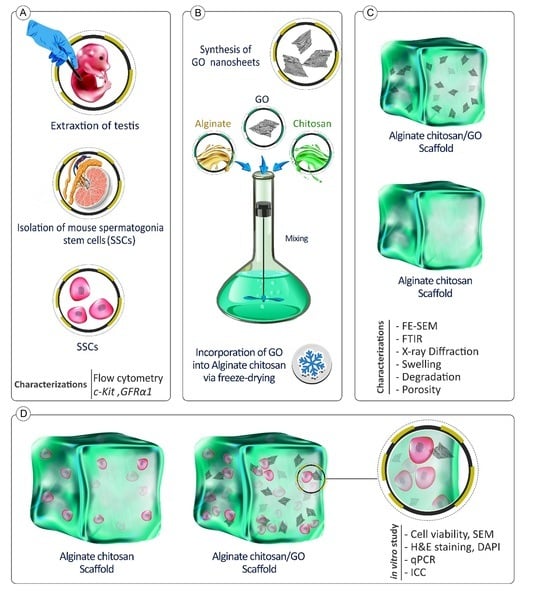
The Effect of Chitosan/Alginate/Graphene Oxide Nanocomposites on Proliferation of Mouse Spermatogonial Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of GO and Preparation of Scaffolds
2.1.1. Synthesis of GO
2.1.2. Fabrication of Alginate/Chitosan Scaffolds
2.1.3. Preparation of ALGCS/GO Scaffolds
2.2. Characterization of the ALGCS Scaffolds
2.2.1. FTIR
2.2.2. Scanning Electron Microscopy
2.2.3. X-ray Diffraction
2.2.4. Mechanical Properties
2.2.5. Swelling Studies
2.2.6. Hydrolytic Degradation
2.2.7. Measurement of Porosity
2.3. Cell Culture and In Vitro Studies
2.3.1. Isolation of Mouse Spermatogonial Stem Cells
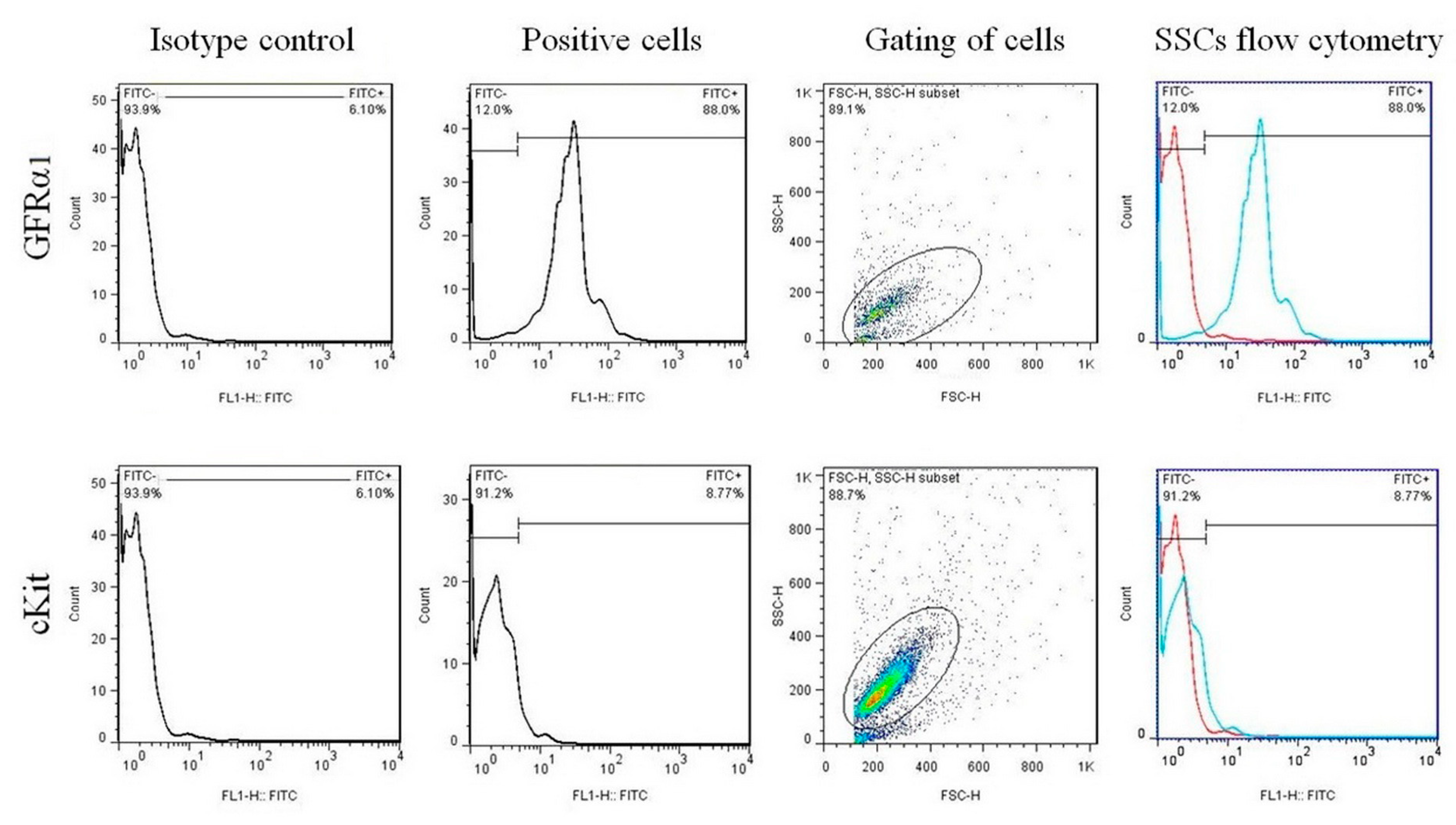
2.3.2. Cell Confirmation by Flow Cytometry
2.3.3. Cell Viability Studies
2.3.4. H&E Staining
2.3.5. Cellular Adhesion
2.3.6. qRT-PCR Assay
2.3.7. Immunocytochemistry Assay
2.4. Statistical Analysis
3. Results
3.1. Flow Cytometry Analysis for SSCs
3.2. Scaffold Characterization
3.2.1. FTIR Spectra
3.2.2. XRD Analysis
3.2.3. Mechanical Properties
3.2.4. Cell Viability on the Scaffolds
3.2.5. Swelling Behavior and Weight Loss
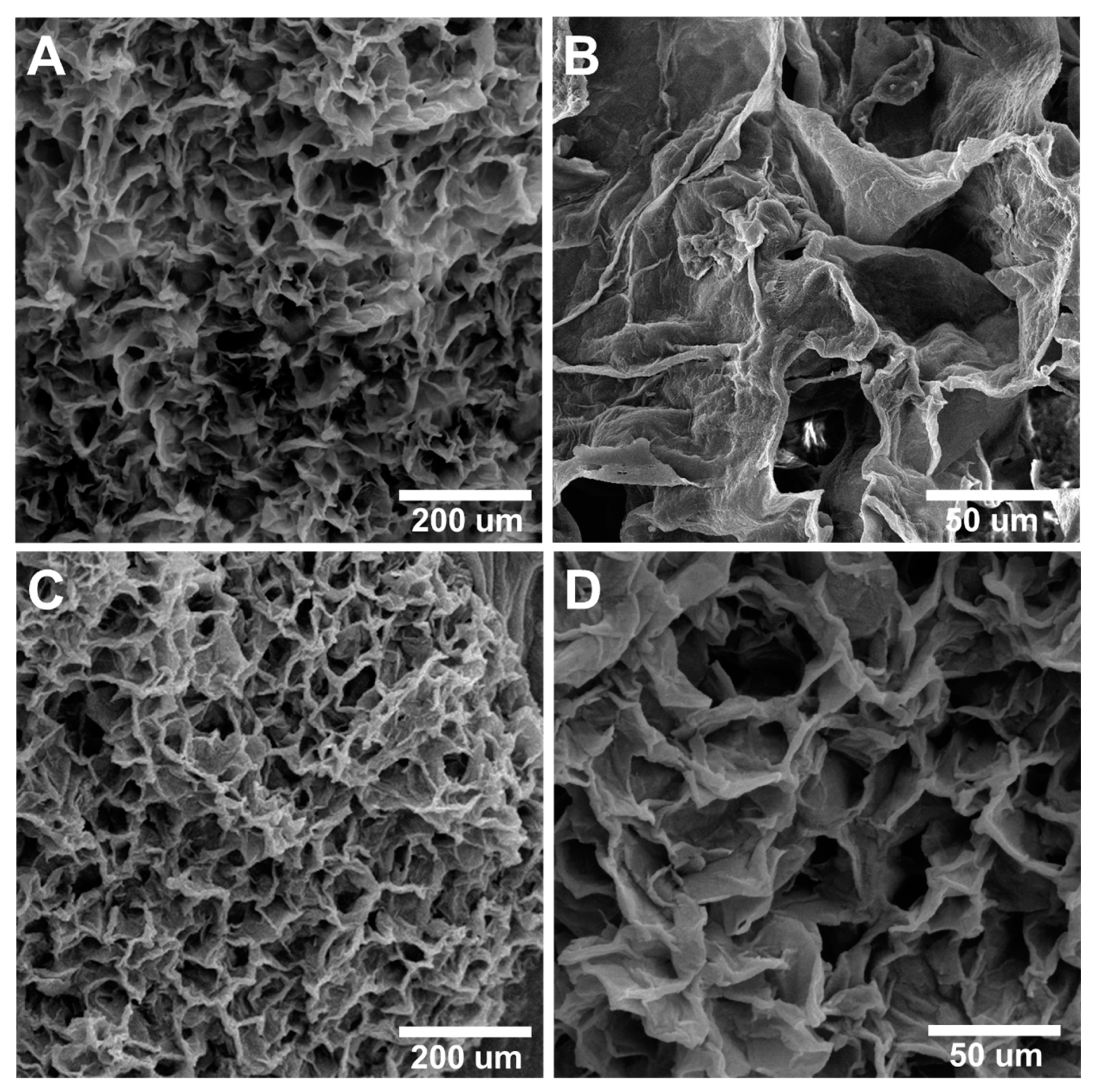
3.2.6. Morphology, Porosity, and Pore Size of the Scaffolds by SEM
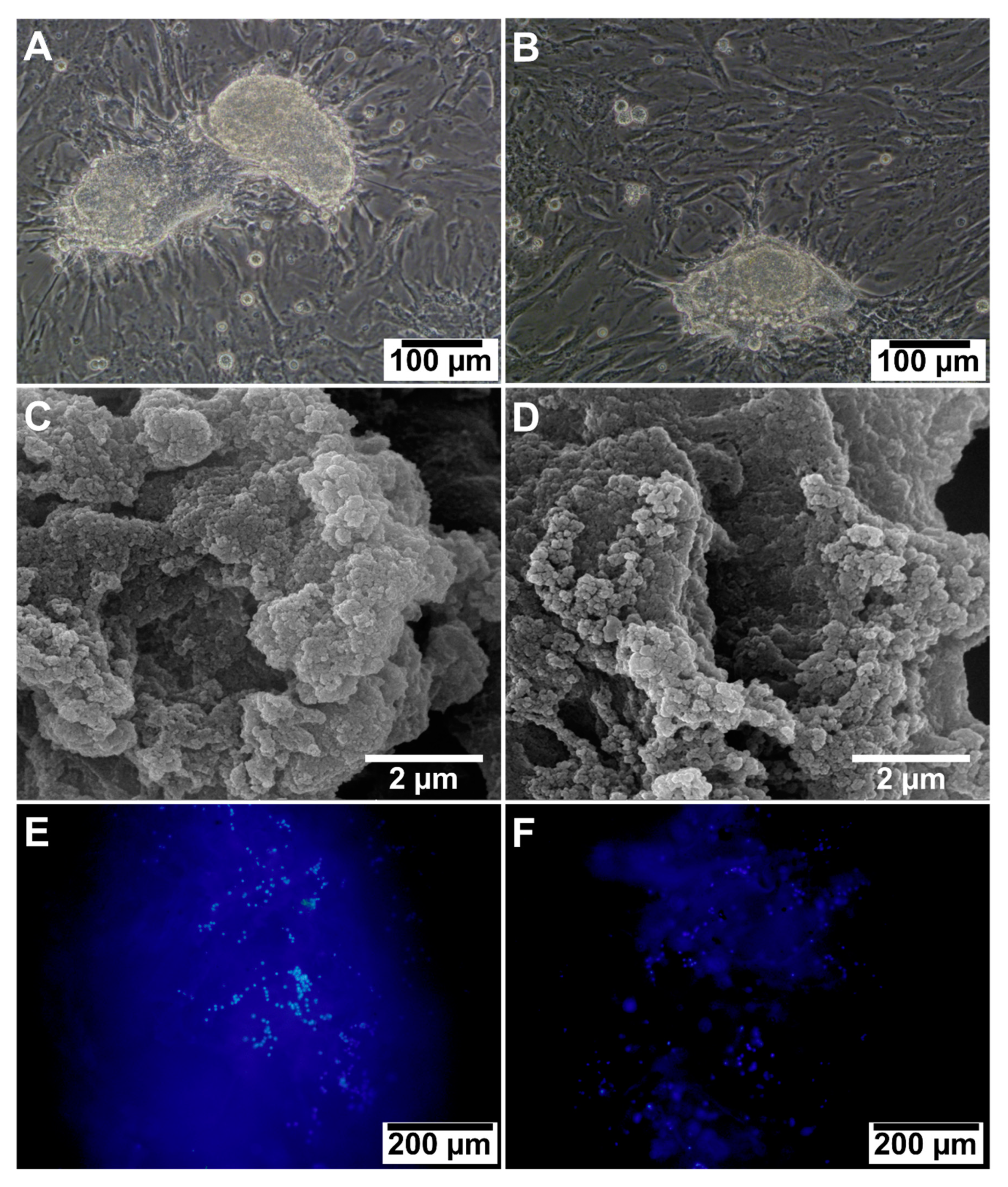
3.2.7. Cell Adhesion and Morphology
3.2.8. H&E Staining
3.2.9. qRT-PCR Analysis
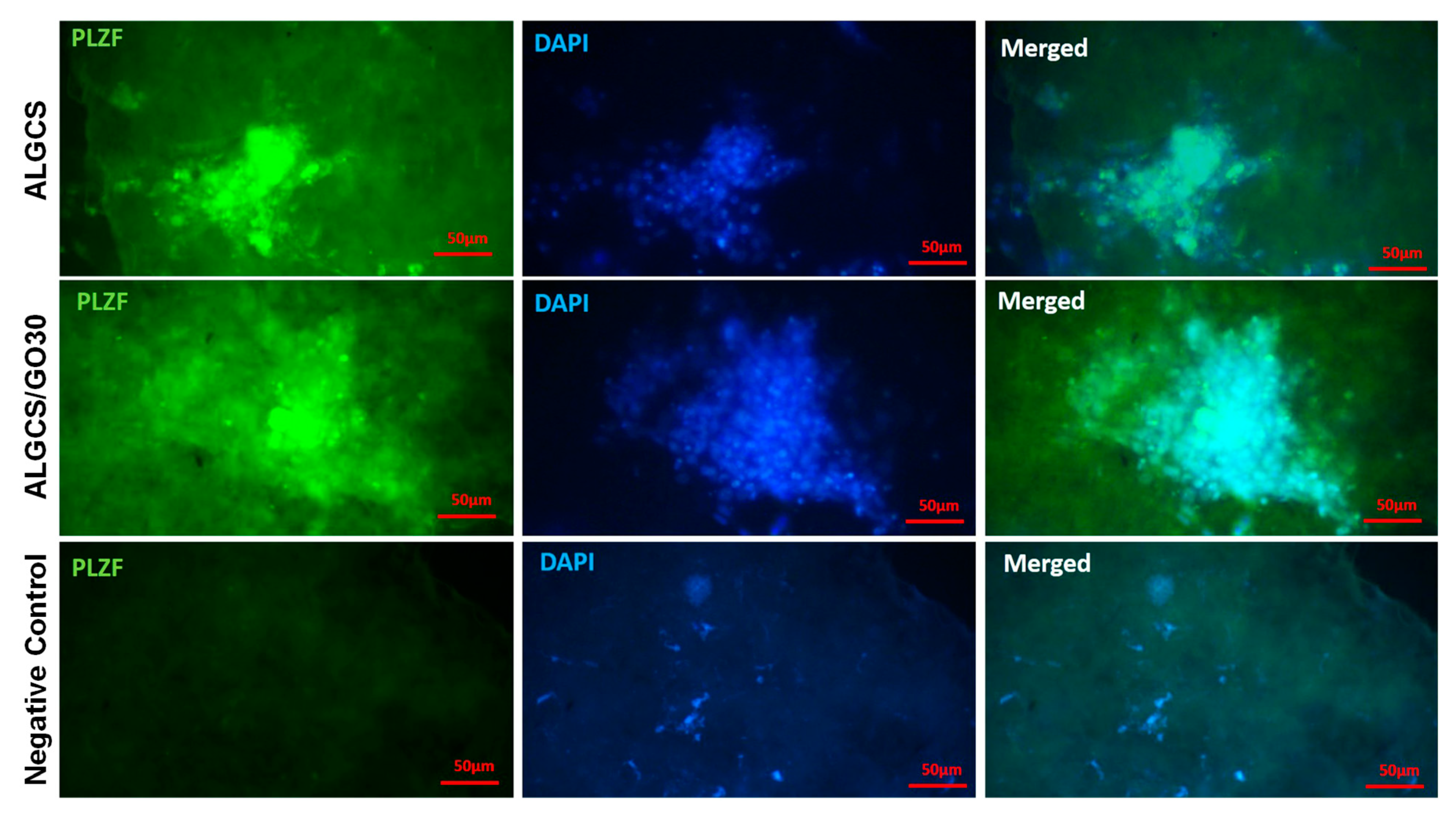
3.2.10. Immunocytochemistry
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinha, N.; Whelan, E.C.; Brinster, R.L. Isolation, cryopreservation, and transplantation of spermatogonial stem cells. Chimera Res. Methods Protoc. 2019, 2005, 205–220. [Google Scholar]
- De Rooij, D.G. The nature and dynamics of spermatogonial stem cells. Development 2017, 144, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S. Elucidating the identity and behavior of spermatogenic stem cells in the mouse testis. Reproduction 2012, 144, 293. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H. Concise review: Spermatogenesis in an artificial three-dimensional system. Stem Cells 2012, 30, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Ryu, B.-Y.; Brinster, C.J.; Avarbock, M.R.; Brinster, R.L. Maintenance of mouse male germ line stem cells in vitro. Biol. Reprod. 2003, 68, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T. Spermatogonial transplantation: The principle and possible applications. J. Mol. Med. 2001, 79, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Teng, S.; Saunders, P.T. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol. Reprod. 2009, 80, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef]
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef]
