Antibacterial and Antihemolytic Activity of New Biomaterial Based on Glycyrrhizic Acid and Quercetin (GAQ) against Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GA–Quercetin Complex (GAQ)
2.3. Measurement of UV–Visible Absorption Spectra
2.4. Measurement of IR Spectra
2.5. Bacterial Strain and Growth Conditions
2.6. Antimicrobial Activity—Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.7. Hemolysis Study
2.8. Measurement of Erythrocytes Membrane Fluidity
2.9. Fluorescence Studies of α-Hemolysin-GAQ Interaction
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristic of a New Biomaterial (GAQ) Based on Quercetin and Glycyrrhizic Acid
3.2. Antibacterial and Antihemolytic Activity of GAQ
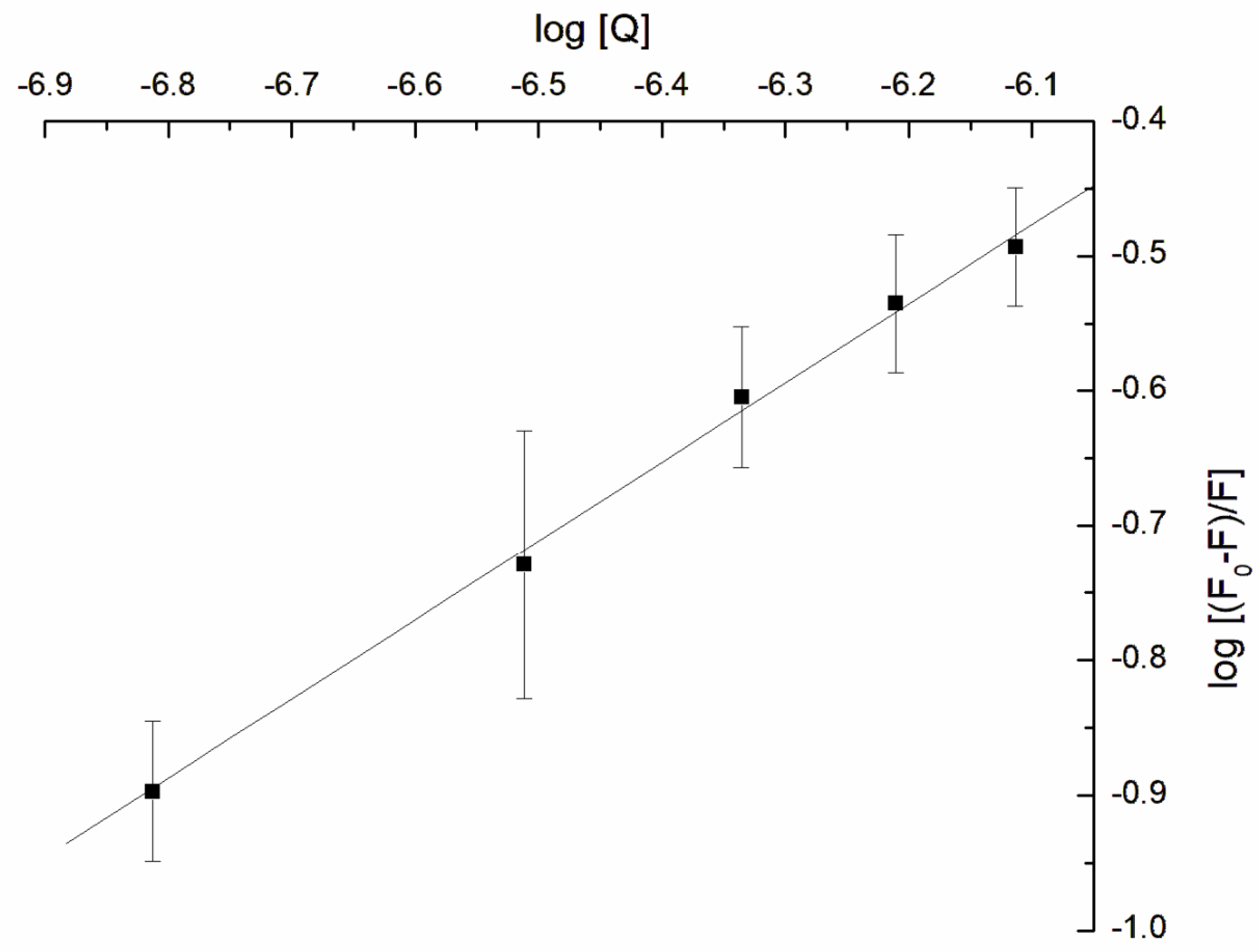
3.3. Influence of GAQ on the Structure of Erythrocytes Membrane
3.4. Fluorescence Characterization of α-Hemolysin–GAQ Interaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechmol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, J.; Xiao, A.; Liu, L. Antibacterial Activity of Polyphenols: Structure-Activity Relationship and Influence of Hyperglycemic Condition. Molecules 2017, 6, 1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolstikov, G.A.; Baltina, L.A.; Shul’ts, E.E.; Pokrovsky, A.G. Glycyrrhizic acid. Russ. J. Bioorganic Chem. 1997, 23, 625–642. [Google Scholar]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Phosphatidylcholine-rutin complex as a potential nanocarrier for food. J. Funct. Food. 2017, 33, 134–141. [Google Scholar] [CrossRef]
- Salcedo, C.L.; Frias, M.A.; Cutro, A.C.; Nazareno, M.A.; Disalvo, E.A. Antiradical activity of gallic acid included in lipid interphases. Biochim. Biophys. Acta 2014, 1838, 2656–2661. [Google Scholar] [CrossRef] [Green Version]
- Naziris, S.; Sekowski, S.; Olchowik-Grabarek, E.; Buczkowski, A.; Balcerzak, Ł.; Chrysostomou, V.; Pispas, S.; Małecka, M.; Bryszewska, M.; Ionov, M. Biophysical interactions of mixed lipid-polymer nanoparticles incorporating curcumin: Potential as antibacterial agent. Biomater. Adv. 2023, 144, 213200. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.S.; Mamdouh, W.; Nasr, M.; ElShaer, A.; Polycarpou, E.; Abdel-Aziz, R.T.A.; Sammour, O.A. Quercetin loaded cosm-nutraceutical electrospun composite nanofibers for acne alleviation: Preparation, characterization and experimental clinical appraisal. Int. J. Pharm. 2022, 612, 121309. [Google Scholar] [CrossRef]
