Evolution of High-Purity Mg Screw Degradation—Local and Systemic Effects of Mg Degradation Products. Acta Biomater. 2018, 71, 215–224. [Google Scholar] [CrossRef]
- Amukarimi, S.; Mozafari, M. Biodegradable Magnesium-based Biomaterials: An Overview of Challenges and Opportunities. MedComm 2021, 2, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Liu, H.; Feng, N.; Bai, J.; Cheng, H.; Liu, J.; Huang, F. Preparation of a Single-Phase Mg–6Zn Alloy via ECAP-Stimulated Solution Treatment. J. Magnes. Alloy. 2019, 7, 305–314. [Google Scholar] [CrossRef]
- Yin, Z.-Z.; Qi, W.-C.; Zeng, R.-C.; Chen, X.-B.; Gu, C.-D.; Guan, S.-K.; Zheng, Y.-F. Advances in Coatings on Biodegradable Magnesium Alloys. J. Magnes. Alloy. 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Ali, M.; Elsherif, M.; Salih, A.E.; Ul-Hamid, A.; Hussein, M.A.; Park, S.; Yetisen, A.K.; Butt, H. Surface Modification and Cytotoxicity of Mg-Based Bio-Alloys: An Overview of Recent Advances. J. Alloys Compd. 2020, 825, 154140. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, Y.-L.; Tian, Y.-Q.; Chen, L.-S. Research Progress on Surface Protective Coatings of Biomedical Degradable Magnesium Alloys. J. Alloys Compd. 2021, 885, 161001. [Google Scholar] [CrossRef]
- Peng, F.; Li, H.; Wang, D.; Tian, P.; Tian, Y.; Yuan, G.; Xu, D.; Liu, X. Enhanced Corrosion Resistance and Biocompatibility of Magnesium Alloy by Mg–Al-Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2016, 8, 35033–35044. [Google Scholar] [CrossRef]
- Zhengjie, L.; Ying, Z.; Zhixiong, Z.; Yanfei, X.; Kelvin, Y. Antibacterial Properties, Hemolysis and Biocompatibility of Biodegradable Medical Magnesium Alloys. Rare Met. Mater. Eng. 2018, 47, 403–408. [Google Scholar]
- Tokunaga, T.; Ohno, M.; Matsuura, K. Coatings on Mg Alloys and Their Mechanical Properties: A Review. J. Mater. Sci. Technol. 2018, 34, 1119–1126. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable Magnesium Alloys for Orthopaedic Applications: A Review on Corrosion, Biocompatibility and Surface Modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef]
- Ghanbari, A.; Bordbar-Khiabani, A.; Warchomicka, F.; Sommitsch, C.; Yarmand, B.; Zamanian, A. PEO/Polymer Hybrid Coatings on Magnesium Alloy to Improve Biodegradation and Biocompatibility Properties. Surf. Interfaces 2023, 36, 102495. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Yu, X.; Sun, X.; Yang, H. Functionalization Treatment of Micro-Arc Oxidation Coatings on Magnesium Alloys: A Review. J. Alloys Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- Esmaeili, M.; Tadayonsaidi, M.; Ghorbanian, B. The Effect of PEO Parameters on the Properties of Biodegradable Mg Alloys: A Review. Surf. Innov. 2021, 9, 184–198. [Google Scholar] [CrossRef]
- Yao, Z.; Gao, H.; Jiang, Z.; Wang, F. Structure and Properties of ZrO2 Ceramic Coatings on AZ91D Mg Alloy by Plasma Electrolytic Oxidation. J. Am. Ceram. Soc. 2008, 91, 555–558. [Google Scholar] [CrossRef]
- Kyrylenko, S.; Warchoł, F.; Oleshko, O.; Husak, Y.; Kazek-Kęsik, A.; Korniienko, V.; Deineka, V.; Sowa, M.; Maciej, A.; Michalska, J. Effects of the Sources of Calcium and Phosphorus on the Structural and Functional Properties of Ceramic Coatings on Titanium Dental Implants Produced by Plasma Electrolytic Oxidation. Mater. Sci. Eng. C 2021, 119, 111607. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Blawert, C.; Zheludkevich, M.L. The Corrosion Performance and Mechanical Properties of Mg-Zn Based Alloys—A Review. Corros. Mater. Degrad. 2020, 1, 7. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- O’Hara, M.; Troughton, S.C.; Francis, R.; Clyne, T.W. The Incorporation of Particles Suspended in the Electrolyte into Plasma Electrolytic Oxidation Coatings on Ti and Al Substrates. Surf. Coatings Technol. 