- Moraveji, S.; Esfandiari, F.; Sharbatoghli, M.; Taleahmad, S.; Nikeghbalian, S.; Shahverdi, A.; Baharvand, H. Optimizing methods for human testicular tissue cryopreservation and spermatogonial stem cell isolation. J. Cell. Biochem. 2019, 120, 613–621. [Google Scholar] [CrossRef]
- Stukenborg, J.-B.; Jahnukainen, K.; Hutka, M.; Mitchell, R.T. Cancer treatment in childhood and testicular function: The importance of the somatic environment. Endocr. Connect. 2018, 7, R69–R87. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Nourashrafeddin, S.; Plant, T.M. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J. Androl. 2015, 17, 972. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.J.; Madihally, S.V. Cell colonization in degradable 3D porous matrices. Cell Adhes. Migr. 2008, 2, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.; Ahn, J.; Park, J.; Ryu, B.-Y.; Park, J.Y. Membrane-bottomed microwell array added to Transwell insert to facilitate non-contact co-culture of spermatogonial stem cell and STO feeder cell. Biofabrication 2020, 12, 045031. [Google Scholar] [CrossRef] [PubMed]
- Gunja, N.J.; Athanasiou, K.A. Biodegradable materials in arthroscopy. Sports Med. Arthrosc. Rev. 2006, 14, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.V.; Vasava, D.V. A glimpse of biodegradable polymers and their biomedical applications. E-Polymers 2019, 19, 385–410. [Google Scholar] [CrossRef]
- Simionescu, B.C.; Ivanov, D. Natural and synthetic polymers for designing composite materials. In Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016; pp. 233–286. [Google Scholar]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Design. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Fathi, M.; Barar, J. Perspective highlights on biodegradable polymeric nanosystems for targeted therapy of solid tumors. BioImpacts BI 2017, 7, 49. [Google Scholar] [CrossRef]
- Kim, N.J.; Lee, S.J.; Atala, A. Biomedical nanomaterials in tissue engineering. In Nanomaterials in Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–25e. [Google Scholar]
- Furuya, D.C.; da Costa, S.A.; de Oliveira, R.C.; Ferraz, H.G.; Pessoa, A.; da Costa, S.M. Fibers obtained from alginate, chitosan and hybrid used in the development of scaffolds. Mater. Res. 2017, 20, 377–386. [Google Scholar] [CrossRef]
- Bushkalova, R.; Farno, M.; Tenailleau, C.; Duployer, B.; Cussac, D.; Parini, A.; Sallerin, B.; Fullana, S.G. Alginate-chitosan PEC scaffolds: A useful tool for soft tissues cell therapy. Int. J. Pharm. 2019, 571, 118692. [Google Scholar] [CrossRef] [PubMed]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef]