- Pinho, E.; Soares, G.; Henriques, M. Evaluation of antibacterial activity of caffeic acid encapsulated by β-cyclodextrins. J. Microencapsul. 2015, 8, 804–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilyich, T.V.; Kovalenia, T.A.; Lapshina, E.A.; Stępniak, A.; Pałecz, B.; Zavodnik, I.B. Thermodynamic parameters and mitochondrial effects of supramolecular complexes of quercetin with β-cyclodextrins. J. Mol. Liq. 2021, 325, 115184. [Google Scholar] [CrossRef]
- Torres, E.; Marin, V.; Aburto, J.; Beltran, H.I.; Shirai, K.; Villanueva, S.; Sandoval, G. Enzymatic modification of chitosan with quercetin and its application as antioxidant edible films. Prikl. Biokhim. Mikrobiol. 2012, 48, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wu, L.; Hu, M.; Dong, W.; Xu, M.; Zhang, P. Glycyrrhizic acid: A promising carrier material for anticancer therapy. Biomed. Pharmacother. 2017, 95, 670–678. [Google Scholar] [CrossRef]
- Salyutina, O.Y.; Polyakov, N.E. Glycyrrhizic acid as a multifunctional drug carrier—From physicochemical properties to biomedical applications: A modern insight on the ancient drug. Int. J. Pharm. 2019, 559, 271–279. [Google Scholar] [CrossRef]
- Baratov, Q.; Mustafakulov, M.; Matchanov, A.; Vypova, N.; Yakubova, R.; Tagayalieva, N. Glycyrrhizic acid and its derivatives as the carries for the poorly soluble flavonoids. Int. J. Disaster Recovery Bus. Contin. 2021, 12, 1–8. [Google Scholar]
- Batiha, G.E.S.; Beshbishy, A.M.; El-MLeeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar]
- Wen, Y.; Chen, H.; Zhang, L.; Wu, M.; Zhang, F.; Yang, D.; Shen, J.; Chen, J. Glycyrrhetinic acid induces oxidative/nitrative stress and drives ferroptosis through activating NADPH oxidases and iNOS, and depriving glutathione in triple-negative breast cancer cells. Free Radic. Biol. Med. 2021, 173, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.M.; Lin, H.J.; Hung, M.S.; Lin, Y.R.; Chan, M.H.; Lin, J.C. Inhibition of nuclear factor kappaB is associated with neuroprotective effects of glycyrrhizic acid on glutamate-induced excitotoxicity in primary neurons. Eur. J. Pharmacol. 2006, 547, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Meng, X.; Zhang, J.; Zhao, Y. Hepatoprotective effect of different combinations of 18α-and 18β-Glycyrrhizic acid against CCl4-induced liver injury in rats. Biomed. Pharmacother. 2020, 122, 109354. [Google Scholar] [CrossRef]
