Influence of Implant Macro-Design, -Length, and -Diameter on Primary Implant Stability Depending on Different Bone Qualities Using Standard Drilling Protocols—An In Vitro Analysis
Abstract
:1. Introduction
2. Materials and Methods
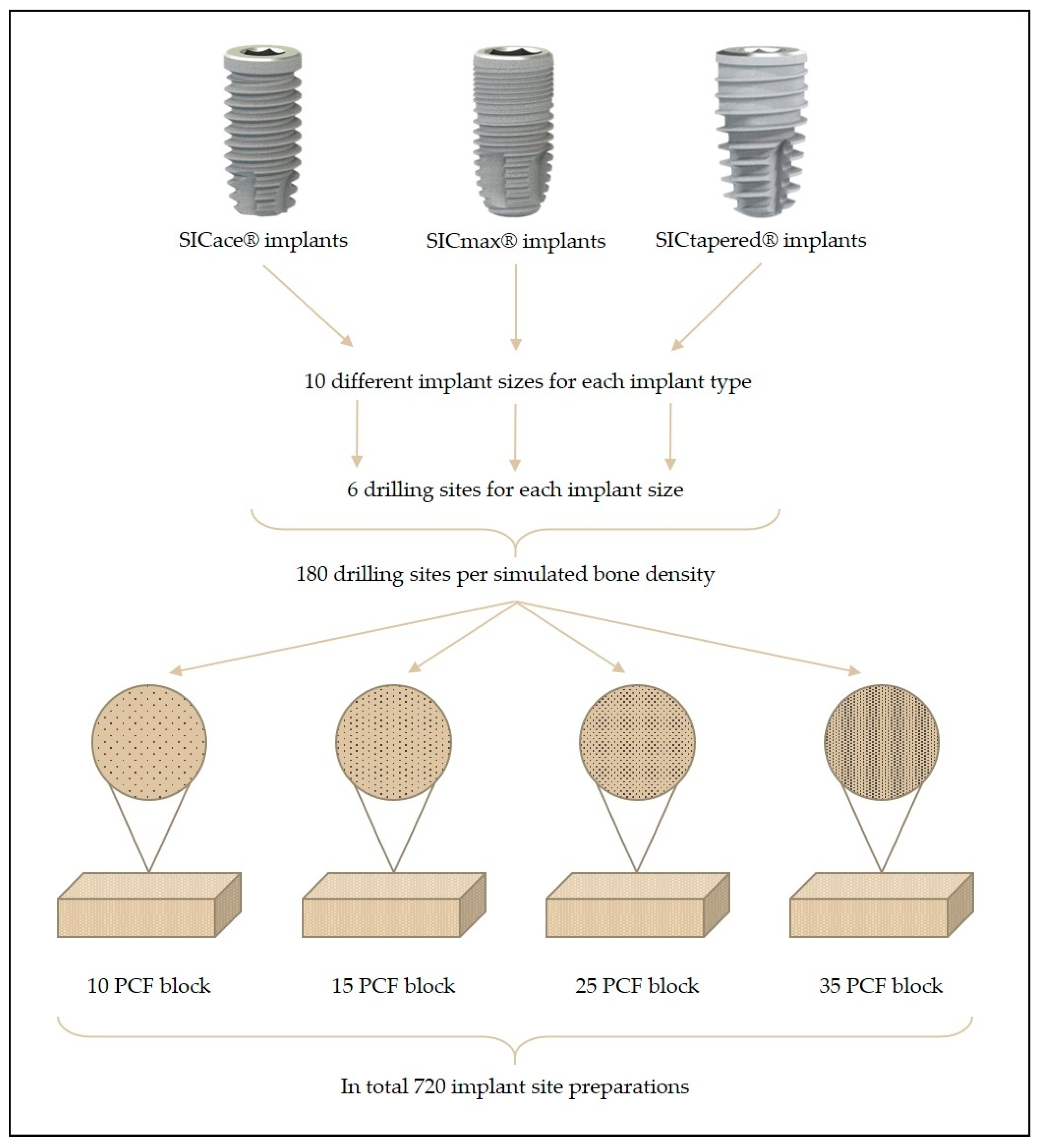
2.1. Implant Characteristics
- -
- Group I: Cylindrical implant (SICace®)
- -
- Group II: Cylindrical implant (SICmax®)
- -
- Group III: Tapered implant (SICtapered®)
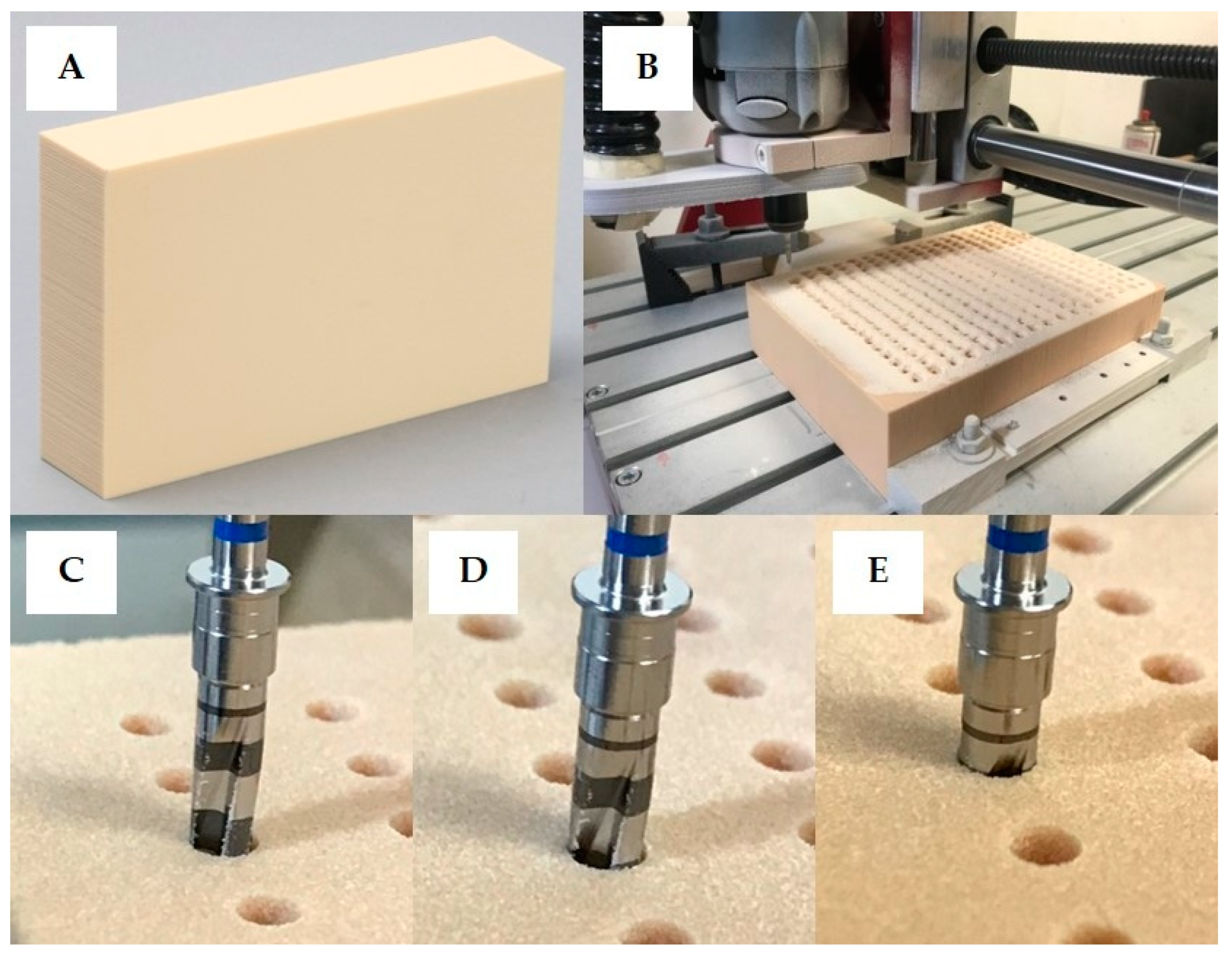
2.2. Implant Site Preparation
2.3. Implant Insertion and Measurement of Implant Stability
2.4. Statistical Analysis
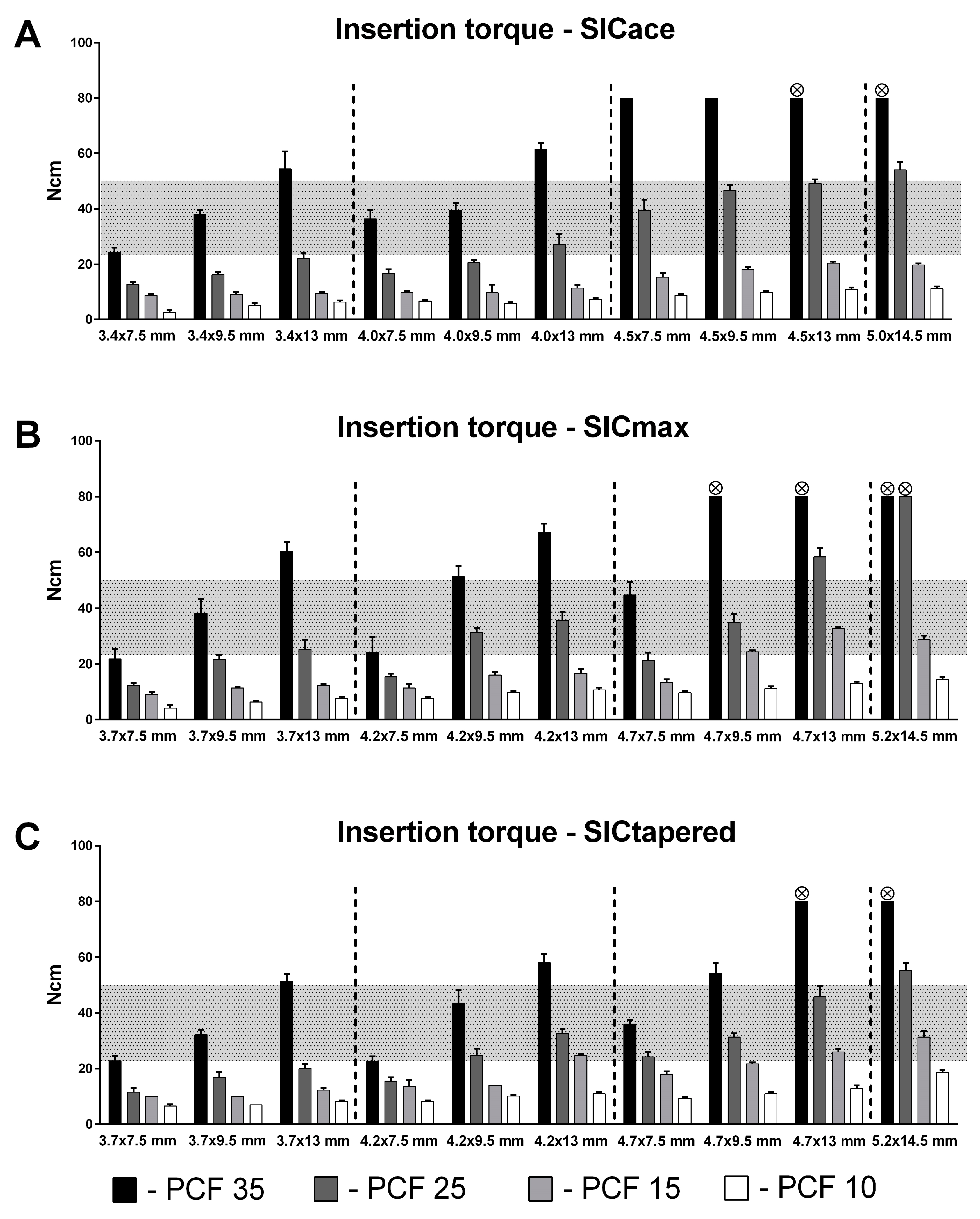
3. Results
4. Discussion
5. Conclusions
- Tapered implants are particularly suitable for achieving higher torques in soft bone compared to standard parallel-walled implants. Therefore, tapered implants seem to be particularly suitable for immediate implantation and immediate loading.
- Larger implant sizes lead to higher insertion torques in soft bone and thus to higher primary stability. The increase in diameter seems to play a greater role than the implant length, so that wider implants are preferable in cases of soft bone and immediate loading.
- The development of the insertion torque must also be observed in the hard bone in order to avoid pressure overloads and necrosis in the peri-implant bone. Even with smaller implant sizes and the standard drilling protocol, critical values (e.g., >50 Ncm) can be exceeded.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leonhardt, A.; Gröndahl, K.; Bergström, C.; Lekholm, U. Long-term follow-up of osseointegrated titanium implants using clinical, radiographic and microbiological parameters. Clin. Oral Implant. Res. 2002, 2, 127–132. [Google Scholar]
- Chug, A.; Shukla, S.; Mahesh, L. Osseointegration—Molecular eventsat the bone–implant interface: A review. J. Oral Maxillofac. Surg. Med. Pathol. 2013, 25, 1–4. [Google Scholar]
- Gallucci, G.O.; Benic, G.I.; Eckert, S.E. Consensus statements and clinical recommendations for implant loading protocols. Int. J. Oral Maxillofac. Implant. 2014, 29, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Puleo, D.; Nanci, A. Understanding and controlling the bone–implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar]
- Atieh, M.A.; Alsabeeha, N.; Duncan, W.J. Stability of Tapered and Parallel-Walled Dental Implants: A Systematic Review and Meta-Analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 634–645. [Google Scholar]
- Herrero-Climent, M.; Lemos, B.F.; Herrero-Climent, F.; Falcao, C.; Oliveira, H.; Herrera, M.; Gil, F.J.; Ros-Carrasco, B.; Ros-Satos, J.V. Influence of Implant Design and Under-Preparation of the Implant Site on Implant Primary Stability. An In Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 4436. [Google Scholar] [CrossRef]
- Waechter, J.; Leite, F.R.; Nascimento, G.G.; Filho, L.C.C.; Faot, F. The Split Crest Technique and Dental Implants: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Möhlhenrich, S.C.; Kniha, K.; Heussen, N.; Hölzle, F.; Modabber, A. Effects on Primary Stability of Three Different Techniques for Implant Site Preparation in Synthetic Bone Models of Different Densities. Br. J. Oral Maxillofac. Surg. 2016, 54, 980–986. [Google Scholar] [CrossRef]
