3,4-Dihydroxiphenylacetic Acid-Based Universal Coating Technique for Magnetic Nanoparticles Stabilization for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CoFe2O4 and Fe3O4 NPs by Thermal Decomposition Method
2.2. Synthesis of CoFe2O4 NPs by Co-Precipitation Method
2.3. Fe3O4-Au Nanoparticles Synthesis
2.4. Synthesis of the MFe2O4 (M = Fe, Co, Mn, Zn)
2.5. Surface Modification Nanoparticles with DOPAC
2.6. Transmission Electron Microscopy (TEM)
2.7. Magnetic Properties Analysis
2.8. Dynamic Light Scattering Studies (DLS)
2.9. Fourier-Transform Infrared Spectroscopy
2.10. Coating of Fe3O4-Au MNPs for Drug Delivery
2.11. Loading of Doxorubicin
2.12. Cytotoxicity Assay
2.13. Confocal Microscopy
3. Results
3.1. Magnetic Core Synthesis
3.2. MNPs Stabilization with DOPAC
3.3. Doxorubicin Delivery to 4T1 Cells by DOPAC Coated Fe3O4-Au NP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y.K. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing Using Magnetic Particle Detection Techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef] [PubMed]
- Dolai, J.; Mandal, K.; Jana, N.R. Nanoparticle Size Effects in Biomedical Applications. ACS Appl. Nano Mater. 2021, 4, 6471–6496. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on In Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, Y. Effects of the Particle Size and Shape of the Magnetic Nanoparticles on the Magnetic Hyperthermia and Exchange Bias Properties☆. Phys. B Condens Matter. 2019, 575, 411689. [Google Scholar] [CrossRef]
- Deatsch, A.E.; Evans, B.A. Heating Efficiency in Magnetic Nanoparticle Hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Plan Sangnier, A.; Van de Walle, A.B.; Curcio, A.; Le Borgne, R.; Motte, L.; Lalatonne, Y.; Wilhelm, C. Impact of Magnetic Nanoparticle Surface Coating on Their Long-Term Intracellular Biodegradation in Stem Cells. Nanoscale 2019, 11, 16488–16498. [Google Scholar] [CrossRef]
