A pH-Responsive DNA Tetrahedron/Methotrexate Drug Delivery System Used for Rheumatoid Arthritis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
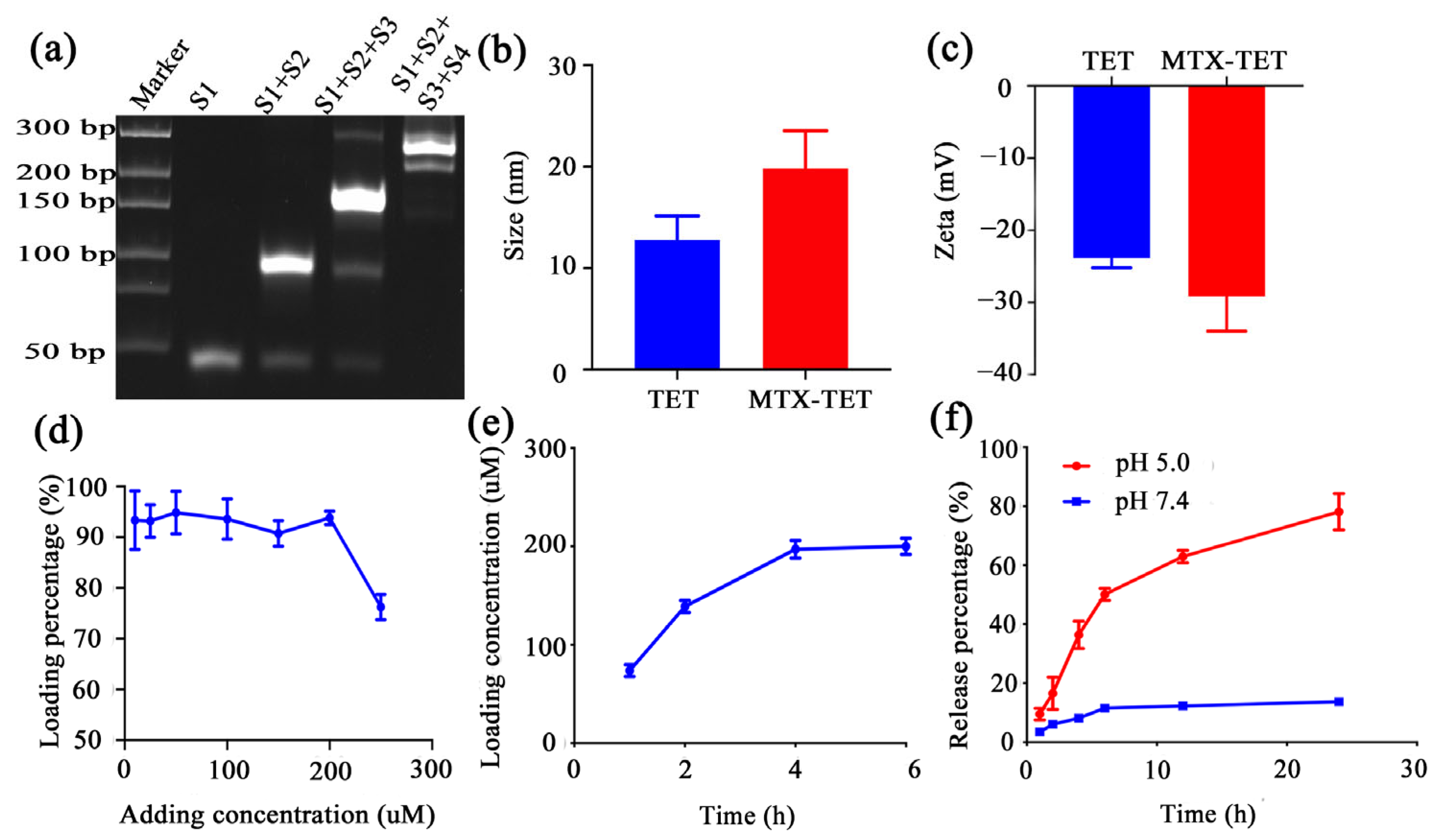
2.2. Preparation and Characterization of MTX-TET
2.3. Cell Culture
2.4. qRT-PCR Analysis
2.5. Establishment and Treatment of the Collagen-Induced Arthritis Model
2.6. Clinical Assessment of Arthritis
2.7. Flow Cytometry
2.8. Immunohistochemical and Histology Analysis
2.9. Micro-CT Analysis
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Statistical Analysis
3. Results
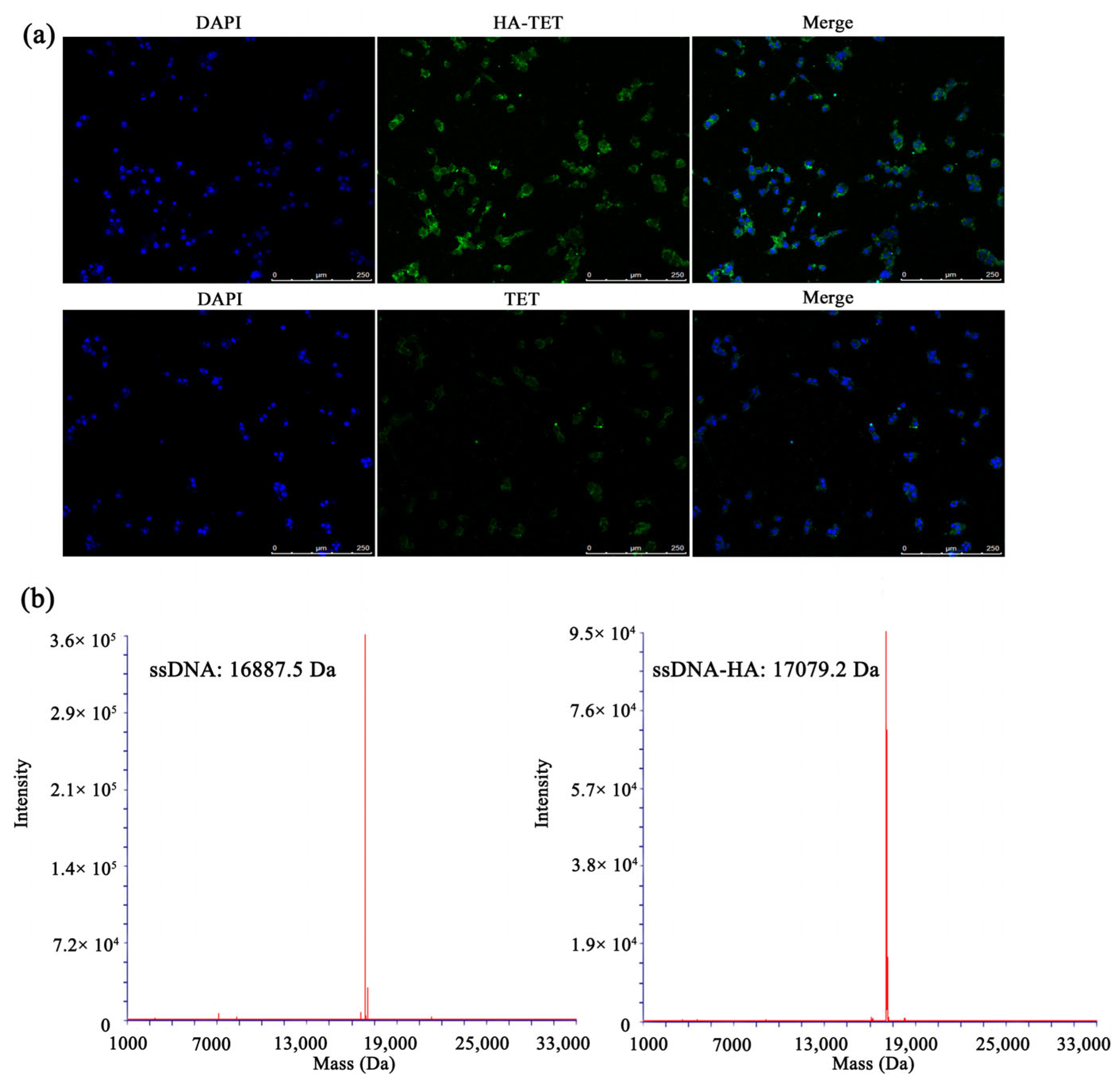
3.1. Characterization of MTX@TET@HA Nanoparticles
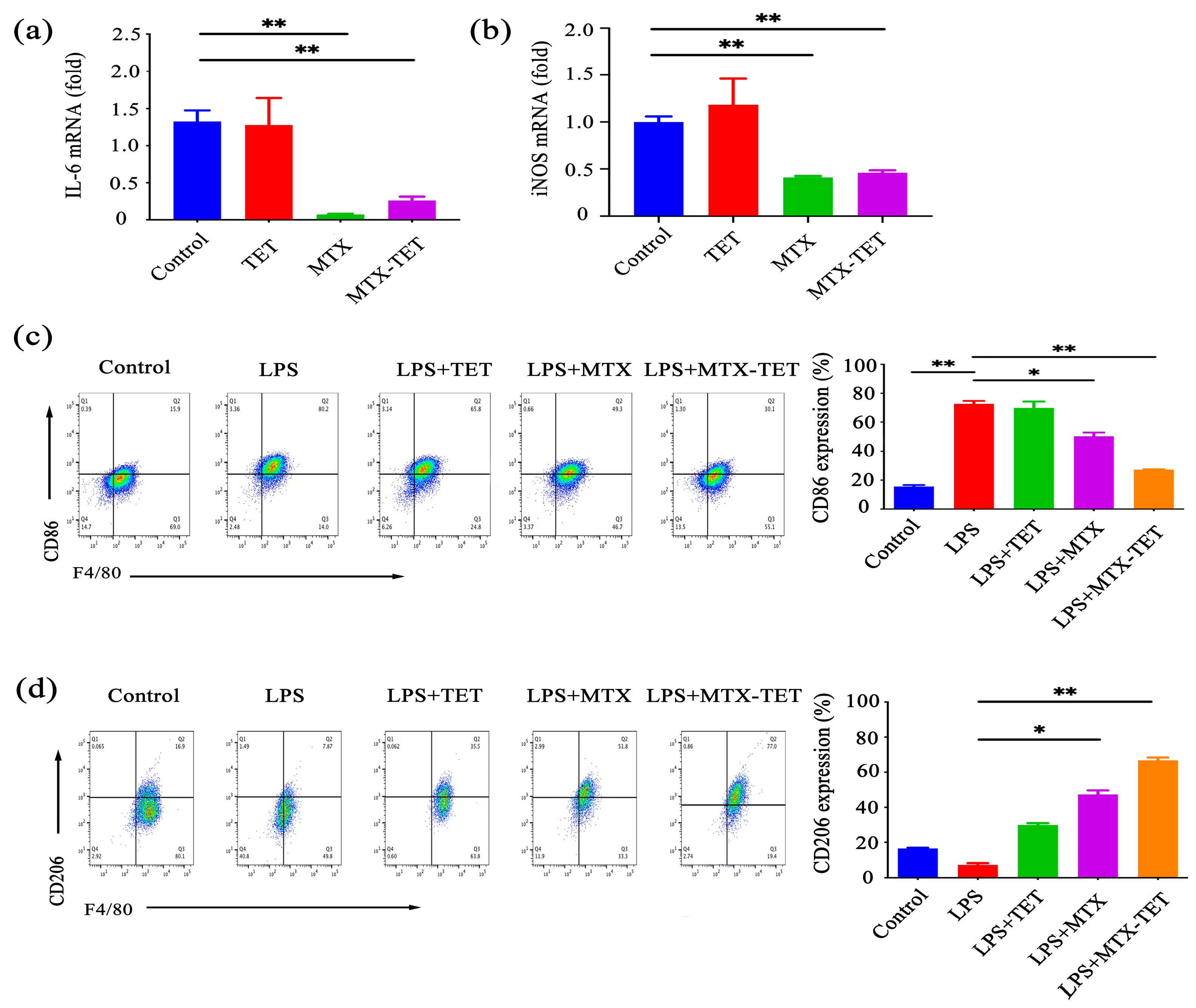
3.2. MTX-TET Could Affect the Polarization of the RAW264.7 Cells
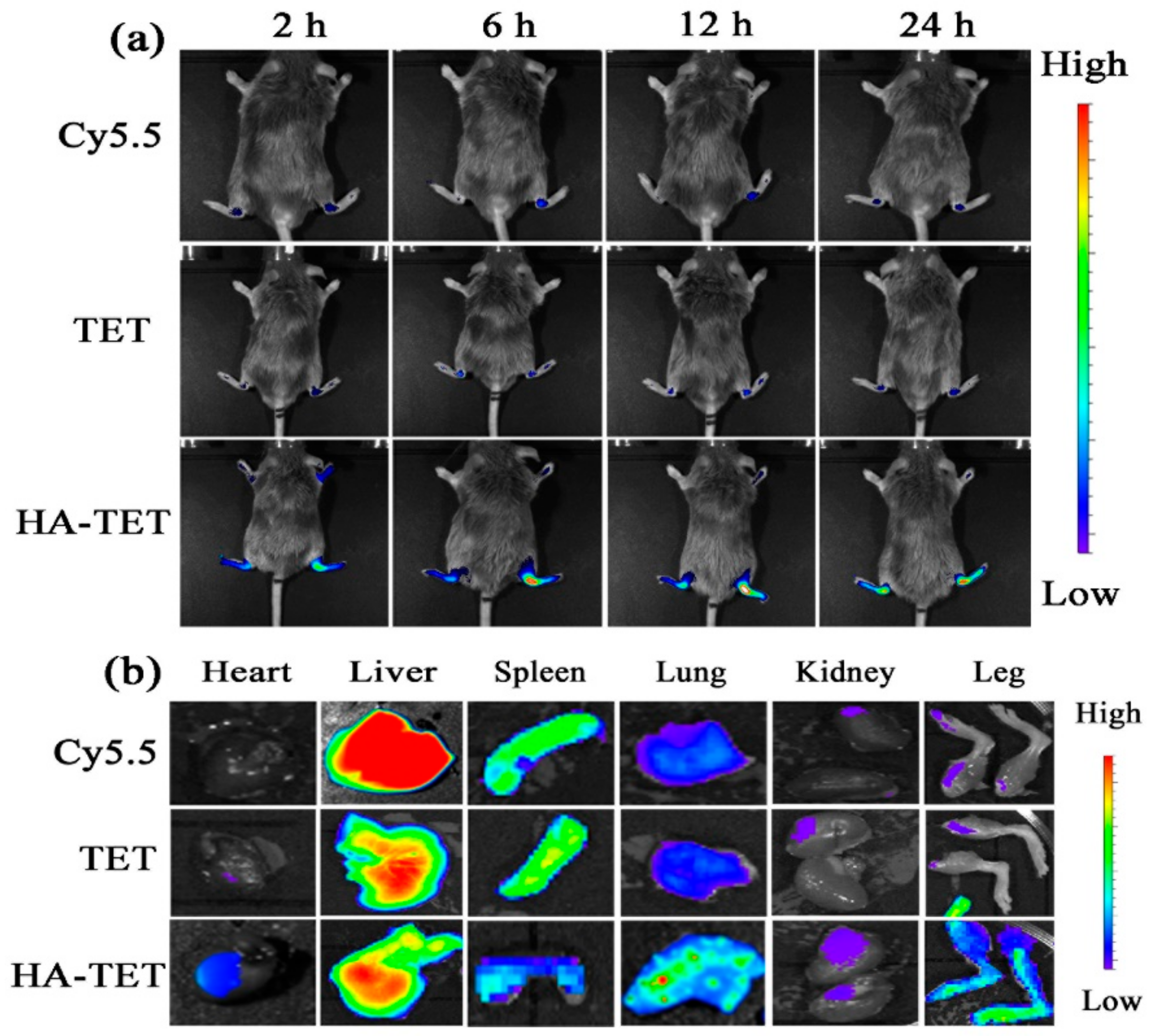
3.3. RA-Targeting Ability of the MTX-TET
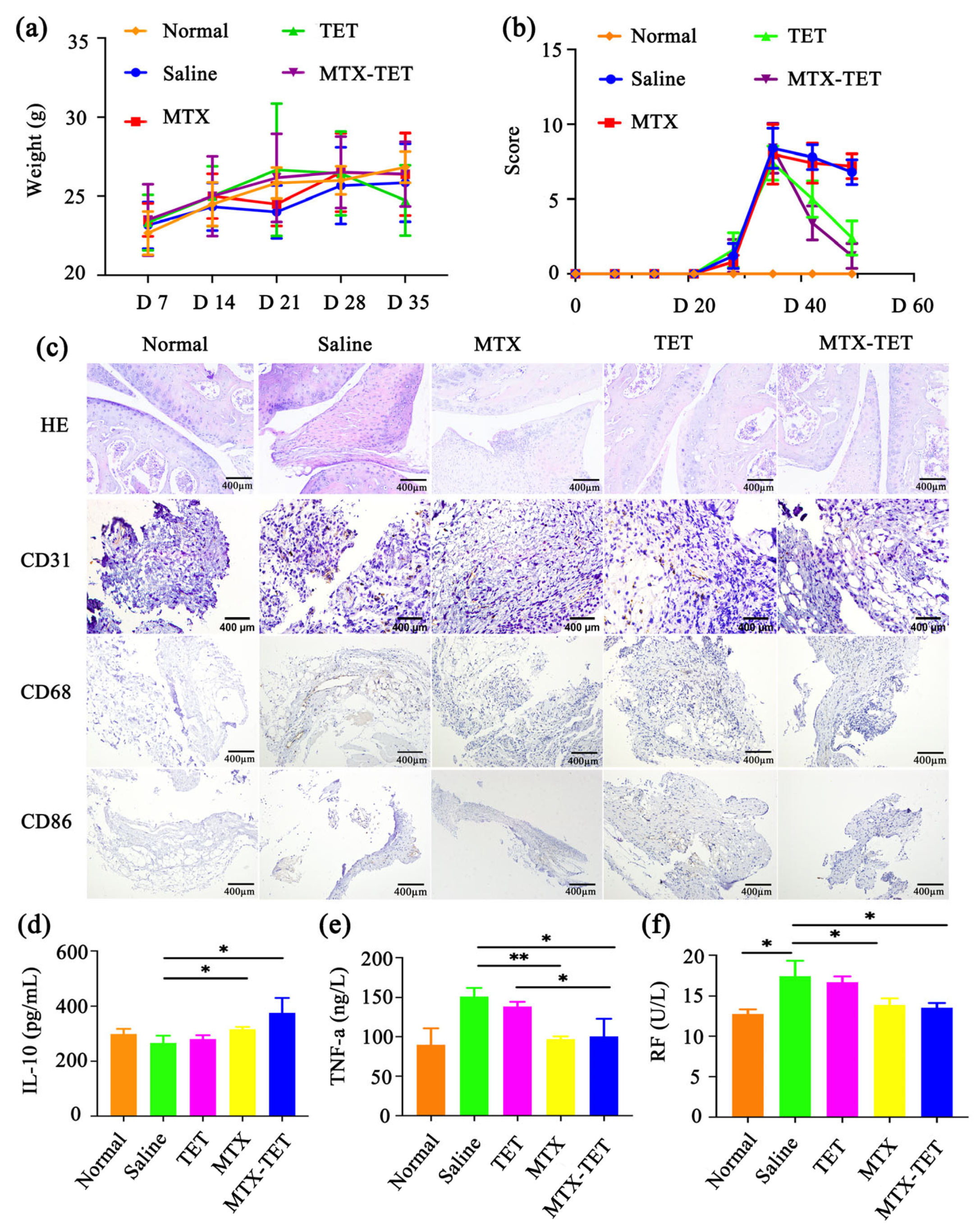
3.4. The Curative Effect of MTX-TET
3.5. Microcomputed Tomography Analysis
3.6. The Safety of the MTX-TET
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coutant, F.; Miossec, P. Evolving concepts of the pathogenesis of rheumatoid arthritis with focus on the early and late stages. Curr. Opin. Rheumatol. 