- Shaheen, T.; Montaser, A.; Li, S. Effect of cellulose nanocrystals on scaffolds comprising chitosan, alginate and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2019, 121, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.-G.; Lin, Z.-T.; Deng, S.-T. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Afshar, H.A.; Ghaee, A. Preparation of aminated chitosan/alginate scaffold containing halloysite nanotubes with improved cell attachment. Carbohydr. Polym. 2016, 151, 1120–1131. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Sun, Q.; Cai, H.; Tan, W.-S. Porous chitosan derivative scaffolds affect proliferation and osteogenesis of mesenchymal stem cell via reducing intracellular ROS. Carbohydr. Polym. 2020, 237, 116108. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Annu; Ali, A.; Sheikh, J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Tao, H.; Jin, L.; Wan, Z.; Dai, F.; Xiang, W.; Deng, H. Carboxymethyl chitosan/sodium alginate-based micron-fibers fabricated by emulsion electrospinning for periosteal tissue engineering. Mater. Des. 2020, 194, 108849. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, L.; Zeng, X.; Sun, Z.; Zhang, D.; Shen, P.; Li, Z.; Han, Y.; Li, P.; et al. Bio-multifunctional alginate/chitosan/fucoidan sponges with enhanced angiogenesis and hair follicle regeneration for promoting full-thickness wound healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Afjoul, H.; Shamloo, A.; Kamali, A. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: An in vitro, in vivo study. Mater. Sci. Eng. C 2020, 113, 110957. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M. Chitosan–alginate as scaffolding material for cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2005, 75, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Bousnaki, M.; Bakopoulou, A.; Papadogianni, D.; Barkoula, N.M.; Alpantaki, K.; Kritis, A.; Chatzinikolaidou, M.; Koidis, P. Fibro/chondrogenic differentiation of dental stem cells into chitosan/alginate scaffolds towards temporomandibular joint disc regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fang, M.; Xia, Y.; Hou, J.; Nan, X.; Zhao, B.; Wang, X. Preparation and biological properties of silk fibroin/nano-hydroxyapatite/graphene oxide scaffolds with an oriented channel-like structure. RSC Adv. 2020, 10, 10118–10128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y. Functional protein-based bioinspired nanomaterials: From coupled proteins, synthetic approaches, nanostructures to applications. Int. J. Mol. Sci. 2019, 20, 3054. [Google Scholar] [CrossRef]
- Bai, R.G.; Hilal-Alnaqbi, A. Polymeric Surface Modification of Graphene. In Biomedical Applications of Graphene and 2D Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 305–320. [Google Scholar]
- Bai, R.G.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Q.; Chen, D.; Lu, P. Enhanced mechanical properties of graphene-based poly (vinyl alcohol) composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar] [CrossRef]