- Portilla, Y.; Fernández-Afonso, Y.; Pérez-Yagüe, S.; Mulens-Arias, V.; Morales, M.P.; Gutiérrez, L.; Barber, D.F. Different Coatings on Magnetic Nanoparticles Dictate Their Degradation Kinetics In Vivo for 15 Months after Intravenous Administration in Mice. J. Nanobiotechnol. 2022, 20, 543. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, M.; Zeng, J.; Huo, L.; Liu, K.; Wei, R.; Ni, K.; Gao, J. Recent Advances in Engineering Iron Oxide Nanoparticles for Effective Magnetic Resonance Imaging. Bioact. Mater. 2022, 12, 214–245. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [PubMed]
- Starsich, F.H.L.; Herrmann, I.K.; Pratsinis, S.E. Nanoparticles for Biomedicine: Coagulation during Synthesis and Applications. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.; et al. Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysts for Localized and PH-Activated Biofilm Disruption. ACS Nano 2019, 13, 4960–4971. [Google Scholar] [CrossRef]
- Predescu, A.M.; Matei, E.; Berbecaru, A.C.; Pantilimon, C.; Drăgan, C.; Vidu, R.; Predescu, C.; Kuncser, V. Synthesis and Characterization of Dextran-Coated Iron Oxide Nanoparticles. R. Soc. Open Sci. 2018, 5, 171525. [Google Scholar] [CrossRef]
- Unterweger, H.; Dézsi, L.; Matuszak, J.; Janko, C.; Poettler, M.; Jordan, J.; Bäuerle, T.; Szebeni, J.; Fey, T.; Boccaccini, A.R.; et al. Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles for Magnetic Resonance Imaging: Evaluation of Size-Dependent Imaging Properties, Storage Stability and Safety. Int. J. Nanomed. 2018, 13, 1899–1915. [Google Scholar] [CrossRef]
- Salunkhe, A.B.; Khot, V.M.; Thorat, N.D.; Phadatare, M.R.; Sathish, C.I.; Dhawale, D.S.; Pawar, S.H. Polyvinyl Alcohol Functionalized Cobalt Ferrite Nanoparticles for Biomedical Applications. Appl. Surf. Sci. 2013, 264, 598–604. [Google Scholar] [CrossRef]