2020, 32, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Gao, J.; Wang, L.; Liu, J.; Zhang, L. Regulation of differentiation and generation of osteoclasts in rheumatoid arthritis. Front. Immunol. 2022, 13, 1034050. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. The immunology of rheumatoid arthritis. Nat. Immunol. 2021, 22, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Caporali, R.; Germinario, S.; Kacsándi, D.; Choy, E.; Szekanecz, Z. Start RA treatment–Biologics or JAK-inhibitors? Autoimmun. Rev. 2023, 103429. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, P. The advances of methotrexate resistance in rheumatoid arthritis. Inflammopharmacology 2020, 28, 1183–1193. [Google Scholar] [CrossRef]
- Pandey, S.; Rai, N.; Mahtab, A.; Mittal, D.; Ahmad, F.J.; Sandal, N.; Neupane, Y.R.; Verma, A.K.; Talegaonkar, S. Hyaluronate-functionalized hydroxyapatite nanoparticles laden with methotrexate and teriflunomide for the treatment of rheumatoid arthritis. Int. J. Biol. Macromol. 2021, 171, 502–513. [Google Scholar] [CrossRef]
- Wei, K.; Jiang, P.; Zhao, J.; Jin, Y.; Zhang, R.; Chang, C.; Xu, L.; Xu, L.; Shi, Y.; Guo, S.; et al. Biomarkers to Predict DMARDs Efficacy and Adverse Effect in Rheumatoid Arthritis. Front. Immunol. 2022, 13, 865267. [Google Scholar] [CrossRef]
