Characterization and Evaluation of Silver Concentrations in Hydroxyapatite Powders
Abstract
:1. Introduction
2. Materials and Methods
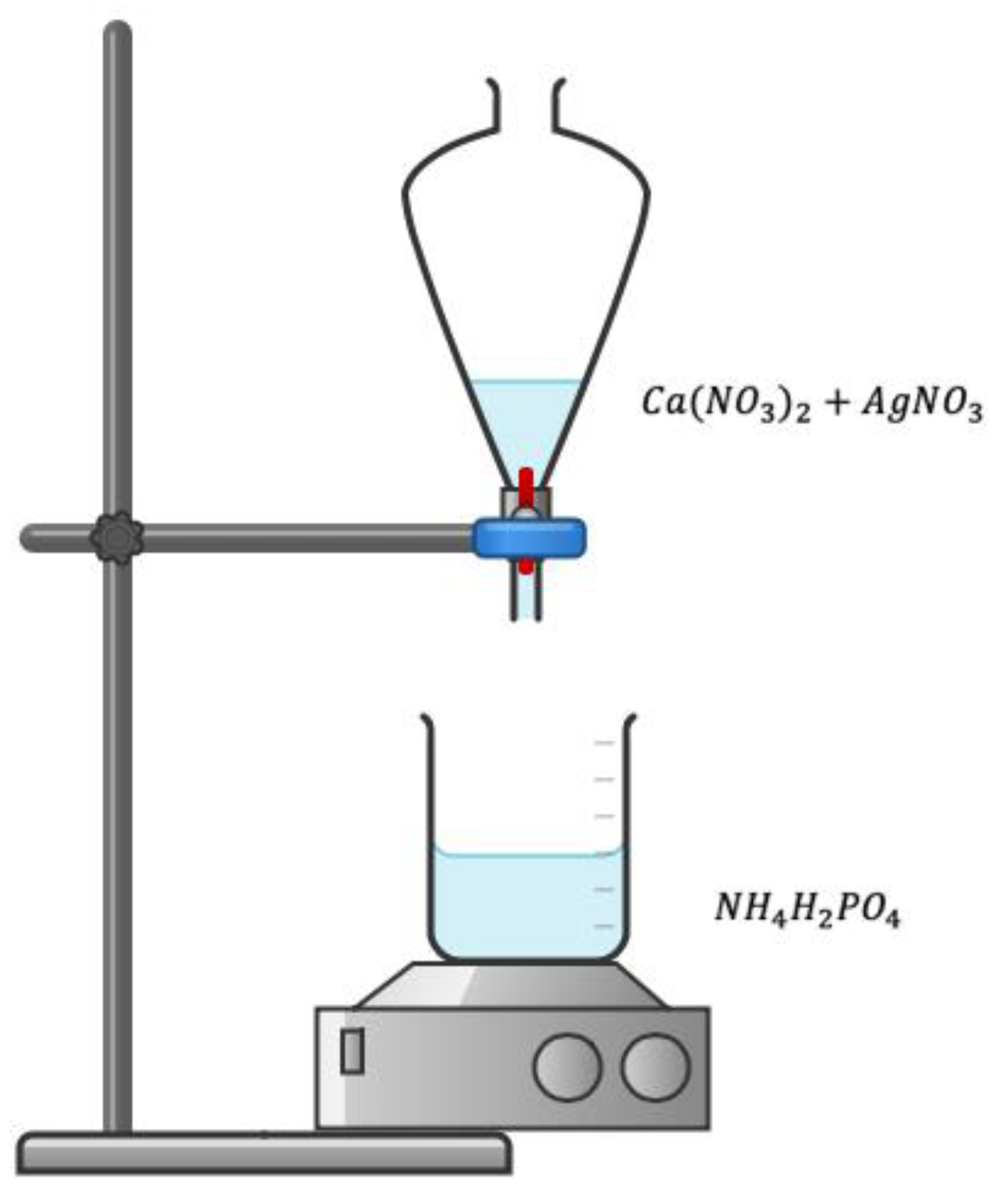
2.1. Synthesis of Silver-Doped Hydroxyapatite Powders (HApAg)
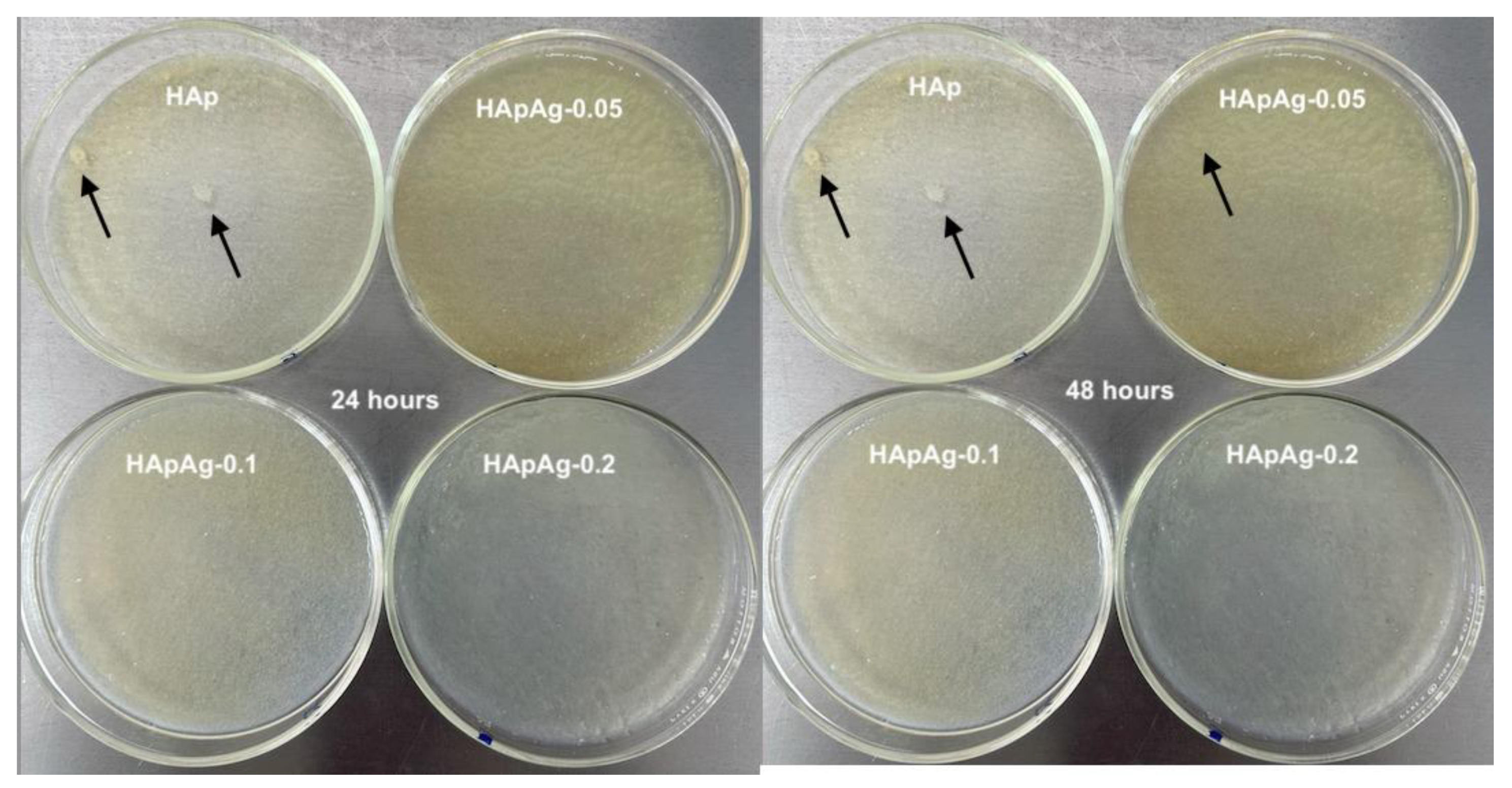
2.2. Antibacterial Evaluation
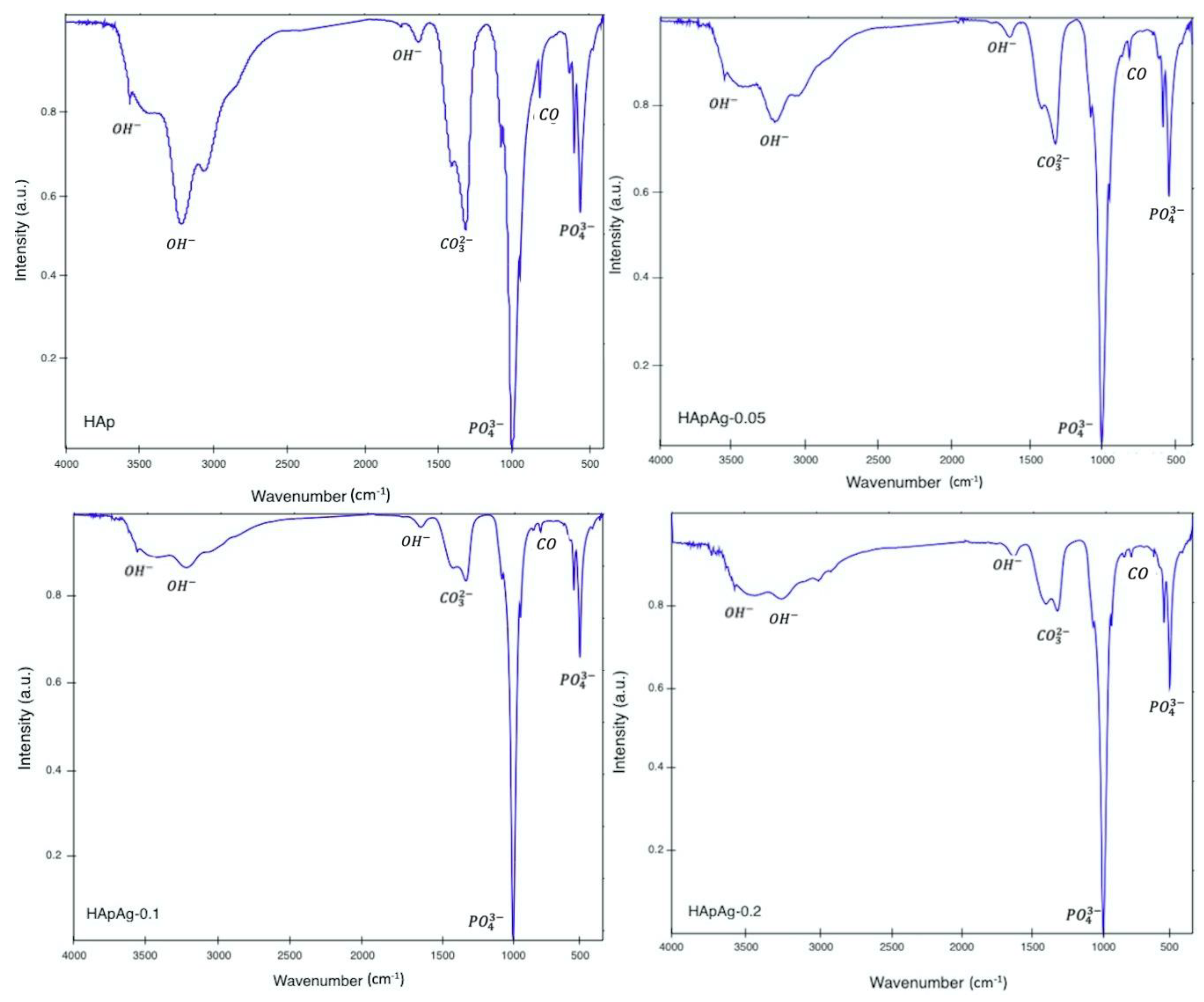
2.3. Functional Groups: Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Vibrational Modes: Raman Spectroscopy
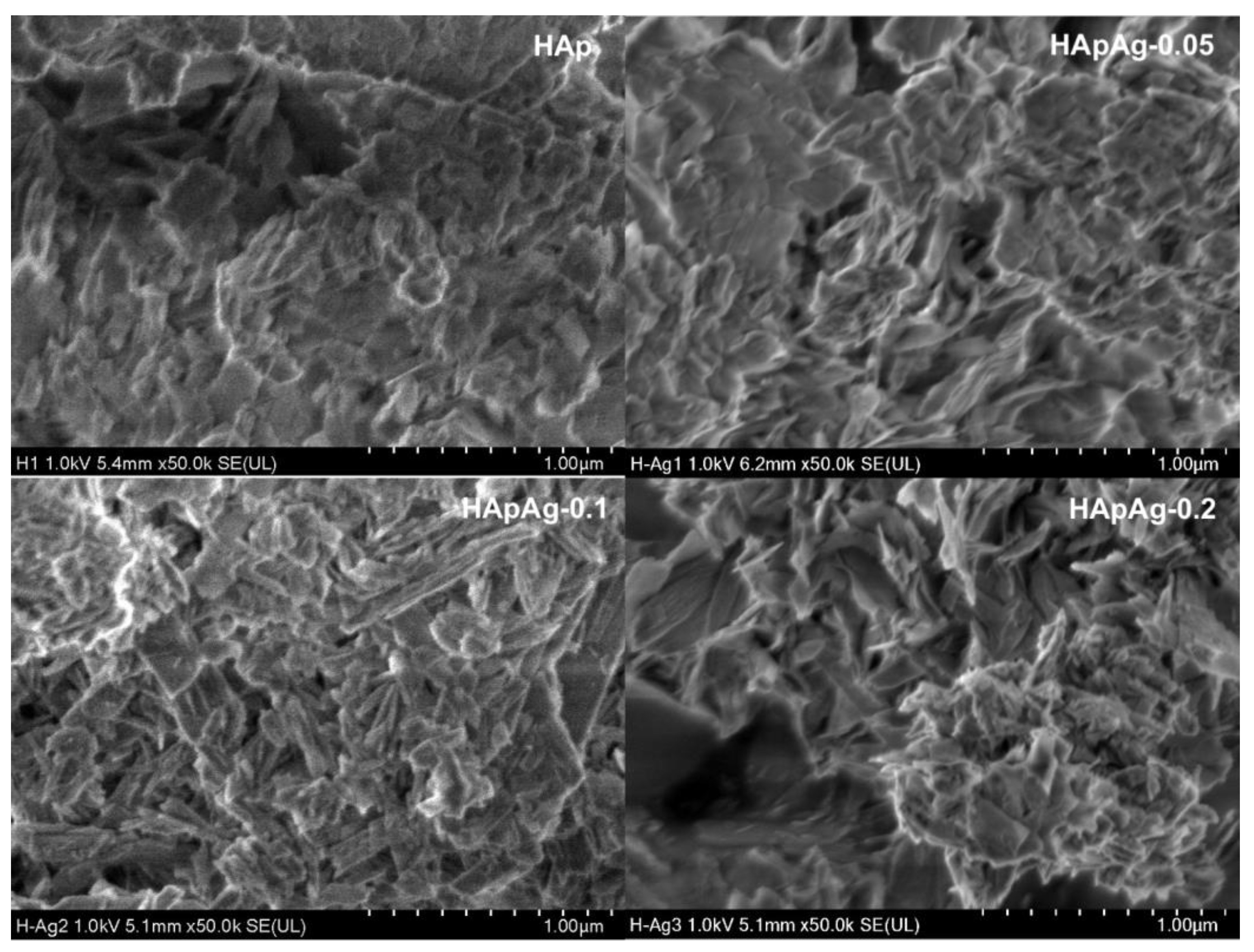
2.5. Morphology and Microstructure: Scanning Electron Microscopy (SEM)
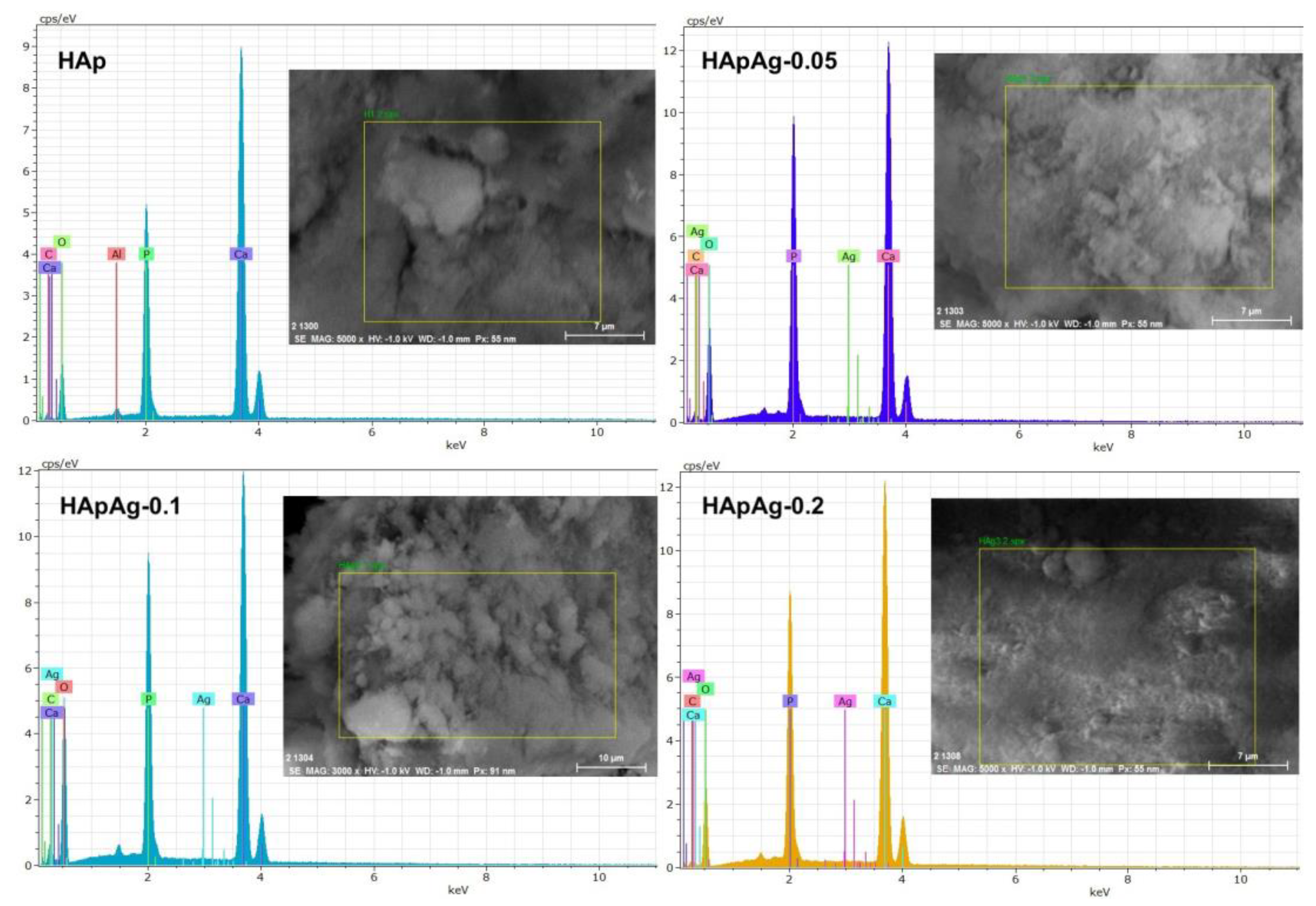
2.6. Elemental Composition: Dispersive Energy Spectroscopy (EDS)
2.7. Phase Composition: X-ray Diffraction (XRD)
3. Results
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. Raman Spectroscopy
3.3. Scanning Electron Microscopy SEM
3.4. Energy Dispersive Spectroscopy (EDS)
3.5. X-ray Diffraction (XRD)
3.6. Antibacterial Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Binyamin, G.; Shafi, B.M.; Mery, C.M. Biomaterials: A primer for surgeons. In Seminars in Pediatric Surgery; WB Saunders: Philadelphia, PE, USA, 2006; Volume 15, pp. 276–283. [Google Scholar]
- Ciobanu, C.S.; Iconaru, S.L.; Pasuk, I.; Vasile, B.S.; Lupu, A.R.; Hermenean, A.; Predoi, D.J.M.S. Structural properties of silver doped hydroxyapatite and their biocompatibility. Mater. Sci. Eng. C 2013, 33, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Andronescu, E.; Pall, L.; Costescu, A.; Le Coustumer, P.; Huneau, F.; Marutescu, L.; Ene, N.E.; Trusca, R.; Iconaru, S.L. Physico-chemical and antibacterial studies on silver doped nano-hydroxyapatite. J. Optoelectron. Adv. Mater. 2013, 15, 918–922. [Google Scholar]