- Ebadi, M.; Bullo, S.; Buskaran, K.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Dual-functional Iron Oxide Nanoparticles Coated with Polyvinyl Alcohol/5-fluorouracil/Zinc-aluminium-layered Double Hydroxide for a Simultaneous Drug and Target Delivery System. Polymer 2021, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Ramnandan, D.; Mokhosi, S.; Daniels, A.; Singh, M. Chitosan, Polyethylene Glycol and Polyvinyl Alcohol Modified MgFe2O4 Ferrite Magnetic Nanoparticles in Doxorubicin Delivery: A Comparative Study In Vitro. Molecules 2021, 26, 3893. [Google Scholar] [CrossRef]
- Kralj, S.; Drofenik, M.; Makovec, D. Controlled Surface Functionalization of Silica-Coated Magnetic Nanoparticles with Terminal Amino and Carboxyl Groups. J. Nanoparticle Res. 2011, 13, 2829–2841. [Google Scholar] [CrossRef]
- Kang, K.; Choi, J.; Nam, J.H.; Lee, S.C.; Kim, K.J.; Lee, S.W.; Chang, J.H. Preparation and Characterization of Chemically Functionalized Silica-Coated Magnetic Nanoparticles as a DNA Separator. J. Phys. Chem. B 2009, 113, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-W.; Fatima, H.; Kim, K.-S. Preparation of Silica Coated Magnetic Nanoparticles for Bioseparation. J. Nanosci. Nanotechnol. 2017, 18, 1414–1418. [Google Scholar] [CrossRef]
- Mejías, R.; Gutiérrez, L.; Salas, G.; Pérez-Yagüe, S.; Zotes, T.M.; Lázaro, F.J.; Morales, M.P.; Barber, D.F. Long Term Biotransformation and Toxicity of Dimercaptosuccinic Acid-Coated Magnetic Nanoparticles Support Their Use in Biomedical Applications. J. Control. Release 2013, 171, 225–233. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Dringen, R.; Petters, C.; Rastedt, W.; Köser, J.; Filser, J.; Stolte, S. Toxicity of Dimercaptosuccinate-Coated and Un-Functionalized Magnetic Iron Oxide Nanoparticles towards Aquatic Organisms. Environ. Sci. Nano 2016, 3, 754–767. [Google Scholar] [CrossRef]