- Zhang, W.; Li, T.; Zhang, X.-J.; Zhu, Z.-Y. Hypoglycemic effect of glycyrrhizic acid, a natural non-carbohydrate sweetener, on streptozotocin-induced diabetic mice. Food Funct. 2020, 11, 4160–4170. [Google Scholar] [CrossRef] [PubMed]
- Baltina, L.; Kondratenko, R. Glycyrrhizic Acid Derivatives as New Antiviral and Immune Modulating Agents. Curr. Bioact. Compd. 2021, 17, 41–58. [Google Scholar] [CrossRef]
- Sun, Z.; He, G.; Huang, N.; Thilakavathy, K.; Lim, J.C.W.; Kumar, S.S.; Xiong, C. Glycyrrhizic acid: A natural plant ingredient as a drug candidate to treat COVID-19. Front. Pharmacol. 2021, 12, 707205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiao, Y.; Xu, L.; Liu, Y.; Jiang, G.; Wang, W.; Li, B.; Zhu, T.; Tan, Q.; Tang, H.; et al. Glycyrrhizic Acid Nanoparticles as Antiviral and Anti-inflammatory Agents for COVID-19 Treatment. ACS Appl. Mater. Interfaces 2021, 13, 20995–21006. [Google Scholar] [CrossRef]
- Gluschenko, O.Y.; Polyakov, N.E.; Leshina, T.V. NMR Relaxation Study of Cholesterol Binding with Plant Metabolites. Appl. Magn. Reson. 2011, 41, 283–294. [Google Scholar] [CrossRef]
- Matchanov, A.D.; Dalimov, D.N.; Zainutdinov, U.N.; Vypova, N.L.; Islamov, A.K.; Bekpolatova, B.M. Preparation and Physicochemical and Biological Properties of Molecular Associates of Lagochilin and Lagochirsine with Glycyrrhizic Acid and its Monoammonium Salt. Chem. Nat. Compd. 2017, 53, 665–669. [Google Scholar] [CrossRef]
- Yang, F.H.; Zhang, Q.; Liang, Q.Y.; Wang, S.Q.; Zhao, B.X.; Wang, Y.T.; Cai, Y.; Li, G.F. Bioavailability Enhancement of Paclitaxel via a Novel Oral Drug Delivery System: Paclitaxel-Loaded Glycyrrhizic Acid Micelles. Molecules 2015, 20, 4337–4356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M. Molecular dynamics simulations of glycyrrhizic acid aggregates as drug-carriers for paclitaxel. Curr. Drug Deliv. 2019, 16, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Kondratenko, R.M.; Baltina, L.A.; Mustafina, S.R.; Ismagilova, A.F.; Zarudii, F.S.; Davydova, V.A.; Bazekin, G.V.; Suleimanova, G.F.; Tolstikov, G.A. Complex compounds of glycyrrihizic acid with antimicrobial drugs. Pharm. Chem. J. 2003, 37, 485–488. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Rahman, F.; Chugtai, S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Liu, D.; Li, J. Pharmacological perspective: Glycyrrhizin may be an efficacious therapeutic agent for COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105995. [Google Scholar] [CrossRef]