- Feng, L.; Yang, X.; Shi, X.; Tan, X.; Peng, R.; Wang, J.; Liu, Z. Polyethylene glycol and polyethylenimine dual-functionalized nano-graphene oxide for photothermally enhanced gene delivery. Small 2013, 9, 1989–1997. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef]
- Hashemi, E.; Akhavan, O.; Shamsara, M.; Daliri, M.; Dashtizad, M.; Farmany, A. Synthesis and cyto-genotoxicity evaluation of graphene on mice spermatogonial stem cells. Colloids Surf. B Biointerfaces 2016, 146, 770–776. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Wang, Y.-H.; Sahu, R.S.; Chen, J.-P.; Dash, B.S.; Chung, P.-J.; Yang, H.-W.; Chuang, E.-Y.; Hwang, T.-L. Mechanism of Nanoformulated Graphene Oxide-Mediated Human Neutrophil Activation. ACS Appl. Mater. Interfaces 2020, 12, 40141–40152. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Gao, X.; Jiang, J.; Xu, C.; Deng, C.; Wang, J. Comparison of carbon nanotubes and graphene oxide coated carbon fiber for improving the interfacial properties of carbon fiber/epoxy composites. Compos. Part B Eng. 2018, 132, 170–177. [Google Scholar] [CrossRef]
- Rafeeqi, T.; Kaul, G. Carbon nanotubes as a scaffold for spermatogonial cell maintenance. J. Biomed. Nanotechnol. 2010, 6, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.; Eyni, H.; Khosrowpour, Z.; Salari Asl, L.; Shabani, R.; Nazari, H.; Mehdizadeh, M.; Ebrahimi Warkiani, M.; Amjadi, F. Spermatogenesis induction of spermatogonial stem cells using nanofibrous poly (l-lactic acid)/multi-walled carbon nanotube scaffolds and naringenin. Polym. Adv. Technol. 2019, 30, 3011–3025. [Google Scholar] [CrossRef]
- Cai, H.; Li, G. Efficacy of alginate-and chitosan-based scaffolds on the healing of diabetic skin wounds in animal experimental models and cell studies: A systematic review. Wound Repair Regen. 2020, 28, 751–771. [Google Scholar] [CrossRef] [PubMed]
- Ziloochi Kashani, M.; Bagher, Z.; Asgari, H.R.; Najafi, M.; Koruji, M.; Mehraein, F. Differentiation of neonate mouse spermatogonial stem cells on three-dimensional agar/polyvinyl alcohol nanofiber scaffold. Syst. Biol. Reprod. Med. 2020, 66, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Wei, Y.; Liu, Q.; Yang, B.; Ma, N.; Li, Z.; Zhao, L.; Chen, W.; Huang, D. Silver-loaded microspheres reinforced chitosan scaffolds for skin tissue engineering. Eur. Polym. J. 2020, 134, 109861. [Google Scholar] [CrossRef]
- Goudarzi, N.; Valipour, S.M.; Nooritahneh, A.; Motaghinejad, M.; Motevalian, M.; Safari, S.; Gholami, M.; Vatandour, S.; Hekmati, M. Pharmacological Evidences for Curcumin Neuroprotective Effects against Lead-Induced Neurodegeneration: Possible Role of Akt/GSK3 Signaling Pathway. Iran. J. Pharm. Res. 2020, 19, 494–508. [Google Scholar]