- Pera, P.; Menini, M.; Pesce, P.; Bevilacqua, M.; Pera, F.; Tealdo, T. Immediate Versus Delayed Loading of Dental Implants Supporting Fixed Full-Arch Maxillary Prostheses: A 10-year Follow-up Report. Int. J. Prosthodont. 2019, 1, 27–31. [Google Scholar]
- Caramês, J.M.M.; Marques, D.N.D.S.; Caramês, G.B.; Francisco, H.C.O.; Vieira, F.A. Implant survival in immediately loaded full-arch rehabilitations following an anatomical classification system—A retrospective study in 1200 edentulous jaws. J. Clin. Med. 2021, 10, 5167. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Farronato, M.; Sforza, C.; Bidra, A.S. Implant-supported immediately loaded complete arch rehabilitations with a mean follow-up of 10 years: A prospective clinical study. J. Prosthodont. 2019, 28, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Malo, P.; Lopes, A.; de Araujo Nobre, M.; Ferro, A. Immediate function dental implants inserted with less than 30 Ncm of torque in full-arch maxillary rehabilitations using the All-on-4 concept: Retrospective study. Int. J. Oral Maxillofac. Surg. 2018, 47, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Wentaschek, S.; Scheller, H.; Schmidtmann, I. Sensitivity and specificity of stability criteria for immediately loaded splinted maxillary implants. Clin. Implant. Dent. Relat. Res. 2015, 17, 542–549. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Sanz-Martín, I.; Figuero, E.; Sanz, M. Clinical efficacy of immediate implant loading protocols compared to conventional loading depending on the type of the restoration: A systematic review. Clin. Oral Implant. Res. 2015, 26, 964–982. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Heussen, N.; Elvers, D.; Steiner, T.; Hölzle, F.; Modabber, A. Compensating for Poor Primary Implant Stability in Different Bone Densities by Varying Implant Geometry: A Laboratory Study. Int. J. Oral Maxillofac. Surg. 2015, 44, 1514–1520. [Google Scholar] [CrossRef]
- Waechter, J.; de Matos Madruga, M.; do Carmo Filho, L.C.; Leite, F.R.M.; Schinestsck, A.R.; Faot, F. Comparison between Tapered and Cylindrical Implants in the Posterior Regions of the Mandible: A Prospective, Randomized, Split-Mouth Clinical Trial Focusing on Implant Stability Changes during Early Healing. Clin. Implant. Dent. Relat. Res. 2017, 19, 733–741. [Google Scholar] [CrossRef]
- Sierra-Rebolledo, A.; Allais-Leon, M.; Maurette-O’Brien, P.; Gay-Escoda, C. Primary Apical Stability of Tapered Implants Through Reduction of Final Drilling Dimensions in Different Bone Density Models: A Biomechanical Study. Implant. Dent. 2016, 25, 775–782. [Google Scholar] [CrossRef]
- Staedt, H.; Heimes, D.; Lehmann, K.M.; Ottl, P.; Bjelopavlovic, M.; Wagner, W.; Al-Nawas, B.; Kämmerer, P.W. Does the Modification of the Apical Geometry of a Dental Implant Affect Its Primary Stability? A Comparative Ex Vivo Study. Materials 2021, 7, 1728. [Google Scholar] [CrossRef]