- Tian, J.; Chen, T.; Huang, B.; Liu, Y.; Wang, C.; Cui, Z.; Xu, H.; Li, Q.; Zhang, W.; Liang, Q. Inflammation specific environment activated methotrexate-loaded nanomedicine to treat rheumatoid arthritis by immune environment reconstruction. Acta Biomater. 2023, 157, 367–380. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Ke, J.; Zhang, H.; Bu, Y.; Gan, P.; Chen, F.; Dong, X.; Wang, Y.; Wu, H. Metabonomic analysis of abnormal sphingolipid metabolism in rheumatoid arthritis synovial fibroblasts in hypoxia microenvironment and intervention of geniposide. Front. Pharmacol. 2022, 13, 969408. [Google Scholar] [CrossRef]
- Fearon, U.; Hanlon, M.; Floudas, A.; Veale, D. Cellular metabolic adaptations in rheumatoid arthritis and their therapeutic implications. Nat. Rev. Rheumatol. 2022, 18, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Fukamachi, T.; Wang, X.; Mochizuki, Y.; Maruyama, C.; Saito, H.; Kobayashi, H. Acidic environments enhance the inhibitory effect of statins on proliferation of synovial cells. Int. Immunopharmacol. 2013, 17, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, M.; Yu, C.; Zhang, X.; Liu, J.; Cheng, X.; Lee, R.J.; Sun, F.; Teng, L.; Li, Y. Multifunctional folate receptor-targeting and pH-responsive nanocarriers loaded with methotrexate for treatment of rheumatoid arthritis. Int. J. Nanomed. 