- Su, X.; Chen, L.; Han, S.; Niu, G.; Ren, J.; Ke, C. Preparation and characterization of a novel triple composite scaffold containing silk fiborin, chitosan, and alginate for 3D culture of colonic carcinoma cells in vitro. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e922935-1. [Google Scholar] [CrossRef]
- Shi, D.; Shen, J.; Zhang, Z.; Shi, C.; Chen, M.; Gu, Y.; Liu, Y. Preparation and properties of dopamine-modified alginate/chitosan–hydroxyapatite scaffolds with gradient structure for bone tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 1615–1627. [Google Scholar] [CrossRef]
- Farokhi, M.; Jonidi Shariatzadeh, F.; Solouk, A.; Mirzadeh, H. Alginate based scaffolds for cartilage tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 230–247. [Google Scholar] [CrossRef]
- Hemadi, M.; Assadollahi, V.; Saki, G.; Pirnia, A.; Alasvand, M.; Zendehdel, A.; Gholami, M. Use of alginate hydrogel to improve long-term 3D culture of spermatogonial stem cells: Stemness gene expression and structural features. Zygote 2022, 30, 312–318. [Google Scholar] [CrossRef]
- Pirnia, A.; Parivar, K.; Hemadi, M.; Yaghmaei, P.; Gholami, M. Stemness of spermatogonial stem cells encapsulated in alginate hydrogel during cryopreservation. Andrologia 2017, 49, e12650. [Google Scholar] [CrossRef] [PubMed]
- Jalayeri, M.; Pirnia, A.; Najafabad, E.P.; Varzi, A.M.; Gholami, M. Evaluation of alginate hydrogel cytotoxicity on three-dimensional culture of type A spermatogonial stem cells. Int. J. Biol. Macromol. 2017, 95, 888–894. [Google Scholar] [CrossRef]
- Naeemi, S.; Eidi, A.; Khanbabaee, R.; Sadri-Ardekani, H.; Kajbafzadeh, A.-M. Differentiation and proliferation of spermatogonial stem cells using a three-dimensional decellularized testicular scaffold: A new method to study the testicular microenvironment in vitro. Int. Urol. Nephrol. 2021, 53, 1543–1550. [Google Scholar] [CrossRef]
- Perrard, M.H.; Sereni, N.; Schluth-Bolard, C.; Blondet, A.; d’ Estaing, S.G.; Plotton, I.; Morel-Journel, N.; Lejeune, H.; David, L.; Durand, P. Complete Human and Rat Ex Vivo Spermatogenesis from Fresh or Frozen Testicular Tissue1. Biol. Reprod. 2016, 95, 89. [Google Scholar] [CrossRef] [PubMed]
- Eslahi, N.; Hadjighassem, M.R.; Joghataei, M.T.; Mirzapour, T.; Bakhtiyari, M.; Shakeri, M.; Pirhajati, V.; Shirinbayan, P.; Koruji, M. The effects of poly L-lactic acid nanofiber scaffold on mouse spermatogonial stem cell culture. Int. J. Nanomed. 2013, 8, 4563–4576. [Google Scholar]
- Shakeri, M.; Kohram, H.; Shahverdi, A.; Shahneh, A.Z.; Tavakolifar, F.; Pirouz, M.; Shahrebabak, H.M.; Koruji, M.; Baharvand, H. Behavior of mouse spermatogonial stem-like cells on an electrospun nanofibrillar matrix. J. Assist. Reprod. Genet. 2013, 30, 325–332. [Google Scholar] [CrossRef]