- Motskin, M.; Wright, D.M.; Muller, K.; Kyle, N.; Gard, T.G.; Porter, A.E.; Skepper, J.N. Hydroxyapatite nano and microparticles: Correlation of particle properties with cytotoxicity and biostability. Biomaterials 2009, 30, 3307–3317. [Google Scholar] [CrossRef] [PubMed]
- Akindoyo, J.O.; Ghazali, S.; Beg, M.D.; Jeyaratnam, N. Characterization and elemental quantification of natural hydroxyapatite produced from cow bone. Chem. Eng. Technol. 2019, 42, 1805–1815. [Google Scholar] [CrossRef]
- Chesley, M.; Kennard, R.; Roozbahani, S.; Kim, S.M.; Kukk, K.; Mason, M. One-step hydrothermal synthesis within situ milling of biologically relevant hydroxyapatite. Mater. Sci. Eng. C 2020, 113, 110962. [Google Scholar] [CrossRef]
- Poovendran, K.; Josephwilson, K.S.; Sakthipandi, K.; Ramanujam, N.R. Assimilation of manganese metal ion doped hydroxyapatite by Co-Precipitation technique. J. Indian Chem. Soc. 2022, 99, 100779. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Fabrication of silver-and zinc-doped hydroxyapatite coatings for enhancing antimicrobial effect. Coatings 2020, 10, 905. [Google Scholar] [CrossRef]
- Kumar, R.; Münstedt, H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 2005, 26, 2081–2088. [Google Scholar] [CrossRef]