- Khayat, P.G.; Milliez, S.N. Prospective clinical evaluation of 835 multi-threaded tapered screw-vent implants: Results after two years of functional loading. J. Oral Implantol. 2007, 33, 225–231. [Google Scholar] [CrossRef]
- Gapski, R.; Wang, H.L.; Mascarenhas, P.; Lang, N.P. Critical review of immediate implant loading. Clin. Oral Implant. Res. 2003, 5, 515–527. [Google Scholar] [CrossRef]
- Javed, F.; George, E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef]
- Sennerby, L.; Roos, J. Surgical determinants of clinical success of osseointegrated oral implants: A review of the litera-ture. Int. J. Prosthodont. 1998, 11, 408–420. [Google Scholar] [PubMed]
- Aparicio, C.; Lang, N.P.; Rangert, B. Validity and clinical significance of biomechanical testing of implant/bone interface. Clin. Oral Implant. Res. 2006, 17, 2–7. [Google Scholar] [CrossRef]
- Heimes, D.; Becker, P.; Pabst, A. How does dental implant macrogeometry affect primary implant stability? A narrative review. Int. J. Implant. Dent. 2023, 1, 20. [Google Scholar] [CrossRef]
- Quesada-García, M.P.; Prados-Sánchez, E.; Olmedo-Gaya, M.V.; Muñoz-Soto, E.; González-Rodríguez, M.P.; Valllecillo-Capilla, M. Measurement of dental implant stability by resonance frequency analysis: A review of the literature. Med. Oral Patol. Oral Cir. Bucal 2009, 10, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, R.C.; Wigren, S.; Flynn, M.C.; Meijer, G.J.; Mylanus, E.A.; Hol, M.K. Application and Interpretation of Resonance Frequency Analysis in Auditory Osseointegrated Implants: A Review of Literature and Establishment of Practical Recommendations. Otol. Neurotol. 2015, 9, 1518–1524. [Google Scholar] [CrossRef]
- Satwalekar, P.; Nalla, S.; Reddy, R.; Chowdary, S.G. Clinical evaluation of osseointegration using resonance frequency analysis. J. Indian Prosthodont. Soc. 2015, 3, 192–199. [Google Scholar] [CrossRef]
- Huang, H.; Wu, G.; Hunziker, E. The clinical significance of implant stability quotient (ISQ) measurements: A literature review. J. Oral Biol. Craniofac. Res. 2020, 4, 629–638. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Chen, C.J.; Chuang, S.K.; Weber, H.P. Implant loading protocols for edentulous patients with fixed prostheses: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2014, 29, 256–270. [Google Scholar] [CrossRef]
- Greenstein, G.; Cavallaro, J. Implant Insertion Torque: Its Role in Achieving Primary Stability of Restorable Dental Implants. Compend. Contin. Educ. Dent. 2017, 2, 88–96. [Google Scholar]
- Atieh, M.A.; Baqain, Z.H.; Tawse-Smith, A.; Ma, S.; Almoselli, M.; Lin, L.; Alsabeeha, N.H.M. The influence of insertion torque values on the failure and complication rates of dental implants: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2021, 3, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Barewal, R.M.; Stanford, C.; Weesner, T.C. A randomized controlled clinical trial comparing the effects of three loading protocols on dental implant stability. Int. J. Oral Maxillofac. Implant. 2012, 27, 945–956. [Google Scholar]