- Paulini, F.; Marangon, A.R.M.; Azevedo, C.L.; Brito, J.L.M.; Lemos, M.S.; Sousa, M.H.; Veiga-Souza, F.H.; Souza, P.E.N.; Lucci, C.M.; Azevedo, R.B. In Vivo Evaluation of DMSA-Coated Magnetic Nanoparticle Toxicity and Biodistribution in Rats: A Long-Term Follow-Up. Nanomaterials 2022, 12, 3513. [Google Scholar] [CrossRef]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, S. Size-Dependent Properties of Magnetic Iron Oxide Nanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; Fernández, C.D.J.; Sangregorio, C. Exploring the Magnetic Properties of Cobalt-Ferrite Nanoparticles for the Development of a Rare-Earth-Free Permanent Magnet. Chem. Mater. 2015, 27, 4048–4056. [Google Scholar] [CrossRef]
- Frolova, L.; Khmelenko, O. Investigation of the Magnetic Properties of Ferrites in the CoO-NiO-ZnO Using Simplex-Lattice Design. J. Nanomater. 2018, 2018, 5686741. [Google Scholar] [CrossRef]
- Cortés-llanos, B.; Ocampo, S.M.; de la Cueva, L.; Calvo, G.F.; Belmonte-beitia, J.; Pérez, L.; Salas, G.; Ayuso-sacido, Á. Influence of Coating and Size of Magnetic Nanoparticles on Cellular Uptake for In Vitro Mri. Nanomaterials 2021, 11, 2888. [Google Scholar] [CrossRef]
- Favela-Camacho, S.E.; Pérez-Robles, J.F.; García-Casillas, P.E.; Godinez-Garcia, A. Stability of Magnetite Nanoparticles with Different Coatings in a Simulated Blood Plasma. J. Nanoparticle Res. 2016, 18, 176. [Google Scholar] [CrossRef]
- Tao, K.; Dou, H.; Sun, K. Facile Interfacial Coprecipitation to Fabricate Hydrophilic Amine-Capped Magnetite Nanoparticles. Chem. Mater. 2006, 18, 5273–5278. [Google Scholar] [CrossRef]
- Frimpong, R.A.; Dou, J.; Pechan, M.; Hilt, J.Z. Enhancing Remote Controlled Heating Characteristics in Hydrophilic Magnetite Nanoparticles via Facile Co-Precipitation. J. Magn. Magn. Mater. 2010, 322, 326–331. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Jie Ying, A.N.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and Strategies for the Synthesis of Diverse Nanoparticles and Their Applications: A Comprehensive Overview. RSC Adv 2015, 5, 105003. [Google Scholar] [CrossRef]