- Yakovishin, L.A.; Korzh, E.N. Molecular complex of quercetin with glycyram. AIP Conf. Proc. 2019, 2063, 040066. [Google Scholar] [CrossRef]
- Zhao, F.-Q.; Wang, G.-F.; Xu, D.; Zhang, H.-Y.; Cui, Y.-L.; Wang, Q.-S. Glycyrrhizin mediated liver-targeted alginate nanogels delivers quercetin to relieve acute liver failure. Int. J. Biol. Macromol. 2021, 168, 93–104. [Google Scholar] [CrossRef]
- Kondratenko, R.M.; Baltina, L.A.; Mikhailova, L.R.; Danilov, V.T.; Gabbasov, T.M.; Murinov, Y.I.; Tolstikov, G.A. Preparation of glycyrrhizic acid and its practically important salts from licorice root extract. Chem. Pharm. J. 2005, 19, 30–35. [Google Scholar]
- Sekowski, S.; Naziris, N.; Chountoulesi, M.; Olchowik-Grabarek, E.; Czerkas, K.; Veiko, A.; Abdulladjanova, N.; Demetzos, C.; Zamaraeva, M. Interaction of Rhus typhina tannin with lipid nanoparticles: Implication for the formulation of a tannin-liposome hybrid biomaterial with antibacterial activity. J. Funct. Biometer. 2023, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Olchowik-Grabarek, E.; Sekowski, S.; Bitiucki, M.; Dobrzynska, I.; Shlyonsky, V.; Ionov, M.; Burzynski, P.; Roszkowska, A.; Swiecicka, I.; Abdulladjanova, N.; et al. Inhibition of interaction between Staphylococcus aureus α-hemolysin and erythrocytes membrane by hydrolysable tannins: Structure-related activity study. Sci. Rep. 2020, 10, 11168. [Google Scholar] [CrossRef] [PubMed]
- Olchowik, E.; Lotkowski, K.; Mavlyanov, S.; Abdullajanova, N.; Ionov, M.; Bryszewska, M.; Zamaraeva, M. Stabilization of erythrocytes against oxidative and hypotonic stress by tannins isolated from sumac leaves (Rhus typhina L.) and grape seeds (Vitis vinifera L.). Cell Mol. Biol. Lett. 2012, 17, 333–348. [Google Scholar] [CrossRef]