- Ostman, P.O.; Hellman, M.; Sennerby, L. Direct implant loading in the edentulous maxilla using a bone density-adapted surgical protocol and primary implant stability criteria for inclusion. Clin. Implant. Dent. Relat. Res. 2005, 7, 60–69. [Google Scholar] [CrossRef]
- Schimmel, M.; Srinivasan, M.; Herrmann, F.R.; Müller, F. Loading protocols for implant-supported overdentures in the edentulous jaw: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2014, 29, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Weigl, P.; Strangio, A. The impact of immediately placed and restored single tooth implants on hard and soft tissues in the anterior maxilla. Eur. J. Oral Implantol. 2016, 9, 89–106. [Google Scholar]
- Lemos, C.A.A.; Verri, F.R.; de Oliveira Neto, O.B. Clinical effect of the high insertion torque on dental implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2021, 4, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Coulthard, P.; Worthington, H.V. Different loading strategies of dental implants: A Cochrane systematic review of randomised controlled clinical trials. Eur. J. Oral Implantol. 2008, 1, 259–276. [Google Scholar]
- Rea, M.; Lang, N.P.; Ricci, S.; Mintrone, F.; Gonzalez, G.; Botticelli, D. Healing of implants installed in over- or un-der-prepared sites: An experimental study in dogs. Clin. Oral Implant. Res. 2015, 26, 442–446. [Google Scholar] [CrossRef]
- Benic, G.I.; Mir-Mari, J.; Hämmerle, C.H. Loading protocols for single-implant crowns: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2014, 29, 222–238. [Google Scholar] [CrossRef]
- Degidi, M.; Nardi, D.; Daprile, G.; Piattelli, A. Nonremoval of immediate abutments in cases involving subcrestally placed postextractive tapered single implants: A randomized controlled clinical study. Clin. Implant. Dent. Relat. Res. 2014, 6, 794–805. [Google Scholar] [CrossRef]
- Barone, A.; Alfonsi, F.; Derchi, G.; Tonelli, P.; Toti, P.; Marchionni, S.; Covani, U. The Effect of Insertion Torque on the Clinical Outcome of Single Implants: A Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2016, 3, 588–600. [Google Scholar] [CrossRef]
- Marconcini, S.; Giammarinaro, E.; Toti, P.; Alfonsi, F.; Covani, U.; Barone, A. Longitudinal analysis on the effect of insertion torque on delayed single implants: A 3-year randomized clinical study. Clin. Implant. Dent. Relat. Res. 2018, 3, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Al-Nawas, B.; Wagner, W.; Grötz, K.A. Insertion torque and resonance frequency analysis of dental implant systems in an animal model with loaded implants. Int. J. Oral Maxillofac. Implant. 2006, 21, 726–732. [Google Scholar]
- Rebaudi, A.; Trisi, P.; Cella, R.; Cecchini, G. Preoperative evaluation of bone quality and bone density using novel CT/microCT-based hard-normal-soft classification system. Int. J. Oral Maxillofac. Implant. 2010, 25, 75–85. [Google Scholar]
- Jaffin, R.A.; Berman, C.L. The excessive loss of Branemark fixtures in type IV bone: A 5-year analysis. J. Periodontal. 1991, 62, 2–4. [Google Scholar] [CrossRef]
