Effect of Mechanical Microenvironment on Collagen Self-Assembly In Vitro
Abstract
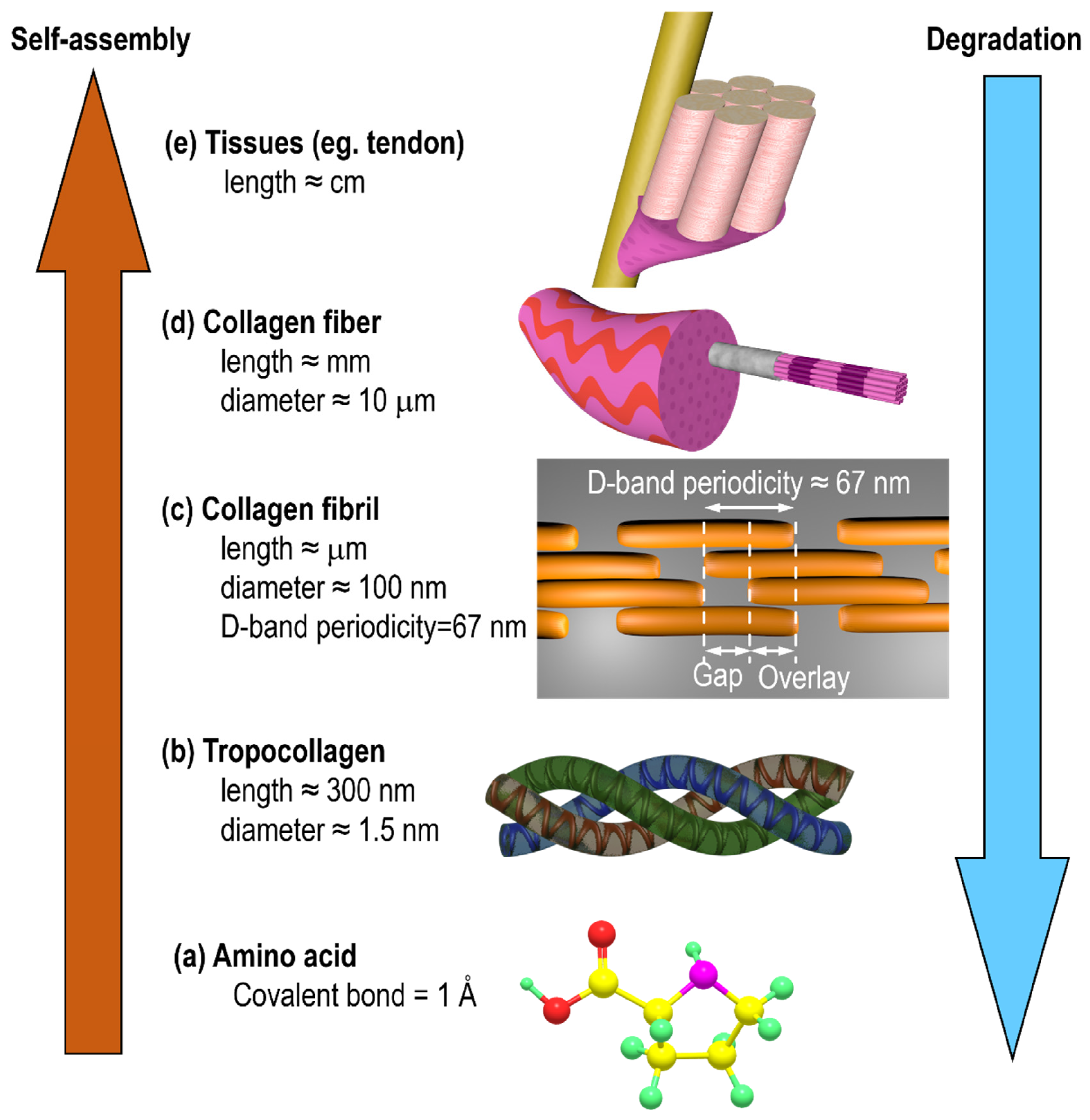
:1. Introduction
2. Methods and Materials
2.1. PDMS Membrane Preparation
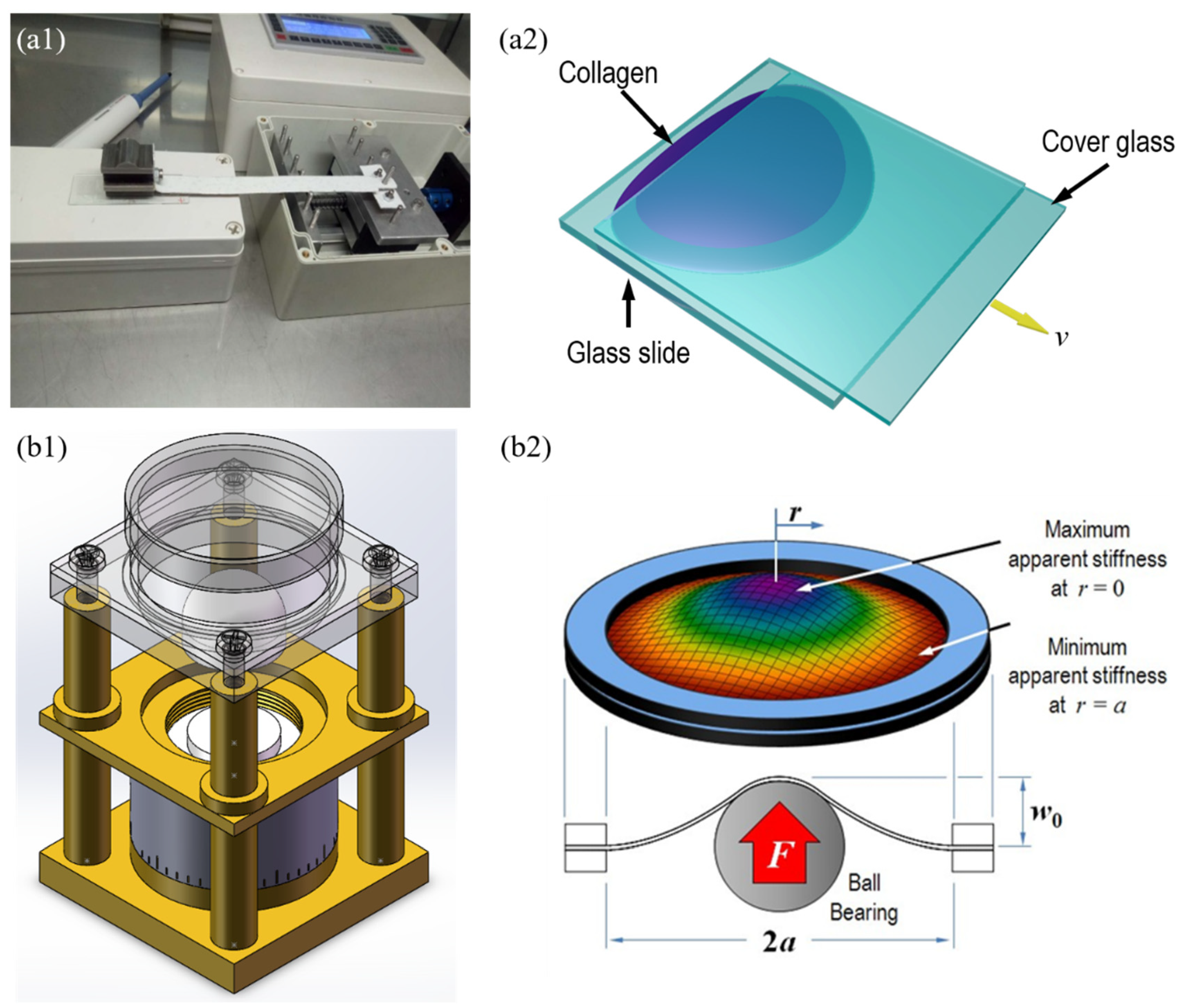
2.2. Collagen Fibers Formed on the Stretching Device
2.3. Observation of Collagen Fibers Formed on Muscovite by SEM
2.4. Observation of Collagen Fibers Formed in Carbon Network by TEM
2.5. AFM Observation of Collagen Fibers Formed under Mechanical Loading Device
3. Results and Discussion
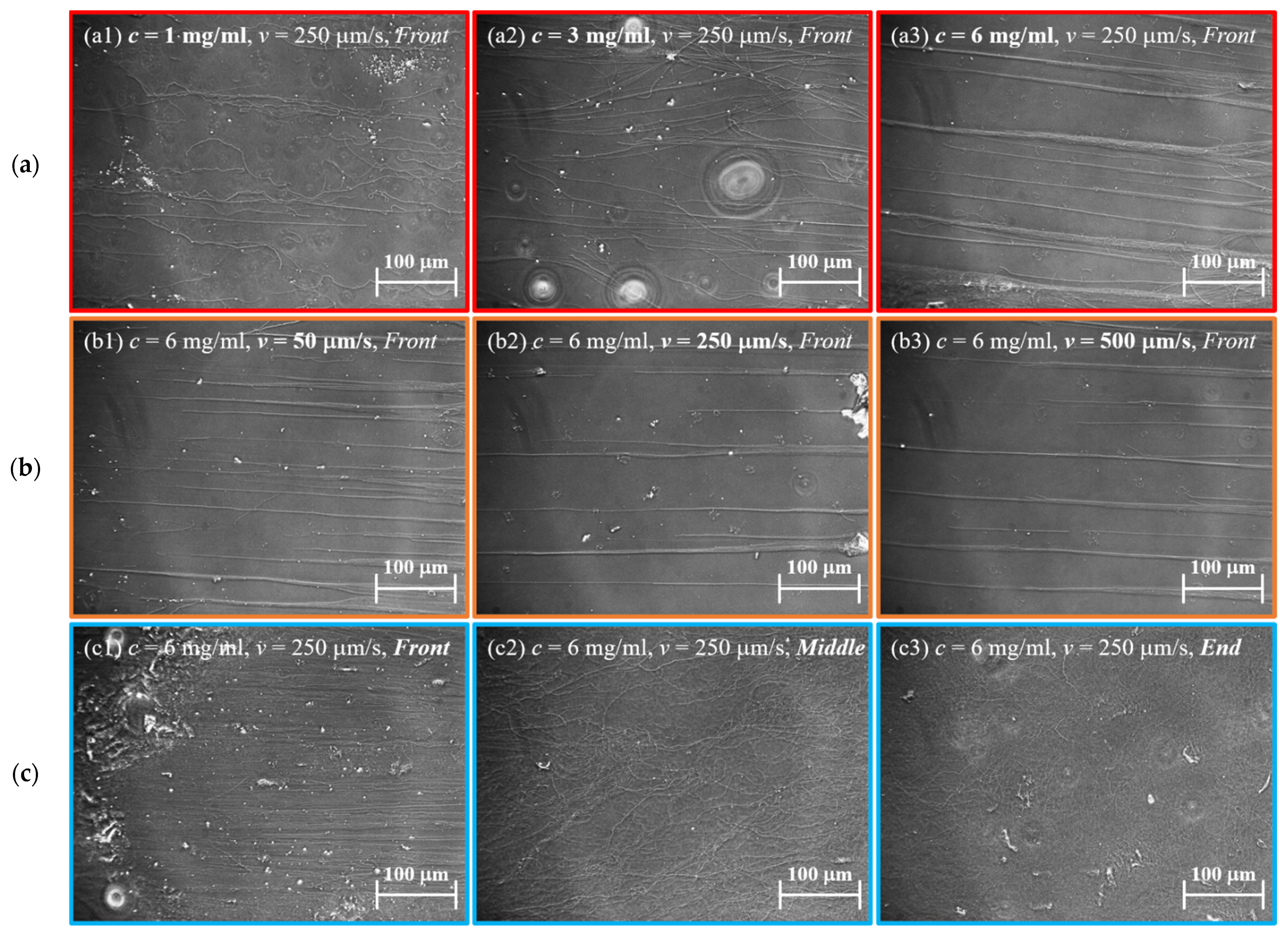
3.1. Observation of Collagen Fibers Formed on the Stretching Device under Optical Microscope
3.2. Observation of Collagen Fibers by SEM and TEM
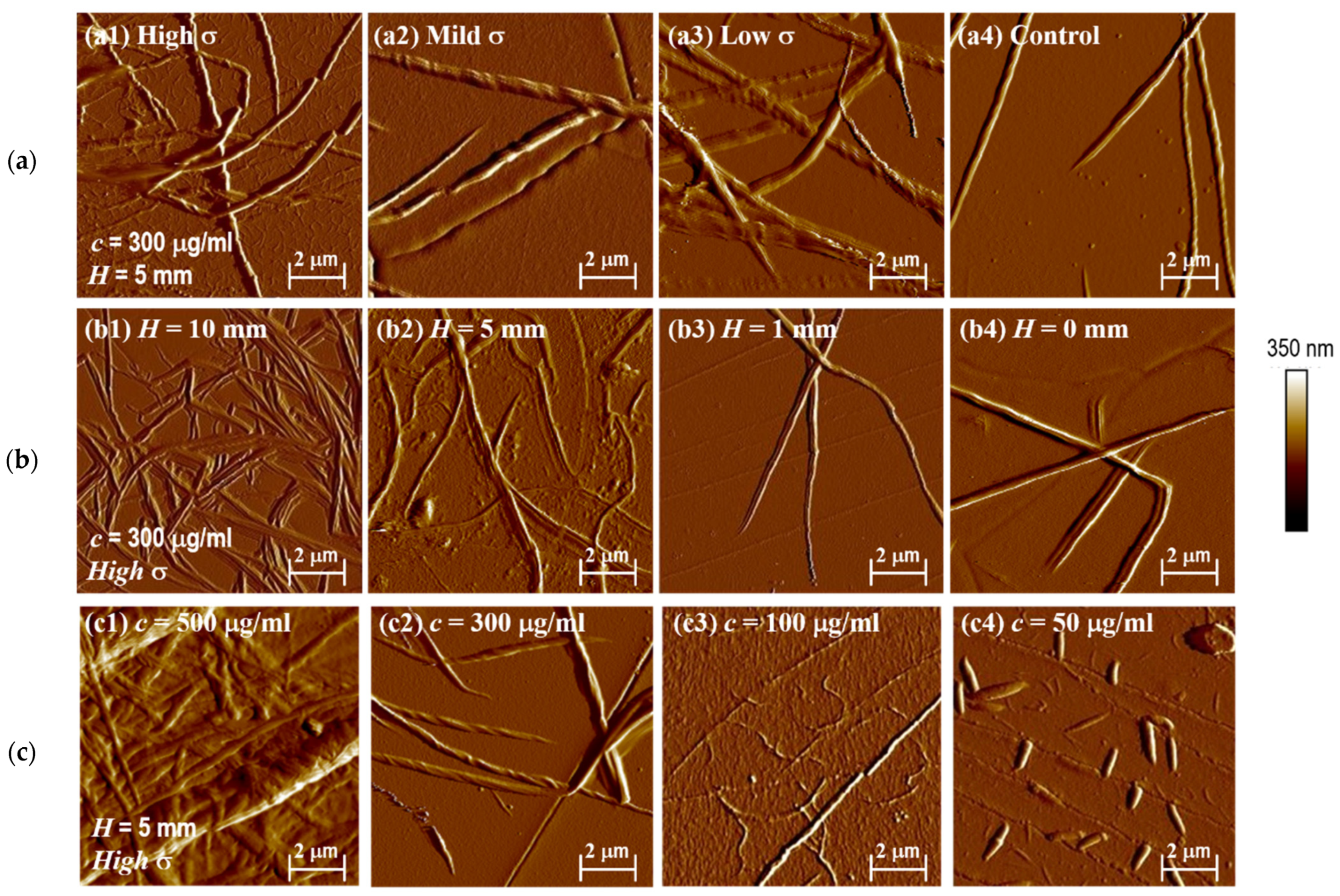
3.3. Observation of Collagen Fibers under AFM
3.4. Effect of Loading Height of Mechanical Loader on Collagen Self-Assembly
3.5. Effect of Concentration on Collagen Self-Assembly
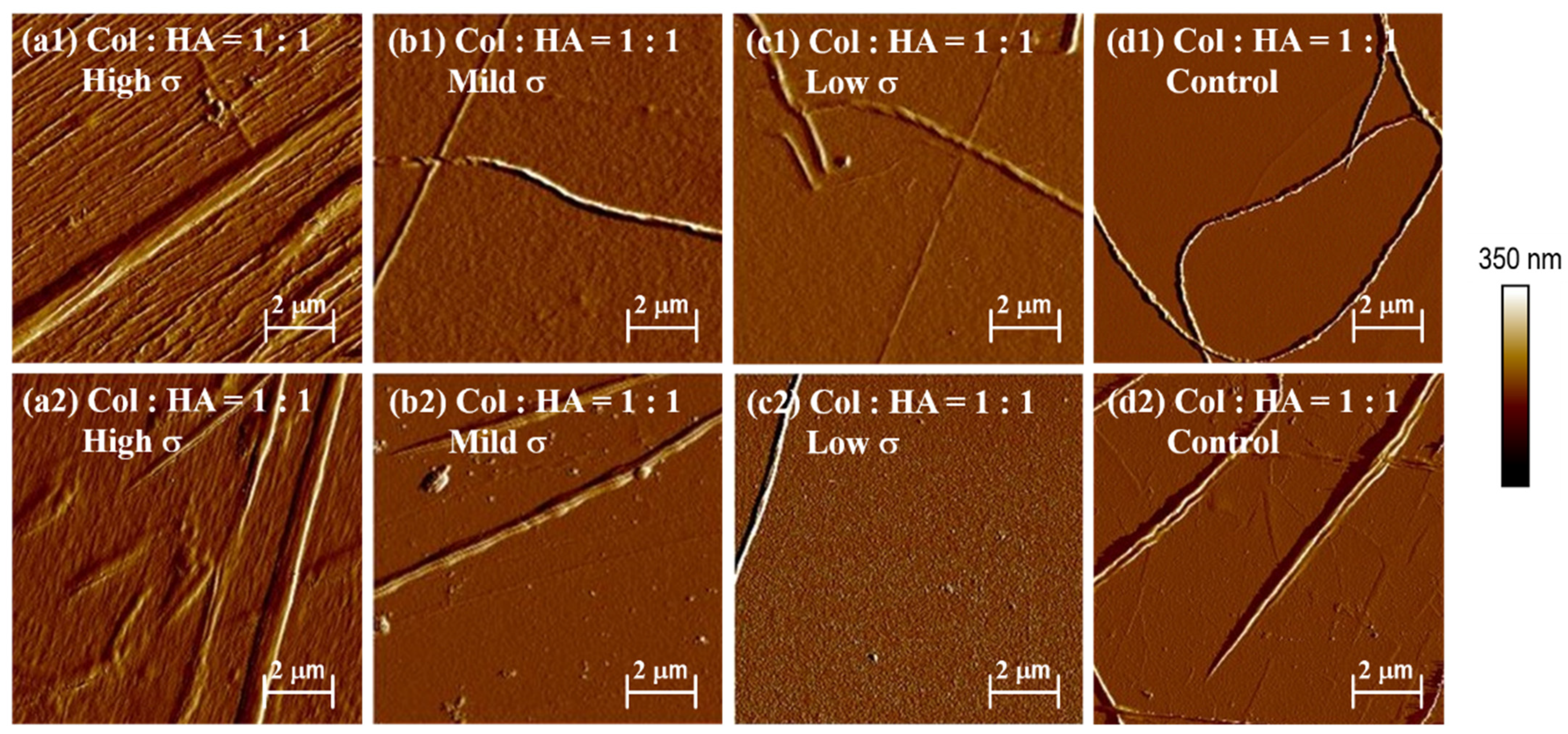
3.6. Effect of HA on Collagen Self-Assembly
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hatami-Marbini, H.; Emu, M.E. Effect of corneal collagen crosslinking on viscoelastic shear properties of the cornea. J. Mech. Behav. Biomed. Mater. 2022, 133, 105300. [Google Scholar] [CrossRef] [PubMed]