- Talebi, A.; Sadighi-Gilani, M.A.; Koruji, M.; Ai, J.; Navid, S.; Rezaie, M.J.; Jabari, A.; Ashouri-Movassagh, S.; Khadivi, F.; Salehi, M.; et al. Proliferation and differentiation of mouse spermatogonial stem cells on a three-dimensional surface composed of PCL/gel nanofibers. Int. J. Morphol. 2019, 37, 1132–1141. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Shayeh, J.S.; Omidi, M.; Yazdian, F.; Alebouyeh, M.; Tayebi, L. A glassy carbon electrode modified with reduced graphene oxide and gold nanoparticles for electrochemical aptasensing of lipopolysaccharides from Escherichia coli bacteria. Microchim. Acta 2019, 186, 787. [Google Scholar] [CrossRef]
- Florczyk, S.J.; Kievit, F.M.; Wang, K.; Erickson, A.E.; Ellenbogen, R.G.; Zhang, M. 3D porous chitosan–alginate scaffolds promote proliferation and enrichment of cancer stem-like cells. J. Mater. Chem. B 2016, 4, 6326–6334. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, S.G.; Pawar, S. Synthesis and characterization of chitosan-alginate scaffolds for seeding human umbilical cord derived mesenchymal stem cells. Bio-Med. Mater. Eng. 2016, 27, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Amroon, M.; Ghahremanzadeh, R.; Mahmoodi, M.; Baheiraei, N. Electroactive graphene oxide-incorporated collagen assisting vascularization for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Garakani, S.S.; Khanmohammadi, M.; Atoufi, Z.; Kamrava, S.K.; Setayeshmehr, M.; Alizadeh, R.; Faghihi, F.; Bagher, Z.; Davachi, S.M.; Abbaspourrad, A. Fabrication of chitosan/agarose scaffolds containing extracellular matrix for tissue engineering applications. Int. J. Biol. Macromol. 2020, 143, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Le, M.N.; Xu, K.; Wang, Z.; Beverung, S.; Steward, R.L.; Florczyk, S.J. Evaluation of the effect of 3D porous Chitosan-alginate scaffold stiffness on breast cancer proliferation and migration. J. Biomed. Mater. Res. Part A 2021, 109, 1990–2000. [Google Scholar] [CrossRef] [PubMed]
- O’hara, E.; Herbst, A.; Kommadath, A.; Aiken, J.M.; McKenzie, D.; Goodarzi, N.; Skinner, P.; Stothard, P. Neural transcriptomic signature of chronic wasting disease in white-tailed deer. BMC Genom. 2022, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Nalini, T.; Basha, S.K.; Mohamed Sadiq, A.M.; Kumari, V.S.; Kaviyarasu, K. Development and characterization of alginate/chitosan nanoparticulate system for hydrophobic drug encapsulation. J. Drug Deliv. Sci. Technol. 2019, 52, 65–72. [Google Scholar] [CrossRef]
- Baysal, G.; Olcay, H.S.; Günneç, Ç. Encapsulation and antibacterial studies of goji berry and garlic extract in the biodegradable chitosan. J. Bioact. Compat. Polym. 2023, 38, 209–219. [Google Scholar] [CrossRef]
- Tavakoli, M.; Karbasi, S.; Bakhtiari, S.S.E. Evaluation of physical, mechanical, and biodegradation of chitosan/graphene oxide composite as bone substitutes. Polym.-Plast. Technol. Mater. 2020, 59, 430–440. [Google Scholar] [CrossRef]