2017, 12, 6735–6746. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, Z.; Zhang, H.; Li, M.; Hou, Z.; Luo, X.; Xue, X. Development of DNA tetrahedron-based drug delivery system. Drug Deliv. 2017, 24, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.J.W.; Stephanopoulos, N. Functionalizing DNA nanostructures for therapeutic applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1729. [Google Scholar] [CrossRef]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef]
- Kim, K.; Kim, D.; Lee, T.; Yhee, J.; Kim, B.; Kwon, I.; Ahn, D.J.C.c. Drug delivery by a self-assembled DNA tetrahedron for overcoming drug resistance in breast cancer cells. Chem. Commun. 2013, 49, 2010–2012. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Q.; Wang, F.; Zhou, Z.; Xu, J.; Cheng, S.; Cheng, Y.J.I.j.o.n. Self-Assembled DNA Nanostructure as a Carrier for Targeted siRNA Delivery in Glioma Cells. Int. J. Nanomed. 2021, 16, 1805–1817. [Google Scholar] [CrossRef]
- Xue, H.; Ding, F.; Zhang, J.; Guo, Y.; Gao, X.; Feng, J.; Zhu, X.; Zhang, C. DNA tetrahedron-based nanogels for siRNA delivery and gene silencing. Chem. Commun. 