- Nkenke, E.; Kloss, F.; Wiltfang, J. Histomorphometric and fluorescence microscopic analysis of bone remodelling after installa-tion of implants using an osteotome technique. Clin. Oral Implant. Res. 2002, 13, 595–602. [Google Scholar] [CrossRef]
- Pantani, F.; Botticelli, D.; Garcia, I.R.; Salata, L.A.; Borges, G.J.; Lang, N.P. Influence of lateral pressure to the implant bed on osseointegration: An experimental study in dogs. Clin. Oral Implant. Res. 2010, 21, 1264–1270. [Google Scholar] [CrossRef]
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontol. 2000 2008, 47, 51–66. [Google Scholar] [CrossRef]
- Silva, R.; Villalon, P.; Caceres, F. Effect of macro-design in the primary stability of short and extra-short implants using resonance frequency analysis. An ex vivo study. J. Oral Biol. Craniofac. Res. 2020, 10, 603–607. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.J.; An, H.W.; Kim, H.S.; Ha, D.G.; Ryo, K.H.; Park, K.B. The effect of the thread depth on the mechanical properties of the dental implant. J. Adv. Prosthodont. 2015, 2, 115–121. [Google Scholar] [CrossRef]
- Makary, C.; Menhall, A.; Zammarie, C.; Lombardi, T.; Lee, S.Y.; Stacchi, C.; Park, K.B. Primary Stability Optimization by Using Fixtures with Different Thread Depth According to Bone Density: A Clinical Prospective Study on Early Loaded Implants. Materials 2019, 12, 2398. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Carrascal, N.; Salomó-Coll, O.; Gilabert-Cerdà, M.; Farré-Pagés, N.; Gargallo-Albiol, J.; Hernández-Alfaro, F. Effect of implant macro-design on primary stability: A prospective clinical study. Med. Oral Patol. Oral Cir. Bucal 2016, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Comuzzi, L.; Tumedei, M.; Di Pietro, N.; Romasco, T.; Heydari Sheikh Hossein, H.; Montesani, L.; Inchingolo, F.; Piattelli, A.; Covani, U. A Comparison of Conical and Cylindrical Implants Inserted in an In Vitro Post-Extraction Model Using Low-Density Polyurethane Foam Blocks. Materials 2023, 14, 5064. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.L.; de Castro, D.T.; Shimano, A.C.; Lepri, C.P.; dos Reis, A.C. Analysis of the influence of implant shape on primary stability using the correlation of multiple methods. Clin. Oral Investig. 2015, 19, 1861–1866. [Google Scholar] [CrossRef]
- Voumard, B.; Maquer, G.; Heuberger, P.; Zysset, P.K.; Wolfram, U. Peroperative estimation of bone quality and primary dental implant stability. J. Mech. Behav. Biomed. Mater. 2019, 92, 24–32. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Shibli, J.A.; Javid, K.; Cotrim, K.; Canabarro, A.; Louro, R.S.; Lowenstein, A.; Mourão, C.F.; Moraschini, V. Comparing the Long-Term Success Rates of Tooth Preservation and Dental Implants: A Critical Review. J. Funct. Biomater. 2023, 3, 142. [Google Scholar] [CrossRef]
- Schropp, L.; Isidor, F.; Kostopoulos, L.; Wenzel, A. Patient experience of, and satisfaction with, delayed-immediate vs. delayed single-tooth implant placement. Clin. Oral Implant. Res. 2004, 4, 498–503. [Google Scholar] [CrossRef]