- Nagesha, D.K.; Plouffe, B.D.; Phan, M.; Lewis, L.H.; Sridhar, S.; Murthy, S.K. Functionalization-Induced Improvement in Magnetic Properties of Fe3O4 Nanoparticles for Biomedical Applications. J. Appl. Phys. 2009, 105, 07B317. [Google Scholar] [CrossRef]
- Gao, F.; Qu, H.; Duan, Y.; Wang, J.; Song, X.; Ji, T.; Cao, L.; Nie, G.; Sun, S. Dopamine Coating as a General and Facile Route to Biofunctionalization of Superparamagnetic Fe3O4 Nanoparticles for Magnetic Separation of Proteins. RSC Adv. 2014, 4, 6657–6663. [Google Scholar] [CrossRef]
- Roca, A.G.; Veintemillas-Verdaguer, S.; Port, M.; Robic, C.; Serna, C.J.; Morales, M.P. Effect of Nanoparticle and Aggregate Size on the Relaxometric Properties of MR Contrast Agents Based on High Quality Magnetite Nanoparticles. J. Phys. Chem. B 2009, 113, 7033–7039. [Google Scholar] [CrossRef]
- Ball, V. Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S. Dumbbell-like Bifunctional Au-Fe3O4 Nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, 1906539. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, J.; Yan, Y.; Li, J.; Shen, M.; Zhang, G.; Mignani, S.; Shi, X. RGD-Functionalized Ultrasmall Iron Oxide Nanoparticles for Targeted T1-Weighted MR Imaging of Gliomas. Nanoscale 2015, 7, 14538–14546. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod Iron Oxide Nanoparticles as High-Performance T 2 Contrast Agents for Magnetic Resonance Imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Nemati Porshokouh, Z.; Kalappattil, V.; Torres, D.; Phan, M.H.; Garaio, E.; García, J.Á.; Sanchez Llamazares, J.L.; Srikanth, H. Tunable High Aspect Ratio Iron Oxide Nanorods for Enhanced Hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Nikitin, A.A.; Ivanova, A.V.; Semkina, A.S.; Lazareva, P.A.; Abakumov, M.A. Magneto-Mechanical Approach in Biomedicine: Benefits, Challenges, and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 11134. [Google Scholar] [CrossRef]