- Molina-Bolivar, J.A.; Carnero Ruiz, C.; Galisteo-Gonzales, F.; Medona-O’ Donnell, M.; Parra, A. Simultaneous presence of dynamic and sphere action component in the fluorescence quenching of human serum albumin by diphthaloylmaslinic acid. J. Lumin. 2016, 178, 259–266. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Cruz-Martins, N.; Nepovimova, E.; Oleksak, P.; Dhanjal, D.S.; Bhardwaj, S.; Singh, R.; Chopra, C.; Verma, R.; et al. Applications of Fruit Polyphenols and Their Functionalized Nanoparticles Against Foodborne Bacteria: A Mini Review. Molecules 2021, 26, 3447. [Google Scholar] [CrossRef]
- Thakur, P.; Chawla, R.; Narula, A.; Goel, R.; Arora, R.; Sharma, R.K. Anti-hemolytic, hemagglutination inhibition and bacterial membrane disruptive properties of selected herbal extracts attenuate virulence of Carbapenem Resistant Escherichia coli. Microb. Pathog. 2016, 95, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, N.; Yahiro, K.; Noda, M. Resveratrol, a natural polyphenolic compound, inhibits cholera toxin-induced cyclic AMP accumulation in Vero cells. Toxicon 2010, 56, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Tombola, F.; Campello, S.; De Luca, L.; Ruggiero, P.; Del Giudice, G.; Papini, E.; Zoratti, M. Plant polyphenols inhibit VacA, a toxin secreted by the gastric pathogen Helicobacter pylori. FEBS Lett. 2003, 543, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostroumova, O.S.; Efimova, S.S.; Schagina, L.S. 5- and 4′-Hydroxylated flavonoids affect voltage gating of single alpha-hemolysin pore. Bioch. Biophys. Acta 2011, 1808, 2051–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olchowik-Grabarek, E.; Sekowski, S.; Mies, F.; Bitiucki, M.; Swiecicka, I.; Abdulladjanova, N.; Shlyonsky, V.; Zamaraeva, M. Electrophysiological and spectroscopic investigation of hydrolysable tannins interaction with α-hemolysin of S. aureus. Bioelectrochemistry 2023, 150, 108318. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef]
- Matchanov, A.D.; Zaynutdinov, U.N.; Islamov, A.K.; Vipova, N.L.; Tashpulatov, F.N.; Matchanov, U.D. Supramolecular Complexes of Glycyrrhizic Acid, its Monoammonium Salt with Diterpenoid Lagochilin and their Hemostatic Activity. Biochem. Ind. J. 2017, 11, 118–123. [Google Scholar]
- Matchanov, A.D.; Zaynutdinov, U.N.; Islamov, A.K.; Vypova, N.L.; Tashpulatov, F.N.; Esanov, R.S.; Matchanov, U.D.; Sobirova, F.A.; Khakberdiev, S.M. Synthesis and hemostatic activity of supramolecular complexes lagochilin. Int. J. Dev. Res. 2018, 8, 19812–19814. [Google Scholar]