- Testori, T.; Del Fabbro, M.; Galli, F.; Francetti, L.; Taschieri, S.; Weinstein, R. Immediate occlusal loading the same day or the after implant placement: Comparison of 2 different time frames in total edentulous lower jaws. J. Oral Implantol. 2004, 5, 307–313. [Google Scholar] [CrossRef]
- Cannizzaro, G.; Leone, M.; Esposito, M. Immediate versus early loading of two implants placed with a flapless technique sup-porting mandibular bar-retained overdentures: A single-blinded, randomised controlled clinical trial. Eur. J. Oral Implantol. 2008, 1, 33–43. [Google Scholar]
- Caneva, M.; Salata, L.A.; de Souza, S.S.; Baffone, G.; Lang, N.P.; Botticelli, D. Influence of implant positioning in extraction sockets on osseointegration: Histomorphometric analyses in dogs. Clin. Oral Implant. Res. 2010, 1, 43–49. [Google Scholar] [CrossRef]
- Araújo, M.G.; Wennström, J.L.; Lindhe, J. Modeling of the buccal and lingual bone walls of fresh extraction sites following implant installation. Clin. Oral Implant. Res. 2006, 6, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Vignoletti, F.; Johansson, C.; Albrektsson, T.; De Sanctis, M.; San Roman, F.; Sanz, M. Early healing of implants placed into fresh extraction sockets: An experimental study in the beagle dog. De novo bone formation. J. Clin. Periodontol. 2009, 3, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Linder, E.; Lindhe, J. Bio-Oss collagen in the buccal gap at immediate implants: A 6-month study in the dog. Clin. Oral Implant. Res. 2011, 1, 1–8. [Google Scholar] [CrossRef]
- Caneva, M.; Botticelli, D.; Pantani, F.; Baffone, G.M.; Rangel, I.G.; Lang, N.P. Deproteinized bovine bone mineral in marginal defects at implants installed immediately into extraction sockets: An experimental study in dogs. Clin. Oral Implant. Res. 2012, 1, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Seyssens, L.; Eeckhout, C.; Cosyn, J. Immediate implant placement with or without socket grafting: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2022, 3, 339–351. [Google Scholar] [CrossRef]
- Pitman, J.; Seyssens, L.; Christiaens, V.; Cosyn, J. Immediate implant placement with or without immediate provisionalization: A systematic review and meta-analysis. J. Clin. Periodontol. 2022, 10, 1012–1023. [Google Scholar] [CrossRef]
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implant. Res. 2012, 5, 1–21. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 4, 162–167. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.; Gold, J.; Somohano Marquez, T.; Romanos, G. Under-Drilling versus Hybrid Osseodensification Technique: Differences in Implant Primary Stability and Bone Density of the Implant Bed Walls. Materials 2020, 2, 390. [Google Scholar] [CrossRef]
- Fernández-Olavarria, A.; Gutiérrez-Corrales, A.; González-Martín, M.; Torres-Lagares, D.; Torres-Carranza, E.; Serrera-Figallo, M. Influence of different drilling protocols and bone density on the insertion torque of dental implants. Med. Oral Patol. Oral Cir. Bucal 2023, 4, 385–394. [Google Scholar] [CrossRef]