- Wasupalli, G.K.; Verma, D. Molecular interactions in self-assembled nano-structures of chitosan-sodium alginate based polyelectrolyte complexes. Int. J. Biol. Macromol. 2018, 114, 10–17. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Shakeel, M.; Dun, Y.; Zuo, Y.; Khan, W.Q.; Tang, Y.; Khan, A.; Nadeem, M. Exploring the Nickel–Graphene Nanocomposite Coatings for Superior Corrosion Resistance: Manipulating the Effect of Deposition Current Density on its Morphology, Mechanical Properties, and Erosion-Corrosion Performance. Adv. Eng. Mater. 2018, 20, 1701166. [Google Scholar] [CrossRef]
- Mohammadsalih, Z.G.; Inkson, B.J.; Chen, B. The effect of dispersion condition on the structure and properties of polystyrene/graphene oxide nanocomposites. Polym. Compos. 2021, 42, 320–328. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Zhang, Y.; Lu, J.; Li, Z.; Zhu, D.; Yu, X.; Li, L. Enhanced cellular functions of hepatocytes in the hyaluronate-alginate-chitosan microcapsules. Int. J. Artif. Organs 2021, 44, 340–349. [Google Scholar] [CrossRef]
- Mohammadrezaei, D.; Golzar, H.; Rad, M.R.; Omidi, M.; Rashedi, H.; Yazdian, F.; Khojasteh, A.; Tayebi, L. In vitro effect of graphene structures as an osteoinductive factor in bone tissue engineering: A systematic review. J. Biomed. Mater. Res. Part A 2018, 106, 2284–2343. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, O.N.; Fernando, K.A.S.; Wang, B.; Brown, N.A.; Luo, P.G.; McNamara, N.D.; Vangsness, M.; Sun, Y.-P.; Bunker, C.E. Graphene oxide: A nonspecific enhancer of cellular growth. ACS Nano 2011, 5, 8100–8107. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Iqbal, M.A.; Qasim, M.; Park, C.H.; Yoo, H.; Hwang, J.H.; Uhm, S.J.; Song, H.; Park, C.; Do, J.T.; et al. Evaluation of Graphene Oxide Induced Cellular Toxicity and Transcriptome Analysis in Human Embryonic Kidney Cells. Nanomaterials 2019, 9, 969. [Google Scholar] [CrossRef]
- Shang, L.; Qi, Y.; Lu, H.; Pei, H.; Li, Y.; Qu, L.; Wu, Z.; Zhang, W. Graphene and graphene oxide for tissue engineering and regeneration. In Theranostic Bionanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–185. [Google Scholar]
- Purohit, S.D.; Singh, H.; Bhaskar, R.; Yadav, I.; Bhushan, S.; Gupta, M.K.; Kumar, A.; Mishra, N.C. Fabrication of graphene oxide and nanohydroxyapatite reinforced gelatin–alginate nanocomposite scaffold for bone tissue regeneration. Front. Mater. 2020, 7, 250. [Google Scholar] [CrossRef]
- Aponte, P.M.; Van Bragt, M.P.; De Rooij, D.G.; Van Pelt, A.M. Spermatogonial stem cells: Characteristics and experimental possibilities. Apmis 2005, 113, 727–742. [Google Scholar] [CrossRef]
- Schrans-Stassen, B.H.; Van De Kant, H.J.; de Rooij, D.G.; van Pelt, A.M. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology 1999, 140, 5894–5900. [Google Scholar] [CrossRef]