2019, 55, 4222–4225. [Google Scholar] [CrossRef] [PubMed]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Tenshin, H.; Teramachi, J.; Ashtar, M.; Hiasa, M.; Inoue, Y.; Oda, A.; Tanimoto, K.; Shimizu, S.; Higa, Y.; Harada, T.; et al. TGF-β-activated kinase-1 inhibitor LL-Z1640-2 reduces joint inflammation and bone destruction in mouse models of rheumatoid arthritis by inhibiting NLRP3 inflammasome, TACE, TNF-α and RANKL expression. Clin. Transl. Immunol. 2022, 11, e1371. [Google Scholar] [CrossRef]
- Zhao, C.; Song, W.; Ma, J.; Wang, N. Macrophage-derived hybrid exosome-mimic nanovesicles loaded with black phosphorus for multimodal rheumatoid arthritis therapy. Biomater. Sci. 2022, 10, 6731–6739. [Google Scholar] [CrossRef] [PubMed]
- Sly, L.M.; McKay, D.M. Macrophage immunotherapy: Overcoming impediments to realize promise. Trends Immunol. 2022, 43, 959–968. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Wang, Y.; Han, C.C.; Cui, D.; Li, Y.; Ma, Y.; Wei, W. Is macrophage polarization important in rheumatoid arthritis? Int. Immunopharmacol. 2017, 50, 345–352. [Google Scholar] [CrossRef]
- Kim, H.; Back, J.H.; Han, G.; Lee, S.J.; Park, Y.E.; Gu, M.B.; Yang, Y.; Lee, J.E.; Kim, S.H. Extracellular vesicle-guided in situ reprogramming of synovial macrophages for the treatment of rheumatoid arthritis. Biomaterials 2022, 286, 121578. [Google Scholar] [CrossRef]