2020, 385, 125354. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma Electrolytic Oxidation Coatings with Particle Additions–A Review. Surf. Coatings Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Keyvani, A.; Zamani, M.; Bahamirian, M.; Nikoomanzari, E.; Fattah-Alhosseini, A.; Sina, H. Role of Incorporation of ZnO Nanoparticles on Corrosion Behavior of Ceramic Coatings Developed on AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation Technique. Surfaces and Interfaces 2021, 22, 100728. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Xu, T.; Li, H.; Jiang, B.; Miao, X. Preparation and Corrosion Resistance of a Self-Sealing Hydroxyapatite-MgO Coating on Magnesium Alloy by Microarc Oxidation. Ceram. Int. 2022, 48, 13676–13683. [Google Scholar] [CrossRef]
- Han, Q.; Li, Y.; Lu, X.; Mei, D.; Chen, Q.; Zhang, T.; Wang, F. Fabrication of Ag Containing Antibacterial PEO Coatings on Pure Mg. Mater. Lett. 2021, 293, 129731. [Google Scholar] [CrossRef]
- Xiao, Y.; Gong, T.; Zhou, S. The Functionalization of Multi-Walled Carbon Nanotubes by in Situ Deposition of Hydroxyapatite. Biomaterials 2010, 31, 5182–5190. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Matranga, C.; Tan, S.; Alba, N.; Cui, X.T. Carbon Nanotube Nanoreservior for Controlled Release of Anti-Inflammatory Dexamethasone. Biomaterials 2011, 32, 6316–6323. [Google Scholar] [CrossRef] [PubMed]
- Daavari, M.; Atapour, M.; Mohedano, M.; Arrabal, R.; Matykina, E.; Taherizadeh, A. Biotribology and Biocorrosion of MWCNTs-Reinforced PEO Coating on AZ31B Mg Alloy. Surf. Interfaces 2021, 22, 100850. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Ustinov, A.Y.; Sukhoverkhov, S.V.; Gnedenkov, S.V. New Polycaprolactone-Containing Self-Healing Coating Design for Enhance Corrosion Resistance of the Magnesium and Its Alloys. Polymers 2022, 15, 202. [Google Scholar] [CrossRef]
- Moreno, L.; Wang, C.; Lamaka, S.V.; Zheludkevich, M.L.; Rodríguez-Hernández, J.; Arrabal, R.; Matykina, E. Ciprofloxacin Release and Corrosion Behaviour of a Hybrid PEO/PCL Coating on Mg3Zn0.4Ca Alloy. J. Funct. Biomater. 2023, 14, 65. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Ustinov, A.Y.; Gnedenkov, S. V Hybrid Coatings for Active Protection against Corrosion of Mg and Its Alloys. Polymers 2023, 15, 3035. [Google Scholar] [CrossRef]
- Rai, R.; Tallawi, M.; Grigore, A.; Boccaccini, A.R. Synthesis, Properties and Biomedical Applications of Poly (Glycerol Sebacate) (PGS): A Review. Prog. Polym. Sci. 2012, 37, 1051–1078. [Google Scholar] [CrossRef]
- Kim, M.J.; Hwang, M.Y.; Kim, J.; Chung, D.J. Biodegradable and Elastomeric Poly (Glycerol Sebacate) as a Coating Material for Nitinol Bare Stent. Biomed Res. Int. 2014, 2014, 956952. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Gonzalez, Z.; Utomo, E.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Poly (Caprolactone)-Based Coatings on 3D-Printed Biodegradable Implants: A Novel Strategy to Prolong Delivery of Hydrophilic Drugs. Mol. Pharm. 2020, 17, 3487–3500. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Zhang, W.; Ullah, I.; Shi, L.; Zhang, Y.; Ou, H.; Zhou, J.; Ullah, M.W.; Zhang, X.; Li, W. Fabrication and Characterization of Porous Polycaprolactone Scaffold via Extrusion-Based Cryogenic 3D Printing for Tissue Engineering. Mater. Des. 2019, 180, 107946. [Google Scholar] [CrossRef]
- Liu, D.; Nie, W.; Li, D.; Wang, W.; Zheng, L.; Zhang, J.; Zhang, J.; Peng, C.; Mo, X.; He, C. 3D Printed PCL/SrHA Scaffold for Enhanced Bone Regeneration. Chem. Eng. J. 2019, 362, 269–279. [Google Scholar] [CrossRef]
- Murugan, S.; Parcha, S.R. Fabrication Techniques Involved in Developing the Composite Scaffolds PCL/HA Nanoparticles for Bone Tissue Engineering Applications. J. Mater. Sci. Mater. Med. 2021, 32, 93. [Google Scholar] [CrossRef]