- Shadan, N.; Mehdi, A.; Hoshino, Y. The effects of melatonin on colonization of neonate spermatogonial mouse stem cells in a three-dimensional soft agar culture system. Stem Cell Res. Ther. 2017, 8, 233. [Google Scholar]
- ATalebi, A.; Navid, S.; Borhani-Haghighi, M.; Hoshino, Y.; Abbasi, M.; Khosravizadeh, Z. Colonization of neonate mouse spermatogonial stem cells co-culture with Sertoli cells in the presence and absence soft agar. Asian Pac. J. Reprod. 2020, 9, 298. [Google Scholar]



| Abbreviations | Scaffolds |
|---|---|
| ALGCS | Alginate/chitosan |
| ALGCS/GO5 | Alginate/chitosan + GO concentration 5 µg/mL |
| ALGCS/GO15 | Alginate/chitosan + GO concentration 15 µg/mL |
| ALGCS/GO30 | Alginate/chitosan + GO concentration 30 µg/mL |
| ALGCS/GO45 | Alginate/chitosan + GO concentration 45 µg/mL |
| ALGCS/GO75 | Alginate/chitosan + GO concentration 75 µg/mL |
| Gene Name | Symbol | Accession Number | Forward/Reverse |
|---|---|---|---|
| GDNF family receptor alpha 1 | GFRα1 | NM_001285457.2 | TCAGATATATTCCGGGCAGTCC CATCGAGGCAGTTGTTCCCT |
| Inhibitor of DNA binding 4 | ID4 | NM_031166.3 | TGAAGCAGCAGTTGACATCTCT GGACACAGGCAATATCCTCATAGAA |
| Proto-oncogene C-Kit | C-Kit | XM_021163091.1 | CTAAAGATGAACCCTCAGCCT GCATAACACATGAACACTCCA |
| glyceraldehyde-3-phosphate dehydrogenase | GAPDH | CCCTTAAGAGGGATGCTGCC GTTCACACCGACCTTCACCA | |
| Promyelocytic leukemia zinc finger | PLZF | NM_001289726.1 | CCCGTTGGGGGTCAGCTAGA CTGCAAGGTGGGGCGGTGTAG |
| Samples | Compression Strength | Modulus |
|---|---|---|
| (MPa) | (MPa) | |
| ALGCS | 0.153 ± 0.11 | 0.754 ± 0.07 |
| ALGCS/GO30 | 0.185 ± 0.09 | 0.856 ± 0.09 |
| ALGCS/GO75 | 0.267 ± 0.13 | 0.911 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moeinzadeh, A.; Ashtari, B.; Garcia, H.; Koruji, M.; Velazquez, C.A.; Bagher, Z.; Barati, M.; Shabani, R.; Davachi, S.M. The Effect of Chitosan/Alginate/Graphene Oxide Nanocomposites on Proliferation of Mouse Spermatogonial Stem Cells. J. Funct. Biomater. 2023, 14, 556. https://doi.org/10.3390/jfb14120556
Moeinzadeh A, Ashtari B, Garcia H, Koruji M, Velazquez CA, Bagher Z, Barati M, Shabani R, Davachi SM. The Effect of Chitosan/Alginate/Graphene Oxide Nanocomposites on Proliferation of Mouse Spermatogonial Stem Cells. Journal of Functional Biomaterials. 2023; 14(12):556. https://doi.org/10.3390/jfb14120556
Chicago/Turabian StyleMoeinzadeh, Alaa, Behnaz Ashtari, Heriberto Garcia, Morteza Koruji, Carlo Alberto Velazquez, Zohreh Bagher, Mahmood Barati, Ronak Shabani, and Seyed Mohammad Davachi. 2023. "The Effect of Chitosan/Alginate/Graphene Oxide Nanocomposites on Proliferation of Mouse Spermatogonial Stem Cells" Journal of Functional Biomaterials 14, no. 12: 556. https://doi.org/10.3390/jfb14120556