- Janvier, A.J.; Janvier, A.J.; Pendleton, E.G.; Mortensen, L.J.; Green, D.C.; Henstock, J.R.; Canty-Laird, E.G. Multimodal analysis of the differential effects of cyclic strain on collagen isoform composition, fibril architecture and biomechanics of tissue engineered tendon. J. Tissue Eng. 2022, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Vedhanayagam, M.; Kumar, A.S.; Nair, B.U.; Sreeram, K.J. Dendrimer-Functionalized Metal Oxide Nanoparticle-Mediated Self-Assembled Collagen Scaffold for Skin Regenerative Application: Function of Metal in Metal Oxides. Appl. Biochem. Biotechnol. 2022, 194, 266–290. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Shang, Y.; Ren, B.; Deng, S.; Wang, Z.; Peng, Y.; Huang, Z.; Ma, S.; Peng, C.; Hou, S. A novel natural-derived tilapia skin collagen mineralized with hydroxyapatite as a potential bone-grafting scaffold. J. Biomater. Appl. 2022, 37, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, H.; Miao, Y.; Xiong, S.; Hu, Y. Recent strategies of collagen-based biomaterials for cartilage repair: From structure cognition to function endowment. J. Leather Sci. Eng. 2022, 4, 11. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Raspanti, M.; Viola, M.; Sonaggere, M.; Tira, M.E.; Tenni, R. Collagen fibril structure is affected by collagen concentration and decorin. Biomacromolecules 2007, 8, 2087–2091. [Google Scholar] [CrossRef]
- Song, X.; Wang, Z.; Tao, S.; Li, G.; Zhu, J. Observing Effects of Calcium/Magnesium Ions and pH Value on the Self-Assembly of Extracted Swine Tendon Collagen by Atomic Force Microscopy. J. Food Qual. 2017, 2017, 9257060. [Google Scholar] [CrossRef]
- Stylianou, A.; Yova, D. Surface nanoscale imaging of collagen thin films by Atomic Force Microscopy. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2947–2957. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Lee, M.-S.; Zhang, Z.; Li, B.; Xuan, H.; Li, W.-J.; Zhang, Y. Collagen and chondroitin sulfate functionalized bioinspired fibers for tendon tissue engineering application. Int. J. Biol. Macromol. 2021, 170, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Gizzi, A.; De Bellis, M.L.; Vasta, M.; Pandolfi, A. Diffusion-based degeneration of the collagen reinforcement in the pathologic human cornea. J. Eng. Math. 2021, 127, 1–10. [Google Scholar] [CrossRef]