- ASTM F1839; Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and Instruments. American Society for Testing and Materials: West Conshohocken, PA, USA, 1997. [CrossRef]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, I.N.; Tonsekar, P.P.; Najafi, B.; Drew, H.J.; Sullivan, A.J.; Petrov, S.D. Comparison of Osteotome and Conventional Drilling Techniques for Primary Implant Stability: An In Vitro Study. J. Oral Implantol. 2016, 42, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Comuzzi, L.; Iezzi, G.; Piattelli, A.; Tumedei, M. An In Vitro Evaluation, on Polyurethane Foam Sheets, of the Insertion Torque (IT) Values, Pull-Out Torque Values, and Resonance Frequency Analysis (RFA) of Nano Short Dental Implants. Polymers 2019, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E. Density of bone: Effect on treatment plans, surgical approach, healing, and progressive boen loading. Int. J. Oral Implantol. 1990, 2, 23–31. [Google Scholar]
- Comuzzi, L.; Tumedei, M.; Romasco, T.; Petrini, M.; Afrashtehfar, K.I.; Inchingolo, F.; Piattelli, A.; Di Pietro, N. Insertion Torque, Removal Torque, and Resonance Frequency Analysis Values of Ultrashort, Short, and Standard Dental Implants: An In Vitro Study on Polyurethane Foam Sheets. J. Funct. Biomater. 2022, 1, 10. [Google Scholar] [CrossRef]
- Bilhan, H.; Geckili, O.; Mumcu, E.; Bozdag, E.; Sünbüloğlu, E.; Kutay, O. Influence of surgical technique, implant shape and diameter on the primary stability in cancellous bone. J. Oral Rehabil. 2010, 12, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Morar, L.; Băciuț, G.; Băciuț, M.; Bran, S.; Colosi, H.; Manea, A.; Almășan, O.; Dinu, C. Analysis of CBCT Bone Density Using the Hounsfield Scale. Prosthesis 2022, 4, 414–423. [Google Scholar] [CrossRef]
- Pauwels, R.; Jacobs, R.; Singer, S.R.; Mupparapu, M. CBCT-based bone quality assessment: Are Hounsfield units applicable? Dentomaxillofac. Radiol. 2015, 1, 20140238. [Google Scholar] [CrossRef]
- Schnitman, P.A.; Hayashi, C.; Han, R.K. Why guided when freehand is easier, quicker, and less costly? J. Oral Implantol. 2014, 6, 670–678. [Google Scholar] [CrossRef]

| Implant Type | Implant Ø (mm) | Implant Length (mm) | Last Drill Ø (mm) | Crestal Drill | Drilling Length |
|---|---|---|---|---|---|
| SICace® | 3.4 | 7.5 | 3.1 (blue) | ✔ | Full (according to implant length) |
| SICmax® | 3.7 | 9.5 | |||
| SICtapered® | 3.7 | 13 | |||
| SICace® | 4.0 | 7.5 | 3.25 (red) | ✔ | Full (according to implant length) |
| SICmax® | 4.2 | 9.5 | |||
| SICtapered® | 4.2 | 13 | |||
| SICace® | 4.5 | 7.5 | 3.75 (yellow) | ✔ | Full (according to implant length) |
| SICmax® | 4.7 | 9.5 | |||
| SICtapered® | 4.7 | 13 | |||
| SICace® | 5.0 | 14.5 | 4.25 (green) | ✔ | Full (according to implant length) |
| SICmax® | 5.2 | ||||
| SICtapered® | 5.2 |
| Implant | Contact Surface Area to Bone (mm2) |
|---|---|
| SICace® Ø 3.4 mm/L 7.5 mm | 103.57 |
| SICace® Ø 3.4 mm/L 9.5 mm | 130.24 |
| SICace® Ø 3.4 mm/L 13.0 mm | 180.27 |
| SICace® Ø 4.0 mm/L 7.5 mm | 123.45 |
| SICace® Ø 4.0 mm/L 9.5 mm | 162.25 |
| SICace® Ø 4.0 mm/L 13.0 mm | 218.32 |
| SICace® Ø 4.5 mm/L 7.5 mm | 141.72 |