- Matmuratov, B.Y.A.; Madraximova, S.D.; Esanov, R.S.; Eshchanov, E.U.; Matchanov, A.D.; Xakberdiev, S.M. Supramolecular complexes of kinetin and adenin with glycyrrhizin acid and its monoammonium salt. Ann. Phytomed. Int. J. 2021, 10, 113–118. [Google Scholar]
- Los, F.C.O.; Randis, T.M.; Raffi, R.V.; Ratner, A.J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Veiko, A.G.; Olchowik-Grabarek, E.; Sekowski, S.; Roszkowska, A.; Lapshina, E.A.; Dobrzynska, I.; Zamaraeva, M.; Zavodnik, I.B. Antimicrobial activity of quercetin, naringenin and catechin: Flavonoids inhibit Staphylococcus aureus-induced hemolysis and modify membranes of bacteria and erythrocytes. Molecules 2023, 28, 1252. [Google Scholar] [CrossRef] [PubMed]
- Escajadillo, T.; Nizet, V. Pharmacological Targeting of Pore-Forming Toxins as Adjunctive Therapy for Invasive Bacterial Infection. Toxins 2018, 10, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divyakolu, S.; Chikkala, R.; Ratnakar, K.S.; Sritharan, V. Hemolysins of Staphylococcus aureus —An Update on Their Biology, Role in Pathogenesis and as Targets for Anti-Virulence Therapy. Adv. Infect. Dis. 2019, 9, 80–104. [Google Scholar]
- Qiu, J.; Niu, X.; Dong, J.; Wang, D.; Wang, J.; Li, H.; Luo, M.; Li, S.; Feng, H.; Deng, X. Baicalin protects mice from Staphylococcus aureus pneumonia via inhibition of the cytolytic activity of α-hemolysin. J. Infect. Dis. 2012, 206, 292–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhou, X.; Liu, S.; Li, G.; Shi, L.; Dong, J.; Li, W.; Deng, X.; Niu, X. Morin hydrate attenuates Staphylococcus aureus virulence by inhibiting the self-assembly of α-hemolysin. J. Appl. Microbiol. 2015, 118, 753–763. [Google Scholar] [CrossRef]
- Silva, L.N.A.; Da Hora, G.C.A.; Soares, T.A.; Bojer, M.S.; Ingmer, H.; Macedo, A.J.; Trentin, D.S. Myricetin protects Galleria mellonella against Staphylococcus aureus infection and inhibits multiple virulence factors. Sci. Rep. 2017, 7, 2823. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Deng, Q.; Liang, B.; Yu, F.; Yu, X.; Guo, D.; Liu, X.; Dong, H. Suppressing Alpha-Hemolysin as Potential Target to Screen of Flavonoids to Combat Bacterial Coinfection. Molecules 2021, 26, 7577. [Google Scholar] [CrossRef] [PubMed]