- Damm, C.; Münstedt, H.; Rösch, A. The antimicrobial efficacy of polyamide 6/silver-nano-and microcomposites. Mater. Chem. Phys. 2008, 108, 61–66. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef]
- Hu, C.; Guo, J.; Qu, J.; Hu, X. Efficient destruction of bacteria with Ti (IV) and antibacterial ions in co-substituted hydroxyapatite films. Appl. Catal. B Environ. 2007, 73, 345–353. [Google Scholar] [CrossRef]
- Díaz, M.; Barba, F.; Miranda, M.; Guitián, F.; Torrecillas, R.; Moya, J.S. Synthesis, and antimicrobial activity of a silver-hydroxyapatite nanocomposite. J. Nanomater. 2009, 2009, 498505. [Google Scholar] [CrossRef]
- Bianco, A.; Cacciotti, I.; Lombardi, M.; Montanaro, L.; Gusmano, G. Thermal stability and sintering behaviour of hydroxyapatite nanopowders. J. Therm. Anal. Calorim. 2007, 88, 237–243. [Google Scholar] [CrossRef]
- Nath, S.; Kalmodia, S.; Basu, B. Densification, phase stability and in vitro biocompatibility property of hydroxyapatite-10 wt% silver composites. J. Mater. Sci. Mater. Med. 2010, 21, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Constantin, L.V.; Predoi, D. Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res. Lett. 2012, 7, 324. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Rameshbabu, N.; Sampath Kumar, T.S.; Prabhakar, T.G.; Sastry, V.S.; Murty, K.V.G.K.; Prasad Rao, K. Antibacterial nanosized silver substituted hydroxyapatite: Synthesis and characterization. J. Biomed. Mater. Res. Part A 2007, 80, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Stanić, V.; Janaćković, D.; Dimitrijević, S.; Tanasković, S.B.; Mitrić, M.; Pavlović, M.S.; Raičević, S. Synthesis of antimicrobial monophase silver-doped hydroxyapatite nanopowders for bone tissue engineering. Appl. Surf. Sci. 2011, 257, 4510–4518. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite based materials for bone tissue engineering: A brief and comprehensive introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, M.; Ramesh, S.; Tan, C.Y.; Chandran, H.; Noor, A.F.M.; Krishnasamy, S.; Alengaram, U.J. Effect of multi-ions doping on the properties of carbonated hydroxyapatite bioceramic. Ceram. Int. 2019, 45, 3473–3477. [Google Scholar] [CrossRef]
- Siek, D.; Ślósarczyk, A.; Przekora, A.; Belcarz, A.; Zima, A.; Ginalska, G.; Czechowska, J. Evaluation of antibacterial activity and cytocompatibility of α-TCP based bone cements with silver-doped hydroxyapatite and CaCO3. Ceram. Int. 2017, 43, 13997–14007. [Google Scholar] [CrossRef]
- Fakharzadeh, A.; Ebrahimi-Kahrizsangi, R.; Nasiri-Tabrizi, B.; Basirun, W.J. Effect of dopant loading on the structural features of silver-doped hydroxyapatite obtained by mechanochemical method. Ceram. Int. 2017, 43, 12588–12598. [Google Scholar] [CrossRef]
- Jelínek, M.; Kocourek, T.; Jurek, K.; Remsa, J.; Mikšovský, J.; Weiserová, M.; Luxbacher, T. Antibacterial properties of Ag-doped hydroxyapatite layers prepared by PLD method. Appl. Phys. A 2010, 101, 615–620. [Google Scholar] [CrossRef]