- Daavari, M.; Atapour, M.; Mohedano, M.; Sánchez, H.M.; Rodríguez-Hernández, J.; Matykina, E.; Arrabal, R.; Taherizadeh, A. Quasi-in Vivo Corrosion Behavior of AZ31B Mg Alloy with Hybrid MWCNTs-PEO/PCL Based Coatings. J. Magnes. Alloy. 2022, 10, 3217–3233. [Google Scholar] [CrossRef]
- Chaudhary, S.; Ghosal, D.; Tripathi, P.; Kumar, S. Cellular Metabolism: A Link Connecting with Physiochemical Properties of Biomaterials for Bone Tissue Engineering. Biomater. Sci. 2023, 11, 2277–2291. [Google Scholar] [CrossRef]
- Jurak, M.; Wiącek, A.E.; Ładniak, A.; Przykaza, K.; Szafran, K. What Affects the Biocompatibility of Polymers? Adv. Colloid Interface Sci. 2021, 294, 102451. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Yin, Z.-Z.; Chen, X.-B.; Xu, D.-K. Corrosion Types of Magnesium Alloys. In Magnesium Alloys: Selected Issue; Books on Demand GmbH: Norderstedt, Germany, 2018; pp. 29–53. [Google Scholar]
- Kim, Y.-K.; Lee, K.-B.; Kim, S.-Y.; Jang, Y.-S.; Kim, J.H.; Lee, M.-H. Improvement of Osteogenesis by a Uniform PCL Coating on a Magnesium Screw for Biodegradable Applications. Sci. Rep. 2018, 8, 13264. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Z.; Xing, Y.; Jia, S.; Mo, Z.; Gong, H. Effect of Microtopography on Osseointegration of Implantable Biomaterials and Its Modification Strategies. Front. Bioeng. Biotechnol. 2022, 10, 981062. [Google Scholar] [CrossRef]
- Saji, V.S. Carbon Nanostructure-Based Superhydrophobic Surfaces and Coatings. Nanotechnol. Rev. 2021, 10, 518–571. [Google Scholar] [CrossRef]
- Li, J.; Mou, X.; Qiu, J.; Wang, S.; Wang, D.; Sun, D.; Guo, W.; Li, D.; Kumar, A.; Yang, X. Surface Charge Regulation of Osteogenic Differentiation of Mesenchymal Stem Cell on Polarized Ferroelectric Crystal Substrate. Adv. Healthc. Mater. 2015, 4, 998–1003. [Google Scholar] [CrossRef]
- Kaivosoja, E.; Barreto, G.; Levon, K.; Virtanen, S.; Ainola, M.; Konttinen, Y.T. Chemical and Physical Properties of Regenerative Medicine Materials Controlling Stem Cell Fate. Ann. Med. 2012, 44, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.; Alves, N.M.; Mano, J.F. Cell Interactions with Superhydrophilic and Superhydrophobic Surfaces. J. Adhes. Sci. Technol. 2014, 28, 843–863. [Google Scholar] [CrossRef]
- Sergeeva, Y.N.; Huang, T.; Felix, O.; Jung, L.; Tropel, P.; Viville, S.; Decher, G. What Is Really Driving Cell–Surface Interactions? Layer-by-Layer Assembled Films May Help to Answer Questions Concerning Cell Attachment and Response to Biomaterials. Biointerphases 2016, 11, 019009. [Google Scholar] [CrossRef] [PubMed]
- Husak, Y.; Michalska, J.; Oleshko, O.; Korniienko, V.; Grundsteins, K.; Dryhval, B.; Altundal, S.; Mishchenko, O.; Viter, R.; Pogorielov, M. Bioactivity Performance of Pure Mg after Plasma Electrolytic Oxidation in Silicate-Based Solutions. Molecules 2021, 26, 2094. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, Y.; Peng, X.; Mao, L.; Yuan, G.; Jiang, Y.; He, Y. Effects and Degradable Mg-Nd-Zn-Zr Alloy on Osteoblastic Cell Function. Int. J. Immunopathol. Pharmacol. 2012, 25, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Farshid, S.; Kharaziha, M.; Atapour, M. A Self-Healing and Bioactive Coating Based on Duplex Plasma Electrolytic Oxidation/Polydopamine on AZ91 Alloy for Bone Implants. J. Magnes. Alloy. 2023, 11, 592–606. [Google Scholar] [CrossRef]