- Olchowik-Grabarek, E.; Mies, F.; Sekowski, S.; Dubis, A.; Laurent, P.; Zamaraeva, M.; Swiecicka, I.; Shlyonsky, V. Enzymatic Synthesis and Characterization of Aryl Iodides of Some Phenolic Acids with Enhanced Antibacterial Properties. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184011. [Google Scholar] [CrossRef]
- Berube, B.J.; Wardenburg, J.B. Staphylococcus aureus α-Toxin: Nearly a Century of Intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, T.; Yamashita, D.; Kato, K.; Peng, Z.; Ueda, J.; Kaneko, J.; Kamio, Y.; Tanaka, Y.; Yao, M. Structural basis for pore-forming mechanism of staphylococcal α-hemolysin. Toxicon 2015, 108, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwiering, M.; Brack, A.; Stork, R.; Hellmann, N. Lipid and phase specificity of α-toxin from S. aureus. Biochim. Biophys. Acta 2013, 1828, 1962–1972. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Zhang, P.; Quan, P.; Cui, M.; Liu, T.; Yin, Y.; Chi, G. Quercetin, a pneumolysin inhibitor, protects mice against Streptococcus pneumoniae infection. Microb. Pathog. 2020, 140, 103934. [Google Scholar] [CrossRef]
- Bose, A. Interaction of tea polyphenols with serum albumins: A fluorescence spectroscopic analysis. J. Lumin. 2016, 169, 220–226. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Papadopoulou, A.; Green, R.J.; Frazier, R.A. Interaction of Flavonoids with Bovine Serum Albumin: A Fluorescence Quenching Study. J. Agric. Food Chem. 2005, 53, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Mateus, N.; de Freitas, V. Interaction of different polyphenols with bovine serum albumin (BSA) and human salivary alpha-amylase (HSA) by fluorescence quenching. J. Agric. Food Chem. 2007, 55, 6726–6735. [Google Scholar] [CrossRef]
- Zhou, N.; Liang, Y.-Z.; Wang, P. 18_-Glycyrrhetinic acid interaction with bovine serum albumin. J. Photochem. Photobiol. A Chem. 2007, 185, 271–276. [Google Scholar] [CrossRef]
- Kameníková, M.; Furtmüller, P.G.; Klacsová, M.; Lopez-Guzman, A.; Toca-Herrera, J.L.; Vitkovská, A.; Devínsky, F.; Mučaji, P.; Nagy, M. Influence of quercetin on the interaction of gliclazide with human serum albumin—Spectroscopic and docking approaches. Luminescence 2017, 32, 1203–1211. [Google Scholar] [CrossRef]
- Vaneková, Z.; Hubčík, L.; Toca-Herrera, J.L.; Furtmüller, P.G.; Valentová, J.; Mučaji, P.; Nagy, M. Study of Interactions between AmLodipine and Quercetin on Human Serum Albumin: Spectroscopic and Modeling Approaches. Molecules 2019, 24, 487. [Google Scholar] [CrossRef] [Green Version]