- Hoogenkamp, H.R.; Bakker, G.-J.; Wolf, L.; Suurs, P.; Dunnewind, B.; Barbut, S.; Friedl, P.; van Kuppevelt, T.H.; Daamen, W.F. Directing collagen fibers using counter-rotating cone extrusion. Acta Biomater. 2015, 12, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Gunn, S.; Sizeland, K.H.; Wells, H.C.; Haverkamp, R.G. Collagen arrangement and strength in sausage casings produced from natural intestines. Food Hydrocoll. 2022, 129, 107612. [Google Scholar] [CrossRef]
- Paten, J.A.; Siadat, S.M.; Susilo, M.E.; Ismail, E.N.; Stoner, J.L.; Rothstein, J.P.; Ruberti, J.W. Flow-Induced Crystallization of Collagen: A Potentially Critical Mechanism in Early Tissue Formation. ACS Nano 2016, 10, 5027–5040. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Chi, Y.; Wang, Y.; Jiang, L.; Xu, N.; Wu, Q.; Feng, Q.; Sun, X. Preparation of oriented collagen fiber scaffolds and its application in bone tissue engineering. Appl. Mater. Today 2021, 22, 100902. [Google Scholar] [CrossRef]
- Ruberti, J.W.; Zieske, J.D. Prelude to corneal tissue engineering–gaining control of collagen organization. Prog. Retin. Eye Res. 2008, 27, 549–577. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Z.; Ma, Y.; Chiou, B.-S.; Liu, F.; Zhong, F. Modulating physicochemical properties of collagen films by cross-linking with glutaraldehyde at varied pH values. Food Hydrocoll. 2022, 124, 107270. [Google Scholar] [CrossRef]
- Lim, H.-S.; Lee, S.H.; Seo, H.; Lee, H.-H.; Yoon, K.; Kim, Y.-U.; Park, M.-K.; Chung, J.H.; Lee, Y.-S.; Lee, D.H.; et al. Early stage ultraviolet irradiation damage to skin collagen can be suppressed by HPA axis control via controlled CYP11B. Biomed. Pharmacother. 2022, 155, 113716. [Google Scholar] [CrossRef]
- Brownfield, D.G.; Venugopalan, G.; Lo, A.; Mori, H.; Tanner, K.; Fletcher, D.A.; Bissell, M.J. Patterned collagen fibers orient branching mammary epithelium through distinct signaling modules. Curr. Biol. 2013, 23, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Pins, G.D.; Huang, E.K.; Christiansen, D.L.; Silver, F.H. Effects of static axial strain on the tensile properties and failure mechanisms of self-assembled collagen fibers. J. Appl. Polym. Sci. 1997, 63, 1429–1440. [Google Scholar] [CrossRef]