- Han, X.; Li, Q.; Zhang, S.; Sun, L.; Liu, W.; Wang, J. Inhibition of NEMO alleviates arthritis by blocking the M1 macrophage polarization. Int. Immunopharmacol. 2023, 117, 109983. [Google Scholar] [CrossRef]
- De Paula, M.C.; Carvalho, S.G.; Silvestre, A.L.P.; Dos Santos, A.M.; Meneguin, A.B.; Chorilli, M. The role of hyaluronic acid in the design and functionalization of nanoparticles for the treatment of colorectal cancer. Carbohydr. Polym. 2023, 320, 121257. [Google Scholar] [CrossRef]
- Kim, K.R.; Kim, H.Y.; Lee, Y.D.; Ha, J.S.; Kang, J.H.; Jeong, H.; Bang, D.; Ko, Y.T.; Kim, S.; Lee, H.; et al. Self-assembled mirror DNA nanostructures for tumor-specific delivery of anticancer drugs. J. Control. Release 2016, 243, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Chen, Y.; Tian, T.; Li, S.; Lin, S.; Zhang, Y.; Shao, X.; Zhang, T.; Lin, Y.; Cai, X. Effects of tetrahedral framework nucleic acid/wogonin complexes on osteoarthritis. Bone Res. 2020, 8, 6. [Google Scholar] [CrossRef]
- Wu, T.; Liu, J.; Liu, M.; Liu, S.; Zhao, S.; Tian, R.; Wei, D.; Liu, Y.; Zhao, Y.; Xiao, H.; et al. A Nanobody-Conjugated DNA Nanoplatform for Targeted Platinum-Drug Delivery. Angew. Chem. Int. Ed. Engl. 2019, 58, 14224–14228. [Google Scholar] [CrossRef]