- Dufour, C.; Dangles, O. Flavonoid-serum albumin complexation: Determination of binding constants and binding sites by fluorescence spectroscopy. Biochim. Biophys. Acta 2005, 1721, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, V.D.; Walekar, L.S.; Gore, A.H.; Anbhule, P.V.; Kolekar, G.B. Spectroscopic analysis on the binding interaction of biologically active pyrimidine derivative with bovine serum albumin. J. Pharm. Anal. 2016, 6, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
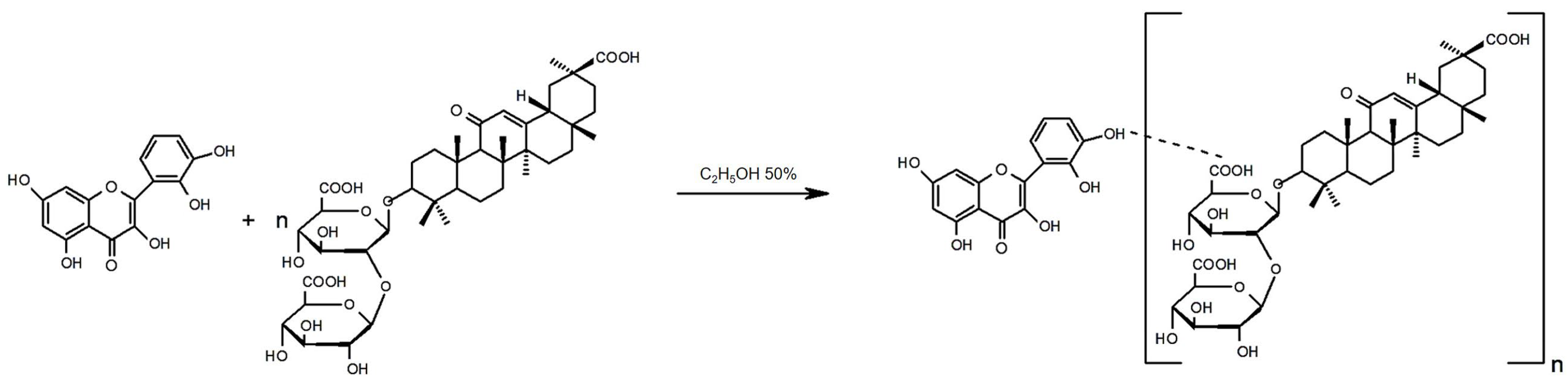
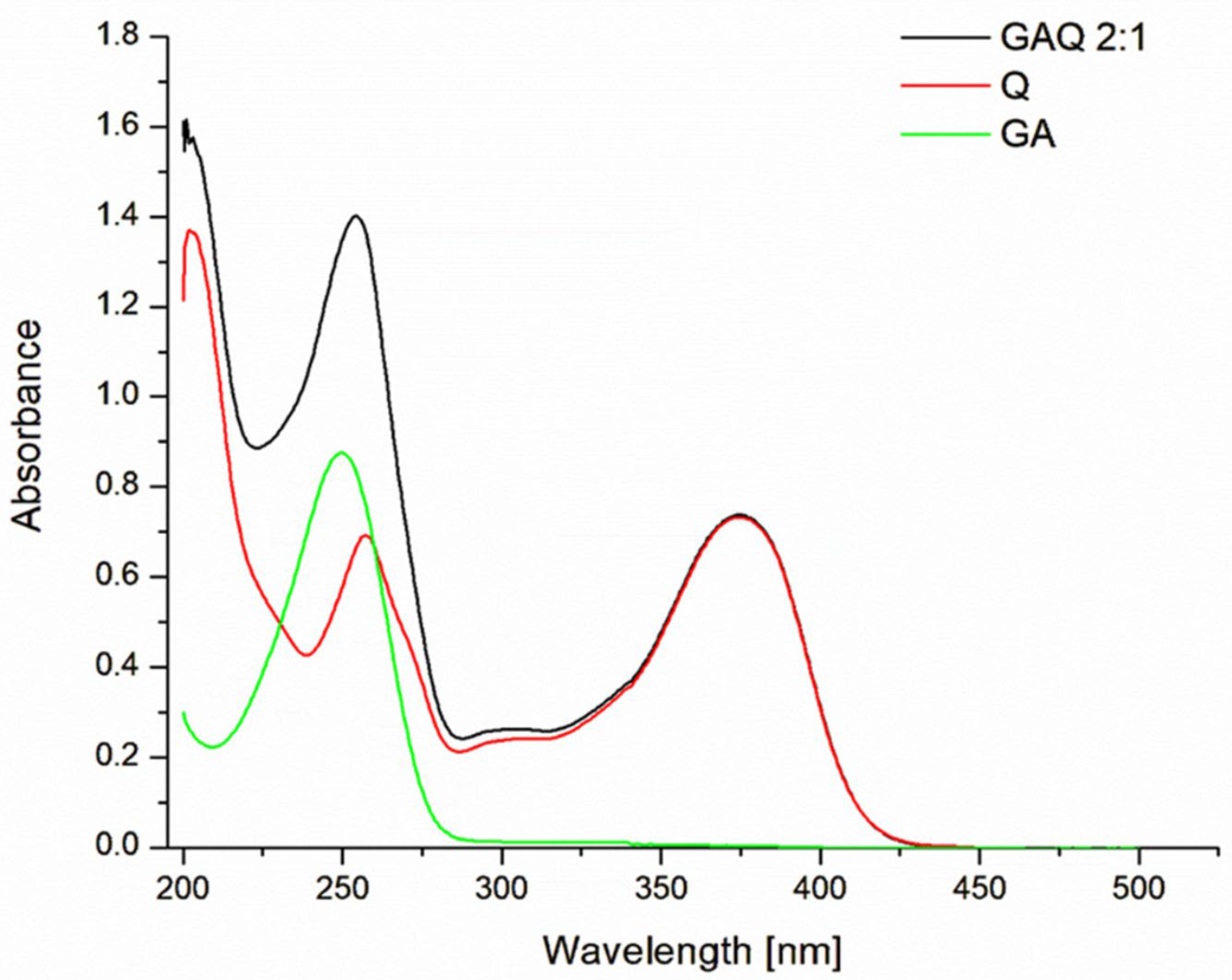


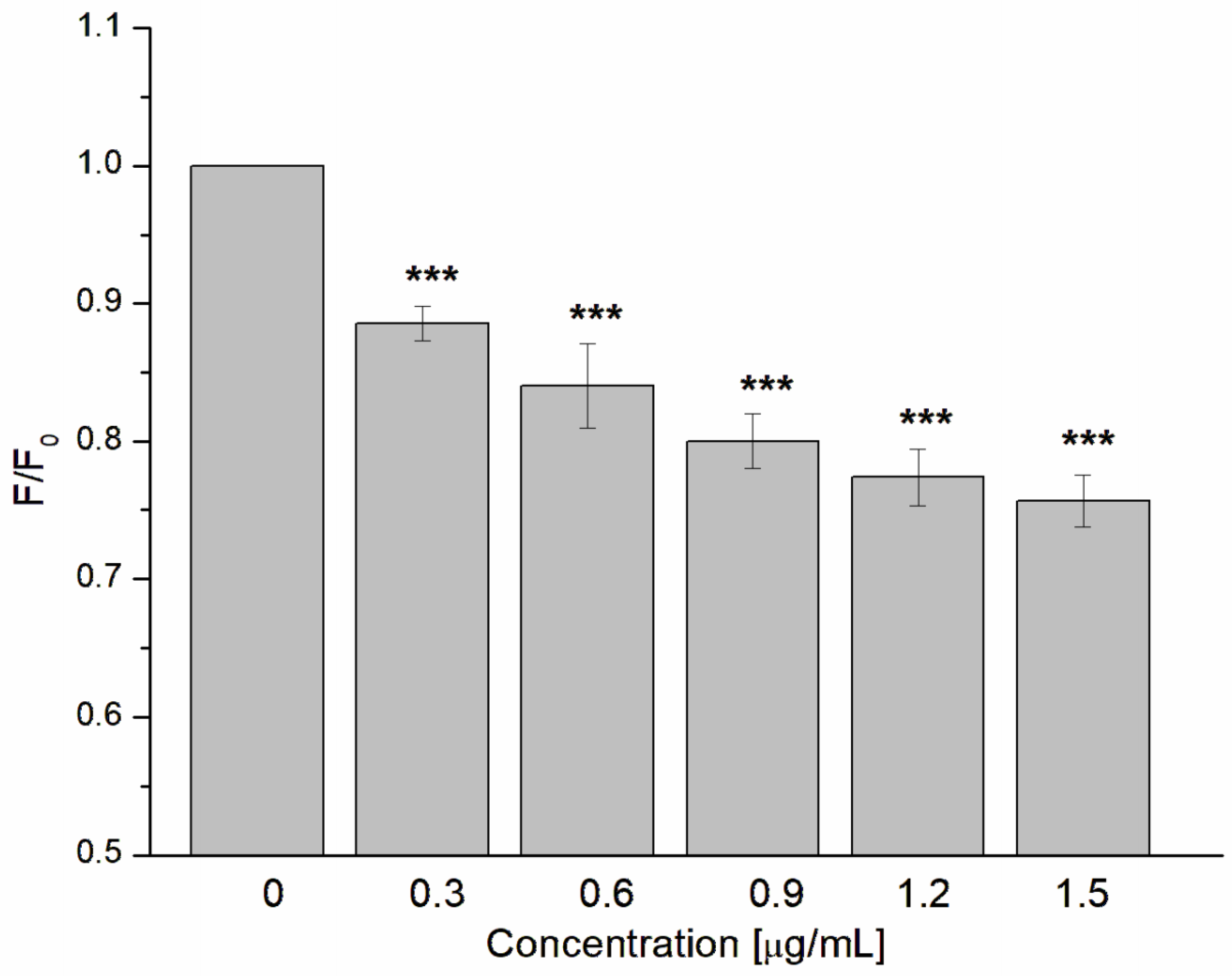
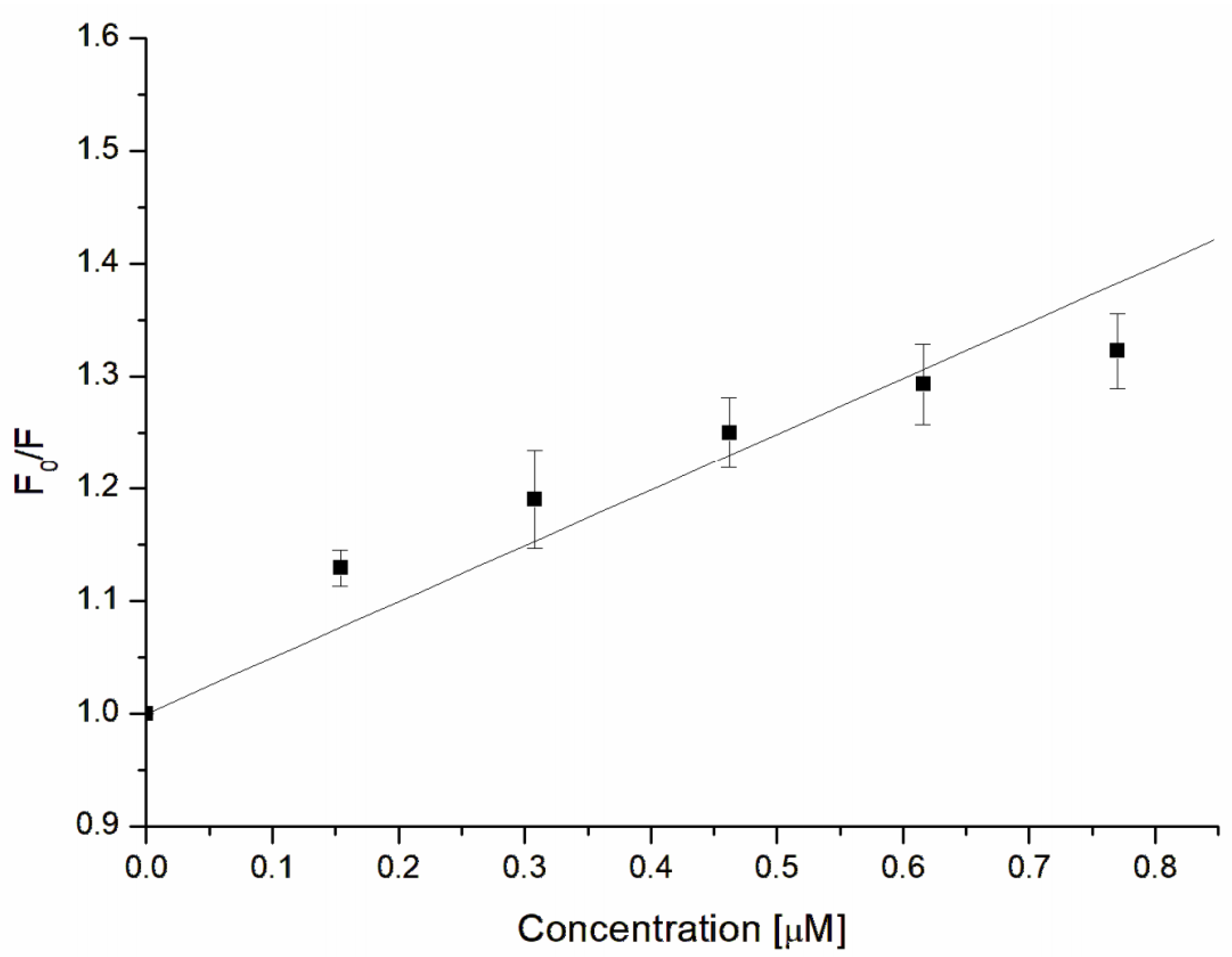
| Complexes | Relation-ships | Melting Point. °C with Decomposition | Water | Ethanol 50% | Acetone | Hexane | Chloroform |
|---|---|---|---|---|---|---|---|
| GAQ | 2:1 | 160–163 | + | + | − | − | − |
| Compounds | IR spectrum, cm−1 | ||||||
| Q | 3600−3000 (OH), 1666−1613 (C = O), 1100−1000 (C-O-C) | ||||||
| GA | 3407 (OH), 1715 (C = O), 1654 (=C-C = O) | ||||||
| GAQ 2:1 | 1600, 1655, 1726 (C = O), 3400−3000 (OH) | ||||||
| MIC | MBC | |
|---|---|---|
| GAQ [µg/mL] | 487 | 1948 |
| GAQ [µM] | 250 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olchowik-Grabarek, E.; Czerkas, K.; Matchanov, A.D.; Esanov, R.S.; Matchanov, U.D.; Zamaraeva, M.; Sekowski, S. Antibacterial and Antihemolytic Activity of New Biomaterial Based on Glycyrrhizic Acid and Quercetin (GAQ) against Staphylococcus aureus. J. Funct. Biomater. 2023, 14, 368. https://doi.org/10.3390/jfb14070368
Olchowik-Grabarek E, Czerkas K, Matchanov AD, Esanov RS, Matchanov UD, Zamaraeva M, Sekowski S. Antibacterial and Antihemolytic Activity of New Biomaterial Based on Glycyrrhizic Acid and Quercetin (GAQ) against Staphylococcus aureus. Journal of Functional Biomaterials. 2023; 14(7):368. https://doi.org/10.3390/jfb14070368
Chicago/Turabian StyleOlchowik-Grabarek, Ewa, Krzysztof Czerkas, Alimjon Davletboevich Matchanov, Rahmat Sulton Esanov, Umarbek Davlatboevich Matchanov, Maria Zamaraeva, and Szymon Sekowski. 2023. "Antibacterial and Antihemolytic Activity of New Biomaterial Based on Glycyrrhizic Acid and Quercetin (GAQ) against Staphylococcus aureus" Journal of Functional Biomaterials 14, no. 7: 368. https://doi.org/10.3390/jfb14070368