- Tian, P.; Peng, F.; Wang, D.; Liu, X. Corrosion Behavior and Cytocompatibility of Fluoride-Incorporated Plasma Electrolytic Oxidation Coating on Biodegradable AZ31 Alloy. Regen. Biomater. 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Ahmad Khalili, A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Ghibaudo, M.; Trichet, L.; Le Digabel, J.; Richert, A.; Hersen, P.; Ladoux, B. Substrate Topography Induces a Crossover from 2D to 3D Behavior in Fibroblast Migration. Biophys. J. 2009, 97, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Ranella, A.; Barberoglou, M.; Bakogianni, S.; Fotakis, C.; Stratakis, E. Tuning Cell Adhesion by Controlling the Roughness and Wettability of 3D Micro/Nano Silicon Structures. Acta Biomater. 2010, 6, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, M.I.; Best, S.M. Effect of Ceramic Scaffold Architectural Parameters on Biological Response. Front. Bioeng. Biotechnol. 2015, 3, 151. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Yokoyama, A.; Matsuoka, M.; Hashimoto, T.; Abe, S.; Uo, M.; Watari, F. Adhesion of Human Osteoblast-like Cells (Saos-2) to Carbon Nanotube Sheets. Biomed. Mater. Eng. 2009, 19, 147–153. [Google Scholar] [CrossRef]
- Imaninezhad, M.; Schober, J.; Griggs, D.; Ruminski, P.; Kuljanishvili, I.; Zustiak, S.P. Cell Attachment and Spreading on Carbon Nanotubes Is Facilitated by Integrin Binding. Front. Bioeng. Biotechnol. 2018, 6, 129. [Google Scholar] [CrossRef]
- Xu, J.; Hu, X.; Jiang, S.; Wang, Y.; Parungao, R.; Zheng, S.; Nie, Y.; Liu, T.; Song, K. The Application of Multi-Walled Carbon Nanotubes in Bone Tissue Repair Hybrid Scaffolds and the Effect on Cell Growth in Vitro. Polymers 2019, 11, 230. [Google Scholar] [CrossRef]
- Ni, S.; Chou, L.; Chang, J. Preparation and Characterization of Forsterite (Mg2SiO4) Bioceramics. Ceram. Int. 2007, 33, 83–88. [Google Scholar] [CrossRef]
- Zomorodian, A.; Garcia, M.P.; Moura e Silva, T.; Fernandes, J.C.S.; Fernandes, M.H.; Montemor, M.d.F. Biofunctional Composite Coating Architectures Based on Polycaprolactone and Nanohydroxyapatite for Controlled Corrosion Activity and Enhanced Biocompatibility of Magnesium AZ31 Alloy. Mater. Sci. Eng. C 2015, 48, 434–443. [Google Scholar] [CrossRef]
- Paul, S.; Hanisch, O.; Nesic, D. Human Gingival Fibroblast Proliferation on Materials Used for Dental Implant Abutments: A Systematic Review. Int. J. Prosthodont. 2021, 34, 811–828. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral Implant Surfaces: Part 1--Review Focusing on Topographic and Chemical Properties of Different Surfaces and in Vivo Responses to Them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Kim, Y.-S.; Shin, S.-Y.; Moon, S.-K.; Yang, S.-M. Surface Properties Correlated with the Human Gingival Fibroblasts Attachment on Various Materials for Implant Abutments: A Multiple Regression Analysis. Acta Odontol. Scand. 2015, 73, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Odén, A.; Wennerberg, A.; Hultenby, K.; Arvidson, K. The Influence of Surface Topography of Ceramic Abutments on the Attachment and Proliferation of Human Oral Fibroblasts. Biomaterials 2005, 26, 373–381. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daavari, M.; Atapour, M.; Mohedano, M.; Matykina, E.; Arrabal, R.; Nesic, D. Biological Performance of Duplex PEO + CNT/PCL Coating on AZ31B Mg Alloy for Orthopedic and Dental Applications. J. Funct. Biomater. 2023, 14, 475. https://doi.org/10.3390/jfb14090475
Daavari M, Atapour M, Mohedano M, Matykina E, Arrabal R, Nesic D. Biological Performance of Duplex PEO + CNT/PCL Coating on AZ31B Mg Alloy for Orthopedic and Dental Applications. Journal of Functional Biomaterials. 2023; 14(9):475. https://doi.org/10.3390/jfb14090475
Chicago/Turabian StyleDaavari, Morteza, Masoud Atapour, Marta Mohedano, Endzhe Matykina, Raul Arrabal, and Dobrila Nesic. 2023. "Biological Performance of Duplex PEO + CNT/PCL Coating on AZ31B Mg Alloy for Orthopedic and Dental Applications" Journal of Functional Biomaterials 14, no. 9: 475. https://doi.org/10.3390/jfb14090475