- Nikitin, A.A.; Yurenya, A.Y.; Zatsepin, T.S.; Aparin, I.O.; Chekhonin, V.P.; Majouga, A.G.; Farle, M.; Wiedwald, U.; Abakumov, M.A. Magnetic Nanoparticles as a Tool for Remote DNA Manipulations at a Single-Molecule Level. ACS Appl. Mater. Interfaces 2021, 13, 14458–14469. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Nikitin, A.A.; Gabashvily, A.N.; Vishnevskiy, D.A.; Abakumov, M.A. Synthesis and Intensive Analysis of Antibody Labeled Single Core Magnetic Nanoparticles for Targeted Delivery to the Cell Membrane. J. Magn. Magn. Mater. 2021, 521, 167487. [Google Scholar] [CrossRef]
- Hermann, R.; Walther, P.; Müller, M. Immunogold Labeling in Scanning Electron Microscopy. Histochem. Cell Biol. 1996, 106, 31–39. [Google Scholar] [CrossRef]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent Progress of Magnetic Nanoparticles in Biomedical Applications: A Review. Nano Sel. 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Efremova, M.V.; Nalench, Y.A.; Myrovali, E.; Garanina, A.S.; Grebennikov, I.S.; Gifer, P.K.; Abakumov, M.A.; Spasova, M.; Angelakeris, M.; Savchenko, A.G.; et al. Size-Selected Fe3O4-Au Hybrid Nanoparticles for Improved Magnetism-Based Theranostics. Beilstein J. Nanotechnol. 2018, 9, 2684–2699. [Google Scholar] [CrossRef]
- Kozenkova, E.; Levada, K.; Efremova, M.V.; Omelyanchik, A.; Nalench, Y.A.; Garanina, A.S.; Pshenichnikov, S.; Zhukov, D.G.; Lunov, O.; Lunova, M.; et al. Multifunctional Fe3O4-Au Nanoparticles for the Mri Diagnosis and Potential Treatment of Liver Cancer. Nanomaterials 2020, 10, 1646. [Google Scholar] [CrossRef] [PubMed]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.S.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.A.; et al. Magnetite-Gold Nanohybrids as Ideal All-in-One Platforms for Theranostics. Sci. Rep. 2018, 8, 11295. [Google Scholar] [CrossRef] [PubMed]