- Brand, D.D.; Latham, K.A.; Rosloniec, E.F. Collagen-induced arthritis. Nat. Protoc. 2007, 2, 1269–1275. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Yamada, A.; Yokota, T.; Moritake, H.; Hirabara, Y.; Ikeda, R. Measurement of methotrexate in human cerebrospinal fluid using a chemiluminescence immunoassay intended for serum and plasma matrices. J. Clin. Lab. Anal. 2021, 35, e23661. [Google Scholar] [CrossRef] [PubMed]
- Bösmüller, H.; Pfefferle, V.; Bittar, Z.; Scheble, V.; Horger, M.; Sipos, B.; Fend, F. Microvessel density and angiogenesis in primary hepatic malignancies: Differential expression of CD31 and VEGFR-2 in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Pathol. Res. Pract. 2018, 214, 1136–1141. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Kemble, S.; Croft, A.P. Critical Role of Synovial Tissue-Resident Macrophage and Fibroblast Subsets in the Persistence of Joint Inflammation. Front. Immunol. 2021, 12, 715894. [Google Scholar] [CrossRef]
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it Up: The Promise of Stimuli-Responsive Polymer Systems in Biomedical Science. Chem. Rec. 2023, e202300217. [Google Scholar] [CrossRef]




| DNA | Sequence |
|---|---|
| Strand 1 | ACA TTC CTA AGT CTG AA AC ATT ACA GCT TGC TAC AC GA GAA GAG CCG CCA TAG TA |