- Méndez-Lozano, N.; Velázquez-Castillo, R.; Rivera-Muñoz, E.M.; Bucio-Galindo, L.; Mondragón-Galicia, G.; Manzano-Ramírez, A.; Apátiga-Castro, L.M. Crystal growth and structural analysis of hydroxyapatite nanofibers synthesized by the hydrothermal microwave-assisted method. Ceram. Int. 2017, 43, 451–457. [Google Scholar] [CrossRef]
- Predoi, D.; Ghita, R.V.; Ungureanu, F.; Negrila, C.C.; Vatasescu-Balcan, R.A.; Costache, M. Characteristics of hydroxyapatite thin films. J. Optoelectron. Adv. Mater. 2007, 9, 3827. [Google Scholar]
- Predoi, D.; Barsan, M.; Andronescu, E.; Vatasescu-Balcan, R.A.; Costache, M. Hydroxyapatite-iron oxide bioceramic prepared using nano-size powders. J. Optoelectron. Adv. Mater. 2007, 9, 3609. [Google Scholar]
- Ciobanu, C.S.; Iconaru, S.L.; Massuyeau, F.; Constantin, L.V.; Costescu, A.; Predoi, D. Synthesis, structure, and luminescent properties of europium-doped hydroxyapatite nanocrystalline powders. J. Nanomater. 2012, 2012, 942801. [Google Scholar] [CrossRef]
- Mendez-Lozano, N.; Apatiga-Castro, M.; Soto, K.M.; Manzano-Ramírez, A.; Zamora-Antunano, M.; Gonzalez-Gutierrez, C. Effect of temperature on crystallite size of hydroxyapatite powders obtained by wet precipitation process. J. Saudi Chem. Soc. 2022, 26, 101513. [Google Scholar] [CrossRef]
- Shirkhanzadeh, M.; Azadegan, M.; Liu, G.Q. Bioactive delivery systems for the slow release of antibiotics: Incorporation of Ag+ ions into micro-porous hydroxyapatite coatings. Mater. Lett. 1995, 24, 7–12. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Li, P.; Jia, Z.; Wang, Q.; Tang, P.; Wang, M.; Wang, K.; Fang, J.; Zhao, C.; Ren, F.; Ge, X.; et al. A resilient and flexible chitosan/silk cryogel incorporated Ag/Sr co-doped nanoscale hydroxyapatite for osteoinductivity and antibacterial properties. J. Mater. Chem. B 2018, 6, 7427–7438. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Predoi, D. Vibrational investigations of silver-doped hydroxyapatite with antibacterial properties. J. Spectrosc. 2013, 2013, 471061. [Google Scholar] [CrossRef]
- Turkoz, M.; Atilla, A.O.; Evis, Z. Silver and fluoride doped hydroxyapatites: Investigation by microstructure, mechanical and antibacterial properties. Ceram. Int. 2013, 39, 8925–8931. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 C. Nanoscale Res. Lett. 2011, 6, 613. [Google Scholar] [CrossRef]
- Kolmas, J.; Piotrowska, U.; Kuras, M.; Kurek, E. Effect of carbonate substitution on physicochemical and biological properties of silver containing hydroxyapatites. Mater. Sci. Eng. C 2017, 74, 124–130. [Google Scholar] [CrossRef]
- Singh, R.P.; Kumar, P. Effect of conventional and microwave heating on the structural and physicochemical properties of silver-doped hydroxyapatite nanopowders. Adv. Appl. Ceram. 2018, 117, 436–444. [Google Scholar] [CrossRef]
- Cullity, B.D. Answers to Problems: Elements of X-ray Diffraction; Addison-Wesley Publishing Company: Boston, MA, USA, 1978. [Google Scholar]