- Kumbham, S.; Paul, M.; Itoo, A.; Ghosh, B.; Biswas, S. Oleanolic Acid-Conjugated Human Serum Albumin Nanoparticles Encapsulating Doxorubicin as Synergistic Combination Chemotherapy in Oropharyngeal Carcinoma and Melanoma. Int. J. Pharm. 2022, 614, 121479. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the Mysteries of Serum Albumin-More than just a Serum Protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef]
- Salas Sanzana, D.; Flores Faúndez, E.; Meléndez, J.; Soto-Arriaza, M. Increased Delivery and Cytotoxicity of Doxorubicin in HeLa Cells Using the Synthetic Cationic Peptide PEM-2 Functionalized Liposomes. Colloids Surf. B Biointerfaces 2023, 228, 113420. [Google Scholar] [CrossRef]
- Malinovskaya, Y.; Melnikov, P.; Baklaushev, V.; Gabashvili, A.; Osipova, N.; Mantrov, S.; Ermolenko, Y.; Maksimenko, O.; Gorshkova, M.; Balabanyan, V.; et al. Delivery of Doxorubicin-Loaded PLGA Nanoparticles into U87 Human Glioblastoma Cells. Int. J. Pharm. 2017, 524, 77–90. [Google Scholar] [CrossRef]
- Kim, D.H.; Tamada, Y.; Ono, T.; Bader, S.D.; Rozhkova, E.A.; Novosad, V. The Effect of Ligands on FePt-Fe3O4 Core-Shell Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 2648–2652. [Google Scholar] [CrossRef]
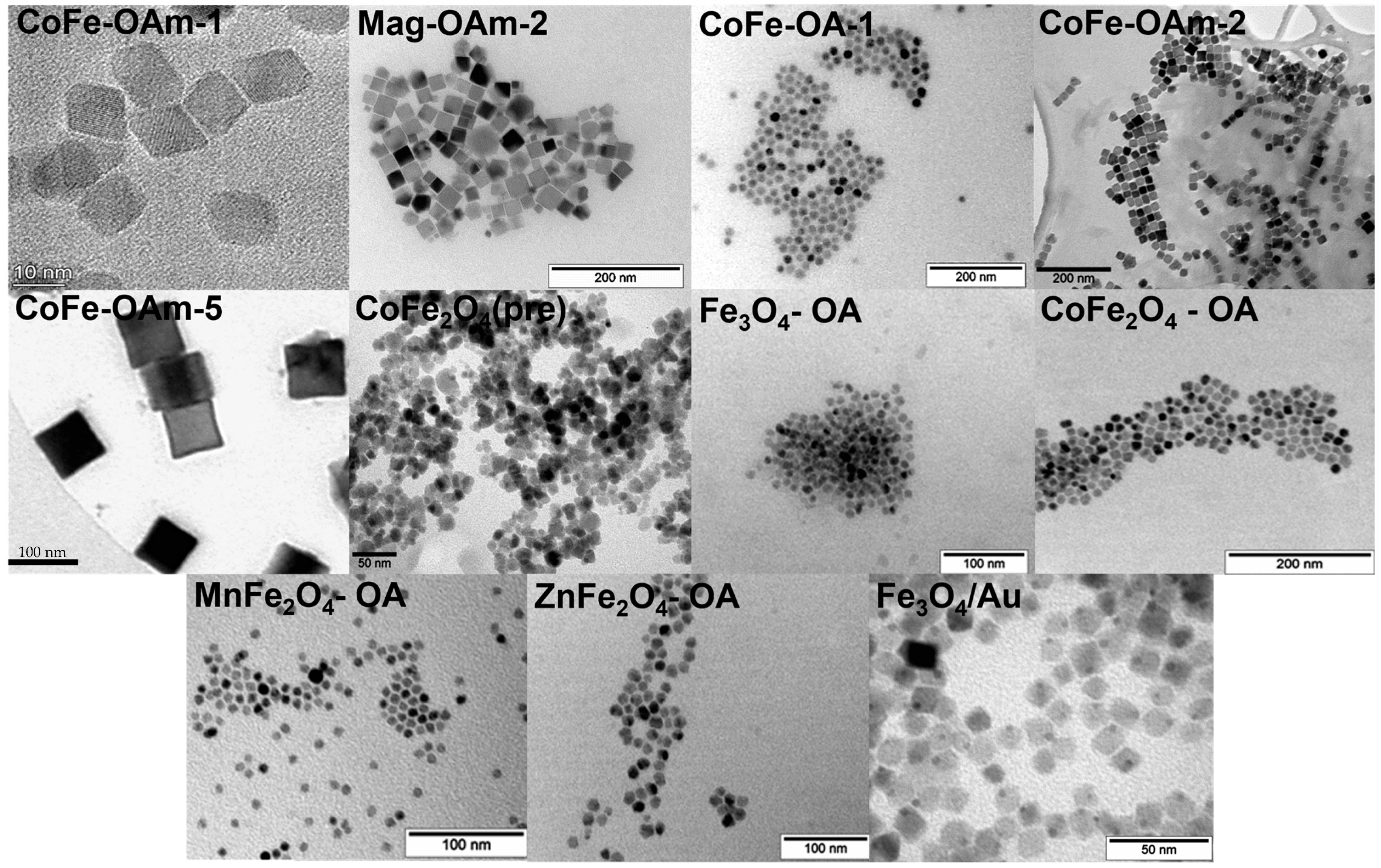
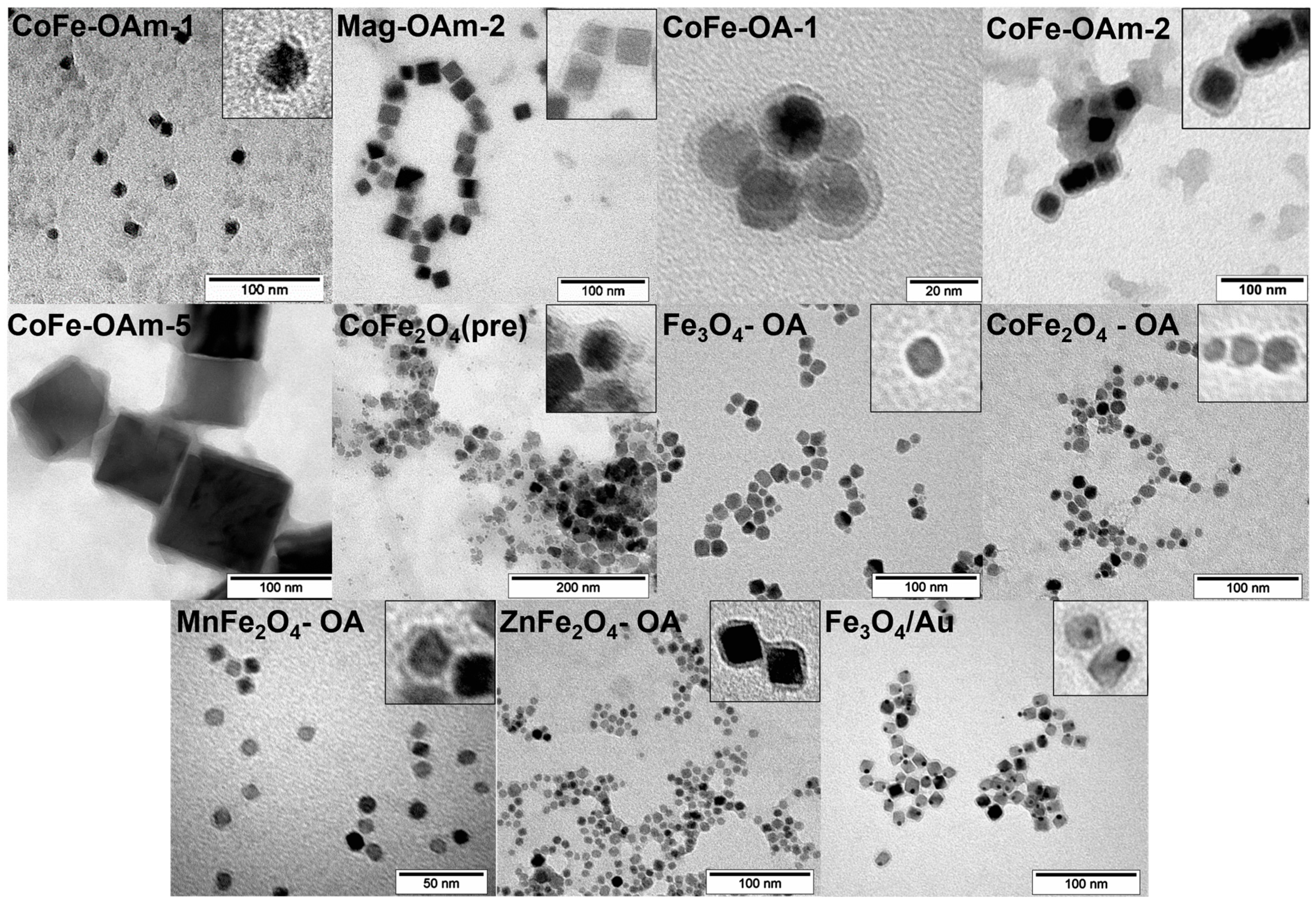
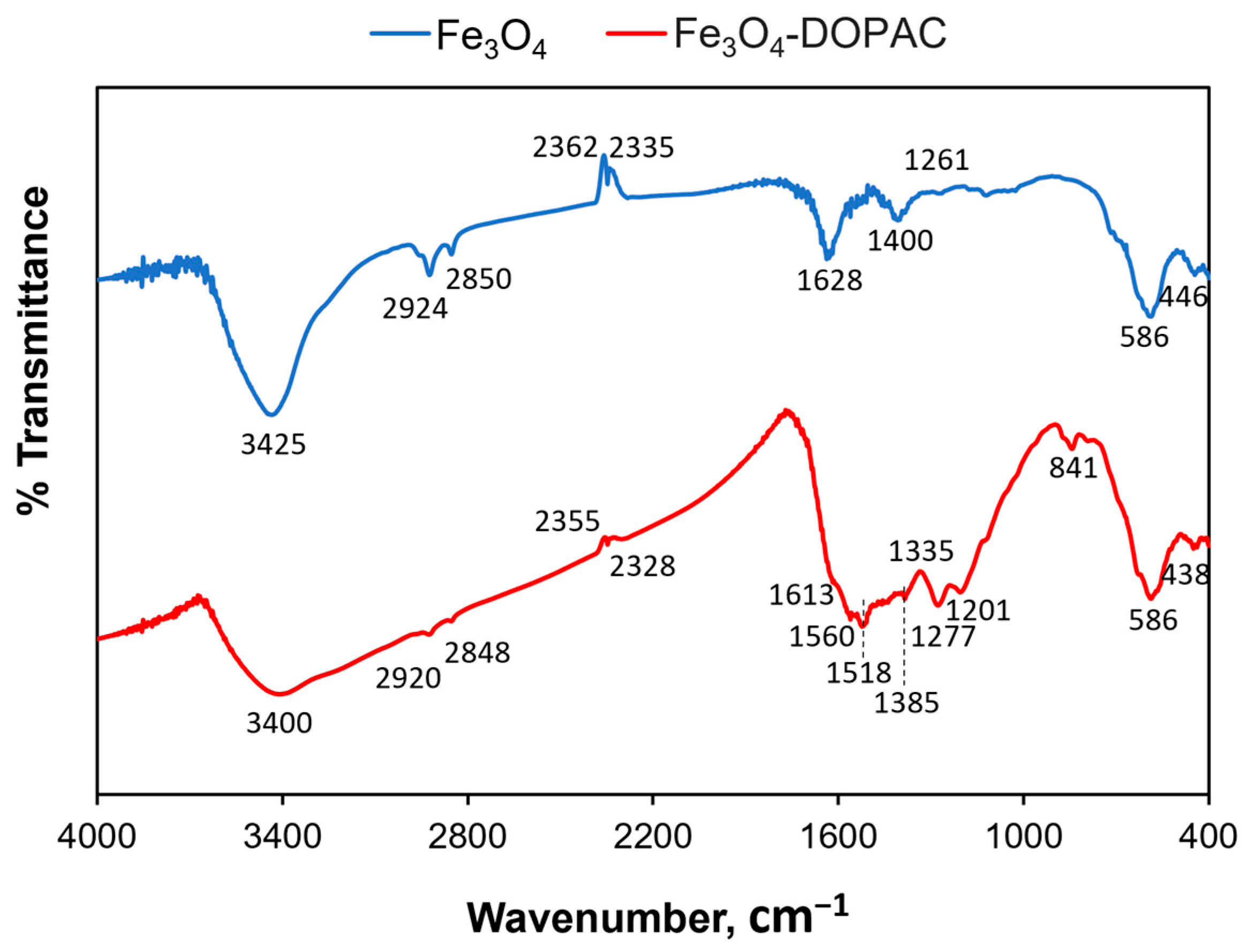

| Sample | Fe(acac)3, mmol | Co(acac)2, mmol | OA, mmol | OAm, mmol | 1,2-HDD | DE, mL | T, °C | Time, h | Phase |
|---|---|---|---|---|---|---|---|---|---|
| CoFe-OAm-1 | 1 | 0.5 | 12 | 2 | - | 40 | 280 °C | 2 | CoFe2O4 |
| CoFe-OAm-2 | 2 | 1 | 12 | 2 | - | 40 | 280 °C | 2 | |
| CoFe-OAm-5 | 4 | 2 | 8 | 2 | - | 40 | 280 °C | 4 | |
| CoFe-OA-1 | 2 | 1 | 6 | 6 | - | 40 | reflux | 4 | |
| Mag-OAm-2 | 0.5 | - | 8 | 2 | 4 | 10 | reflux | 4 | Fe3O4 |