| Strand 2 | TAT CAC CAG GCA GTT GA CA GTG TAG CAA GCT GTA AT AG ATG CGA GGG TCC AAT AC |
| Strand 3 | TCA ACT GCC TGG TGA TA AA ACG ACA CTA CGT GGG AA TC TAC TAT GGC GGC TCT TC |
| Strand 4 | TTC AGA CTT AGG AAT GT GC TTC CCA CGT AGT GTC GT TT GTA TTG GAC CCT CGC AT |
| Strand 1M | ACA TTC CTA AGT CTG AA AC ATT ACA GCT TGC TAC AC GA GAA GAG CCG CCA TAG TA-NH2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Ge, X.; Xu, Y.; Wang, S.; Lu, Q.; Deng, A.; Li, J.; Gu, Z. A pH-Responsive DNA Tetrahedron/Methotrexate Drug Delivery System Used for Rheumatoid Arthritis Treatment. J. Funct. Biomater. 2023, 14, 541. https://doi.org/10.3390/jfb14110541
Jin Y, Ge X, Xu Y, Wang S, Lu Q, Deng A, Li J, Gu Z. A pH-Responsive DNA Tetrahedron/Methotrexate Drug Delivery System Used for Rheumatoid Arthritis Treatment. Journal of Functional Biomaterials. 2023; 14(11):541. https://doi.org/10.3390/jfb14110541
Chicago/Turabian StyleJin, Yi, Xingyu Ge, Yinjin Xu, Siyi Wang, Qian Lu, Aidong Deng, Jingjing Li, and Zhifeng Gu. 2023. "A pH-Responsive DNA Tetrahedron/Methotrexate Drug Delivery System Used for Rheumatoid Arthritis Treatment" Journal of Functional Biomaterials 14, no. 11: 541. https://doi.org/10.3390/jfb14110541