- Riaz, M.; Zia, R.; Saleemi, F.; Hussain, T.; Bashir, F.; Ikhram, H. Effect of Ti+4 on in vitro bioactivity and antibacterial activity of silicate glass-ceramics. Mater. Sci. Eng. C 2016, 69, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Dubnika, A.; Loca, D.; Rudovica, V.; Parekh, M.B.; Berzina-Cimdina, L. Functionalized silver doped hydroxyapatite scaffolds for controlled simultaneous silver ion and drug delivery. Ceram. Int. 2017, 43, 3698–3705. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Mojab, F.; Vahidi, H.; Marashi, B.; Talank, N.; Hosseini, O.; Saravanan, M. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg. Chem. Commun. 2021, 129, 108647. [Google Scholar] [CrossRef]
- Raimondi, F.; Scherer, G.G.; Kötz, R.; Wokaun, A. Nanoparticles in energy technology: Examples from electrochemistry and catalysis. Angew. Chem. Int. Ed. 2005, 44, 2190–2209. [Google Scholar] [CrossRef] [PubMed]



| Method | Advantages | Disadvantages |
|---|---|---|
| Chemical method | Morphology control, low cost | Low reproducibility, phase mixing |
| Sol-gel | Particle homogeneity, energy saving | Cost of reagents, processing times |
| Mechanochemical method | No use of solvents, low energy consumption | Sensitive efficiency, energy expenditure in grinding |
| PLD method | Multilayer growth, homogeneous coatings | Sensitivity in doping, high costs |
| Concentration Ag (mol) | Ca(NO3)2 (g) | NH4H2PO4 (g) | AgNO3 (g) |
|---|---|---|---|
| 0 | 11.75 | 3.43 | 0 |
| 0.05 | 11.65 | 3.42 | 0.042 |
| 0.1 | 11.55 | 3.41 | 0.084 |
| 0.2 | 11.36 | 3.38 | 0.167 |
| Peak (cm−1) | Assignment |
|---|---|
| 3590, 3490, 3100 | Water stretching [vs (O-H)] |
| 1650 | Bending of water δ (O-H) |