| MNPs Core Name | Shape | Size, nm |
|---|---|---|
| CoFe-OAm-1 | Cubic | 14 ± 1 |
| Mag-OAm-2 | Cubic | 37 ± 6 |
| CoFe-OA-1 | Spherical | 15 ± 3 |
| CoFe-OAm-2 | Cubic | 27 ± 4 |
| CoFe-OAm-5 | Cubic | 99 ± 11 |
| CoFe2O4 (precipitation) | Spherical | 11 ± 2 |
| Fe3O4-OA | Spherical | 11 ± 2 |
| CoFe2O4-OA | Spherical | 16 ± 3 |
| MnFe2O4-OA | Spherical | 8 ± 1 |
| ZnFe2O4-OA | Spherical | 13 ± 2 |
| Fe3O4/Au | Octahedron/Spherical | 14 ± 2/4 ± 1 |
| DOPAC Coated MNPs Name | Z-Average, nm | PDI |
|---|---|---|
| CoFe-OAm-1@DOPAC | 32 | 0.200 |
| Mag-OAm-2@DOPAC | 125 | 0.300 |
| CoFe-OA-1@DOPAC | 307 | 0.330 |
| CoFe-OAm-2@DOPAC | 98 | 0.340 |
| CoFe-OAm-5@DOPAC | 251 | 0.450 |
| CoFe2O4(pre)@DOPAC | 125 | 0.220 |
| Fe3O4@DOPAC | 30 | 0.267 |
| CoFe2O4@DOPAC | 35 | 0.368 |
| MnFe2O4@DOPAC | 29 | 0.336 |
| ZnFe2O4@DOPAC | 35 | 0.353 |
| Fe3O4-Au@DOPAC | 51 | 0.215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semkina, A.; Nikitin, A.; Ivanova, A.; Chmelyuk, N.; Sviridenkova, N.; Lazareva, P.; Abakumov, M. 3,4-Dihydroxiphenylacetic Acid-Based Universal Coating Technique for Magnetic Nanoparticles Stabilization for Biomedical Applications. J. Funct. Biomater. 2023, 14, 461. https://doi.org/10.3390/jfb14090461
Semkina A, Nikitin A, Ivanova A, Chmelyuk N, Sviridenkova N, Lazareva P, Abakumov M. 3,4-Dihydroxiphenylacetic Acid-Based Universal Coating Technique for Magnetic Nanoparticles Stabilization for Biomedical Applications. Journal of Functional Biomaterials. 2023; 14(9):461. https://doi.org/10.3390/jfb14090461
Chicago/Turabian StyleSemkina, Alevtina, Aleksey Nikitin, Anna Ivanova, Nelly Chmelyuk, Natalia Sviridenkova, Polina Lazareva, and Maxim Abakumov. 2023. "3,4-Dihydroxiphenylacetic Acid-Based Universal Coating Technique for Magnetic Nanoparticles Stabilization for Biomedical Applications" Journal of Functional Biomaterials 14, no. 9: 461. https://doi.org/10.3390/jfb14090461