- Wilson, D.L.; Martin, R.; Hong, S.; Cronin-Golomb, M.; Mirkin, C.A.; Kaplan, D.L. Surface organization and nanopatterning of collagen by dip-pen nanolithography. Proc. Natl. Acad. Sci. USA 2001, 98, 13660–13664. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, K.K.; Lakhani, P.; Kumar, S.; Kumar, N. Frequency dependent inelastic response of collagen architecture of pig dermis under cyclic tensile loading: An experimental study. J. Mech. Behav. Biomed. Mater. 2020, 112, 104030. [Google Scholar] [CrossRef]
- Buehler, M.J. Nanomechanics of collagen fibrils under varying cross-link densities: Atomistic and continuum studies. J. Mech. Behav. Biomed. Mater. 2008, 1, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Depalle, B.; Qin, Z.; Shefelbine, S.J.; Buehler, M.J. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J. Mech. Behav. Biomed. Mater. 2015, 52, 1–13. [Google Scholar] [CrossRef]
- Uzel, S.G.; Buehler, M.J. Molecular structure, mechanical behavior and failure mechanism of the C-terminal cross-link domain in type I collagen. J. Mech. Behav. Biomed. Mater. 2011, 4, 153–161. [Google Scholar] [CrossRef]
- Davidenko, N.; Campbell, J.J.; Thian, E.S.; Watson, C.J.; Cameron, R.E. Collagen-hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef]
- Gisbert, V.G.; Benaglia, S.; Uhlig, M.R.; Proksch, R.; Garcia, R. High-Speed Nanomechanical Mapping of the Early Stages of Collagen Growth by Bimodal Force Microscopy. ACS Nano 2021, 15, 1850–1857. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Lin, J.; Du, C.; Zhang, C.; Wang, X.; Feng, Q. Effect of Mechanical Microenvironment on Collagen Self-Assembly In Vitro. J. Funct. Biomater. 2023, 14, 235. https://doi.org/10.3390/jfb14040235
Han L, Lin J, Du C, Zhang C, Wang X, Feng Q. Effect of Mechanical Microenvironment on Collagen Self-Assembly In Vitro. Journal of Functional Biomaterials. 2023; 14(4):235. https://doi.org/10.3390/jfb14040235
Chicago/Turabian StyleHan, Leihan, Jiexiang Lin, Chengfei Du, Chunqiu Zhang, Xin Wang, and Qijin Feng. 2023. "Effect of Mechanical Microenvironment on Collagen Self-Assembly In Vitro" Journal of Functional Biomaterials 14, no. 4: 235. https://doi.org/10.3390/jfb14040235