| 1200 | Hydroxyl group HPO |
| 1100 | Stretching (v3 P-O) |
| 870 | Bending (v1 C-O) |
| 520 | Bending (v4 P-O) |
| Peak (cm−1) | Raman Shifts |
|---|---|
| 430, 467 | Mode () of the group |
| 610 | Mode () of the group |
| 980, 1013 | Stretching mode () of the group |
| Sample | Wavelength (Å) | Peak Width (°) | Crystallite Size (nm) | Lattice Constant a, b, c (Å) |
|---|---|---|---|---|
| HAp | 1.54 | 0.19 | 50.12 | 9.14, 9.13, 6.72 |
| HApAg-0.05 | 1.54 | 0.21 | 48.34 | 9.22, 9.22, 6.78 |
| HApAg-0.1 | 1.54 | 0.20 | 49.53 | 9.21, 9.21, 6.79 |
| HApAg-0.2 | 1.54 | 0.22 | 47.51 | 9.25, 9.25, 6.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Lozano, N.; Apatiga-Castro, M.; Ruíz-Baltazar, A.d.J.; Luz-Asunción, M.d.l.; Pérez-Ramírez, E.E. Characterization and Evaluation of Silver Concentrations in Hydroxyapatite Powders. J. Funct. Biomater. 2023, 14, 467. https://doi.org/10.3390/jfb14090467
Méndez-Lozano N, Apatiga-Castro M, Ruíz-Baltazar AdJ, Luz-Asunción Mdl, Pérez-Ramírez EE. Characterization and Evaluation of Silver Concentrations in Hydroxyapatite Powders. Journal of Functional Biomaterials. 2023; 14(9):467. https://doi.org/10.3390/jfb14090467
Chicago/Turabian StyleMéndez-Lozano, Néstor, Miguel Apatiga-Castro, Alvaro de Jesús Ruíz-Baltazar, Miguel de la Luz-Asunción, and Eduardo E. Pérez-Ramírez. 2023. "Characterization and Evaluation of Silver Concentrations in Hydroxyapatite Powders" Journal of Functional Biomaterials 14, no. 9: 467. https://doi.org/10.3390/jfb14090467