| SICace® Ø 4.5 mm/L 9.5 mm | 186.64 |
| SICace® Ø 4.5 mm/L 13.0 mm | 251.34 |
| SICace® Ø 5.0 mm/L 14.5 mm | 333.53 |
| SICmax® Ø 3.7 mm/L 7.5 mm | 106.57 |
| SICmax® Ø 3.7 mm/L 9.5 mm | 137.36 |
| SICmax® Ø 3.7 mm/L 13.0 mm | 198.26 |
| SICmax® Ø 4.2 mm/L 7.5 mm | 120.02 |
| SICmax® Ø 4.2 mm/L 9.5 mm | 162.71 |
| SICmax® Ø 4.2 mm/L 13.0 mm | 217.26 |
| SICmax® Ø 4.7 mm/L 7.5 mm | 143.17 |
| SICmax® Ø 4.7 mm/L 9.5 mm | 191.82 |
| SICmax® Ø 4.7 mm/L 13.0 mm | 277.13 |
| SICmax® Ø 5.2 mm/L 14.5 mm | 345.59 |
| SICtapered® Ø 3.7 mm/L 7.5 mm | 119.45 |
| SICtapered® Ø 3.7 mm/L 9.5 mm | 139.11 |
| SICtapered® Ø 3.7 mm/L 13.0 mm | 218.21 |
| SICtapered® Ø 4.2 mm/L 7.5 mm | 110.28 |
| SICtapered® Ø 4.2 mm/L 9.5 mm | 139.52 |
| SICtapered® Ø 4.2 mm/L 13.0 mm | 213.71 |
| SICtapered® Ø 4.7 mm/L 7.5 mm | 138.47 |
| SICtapered® Ø 4.7 mm/L 9.5 mm | 176.32 |
| SICtapered® Ø 4.7 mm/L 13.0 mm | 265.83 |
| SICtapered® Ø 5.2 mm/L 14.5 mm | 329.49 |
| PCF 35 | PCF 25 | PCF 15 | PCF 10 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b (%) | CI95 | CI95 | b (%) | CI95 | CI95 | b (%) | CI95 | CI95 | b (%) | CI95 | CI95 | ||
| (Low) | (High) | (Low) | (High) | (Low) | (High) | (Low) | (High) | ||||||
| SICace® | Length | 12.3 * | 10.7 | 13.8 | 7.8 | 5.5 | 10.1 | 3.2 § | 1.5 | 4.8 | 7.4 | 4.6 | 10.2 |
| Diameter | 40.3 * | 25.5 | 56.9 | 143.3 § | 122.3 | 166.3 | 81.7 § | 69.5 | 94.7 | 109.2 | 80.8 | 142.1 | |
| Ratio | 3.2 | 18.3 | 25.5 | 14.7 | |||||||||
| SICmax® | Length | 19.4 * | 16.9 | 22 | 15.7 * | 13.5 | 17.9 | 9.3 * | 7.2 | 11.5 | 6.6 | 5.3 | 8 |
| Diameter | 40.8 * | 10.5 | 79.2 | 86.0 | 66.7 | 107.6 | 103.1 | 81.5 | 127.3 | 83.2 | 70.2 | 97.1 | |
| Ratio | 2.1 | 5.4 | 11.0 | 12.6 | |||||||||
| SICtapered® | Length | 16.4 * | 14.4 | 18.4 | 15.0 * | 12.6 | 17.6 | 7.5 * | 5.8 | 9.2 | 4.9 | 3.9 | 5.8 |
| Diameter | 62.5 § | 51.4 | 74.3 | 76.3 * | 53.7 | 102.3 | 100.5 * | 89.5 | 112.2 | 51.0 | 44.0 | 58.5 | |
| Ratio | 3.8 | 5.0 | 13.4 | 10.4 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoilov, M.; Shafaghi, R.; Stark, H.; Marder, M.; Kraus, D.; Enkling, N. Influence of Implant Macro-Design, -Length, and -Diameter on Primary Implant Stability Depending on Different Bone Qualities Using Standard Drilling Protocols—An In Vitro Analysis. J. Funct. Biomater. 2023, 14, 469. https://doi.org/10.3390/jfb14090469
Stoilov M, Shafaghi R, Stark H, Marder M, Kraus D, Enkling N. Influence of Implant Macro-Design, -Length, and -Diameter on Primary Implant Stability Depending on Different Bone Qualities Using Standard Drilling Protocols—An In Vitro Analysis. Journal of Functional Biomaterials. 2023; 14(9):469. https://doi.org/10.3390/jfb14090469
Chicago/Turabian StyleStoilov, Milan, Ramin Shafaghi, Helmut Stark, Michael Marder, Dominik Kraus, and Norbert Enkling. 2023. "Influence of Implant Macro-Design, -Length, and -Diameter on Primary Implant Stability Depending on Different Bone Qualities Using Standard Drilling Protocols—An In Vitro Analysis" Journal of Functional Biomaterials 14, no. 9: 469. https://doi.org/10.3390/jfb14090469






