Antifungal Activity of Biosynthesized Silver Nanoparticles (AgNPs) against Aspergilli Causing Aspergillosis: Ultrastructure Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Isolation of Bacterial Isolates
2.2. Identification of Bacterial Strain
2.2.1. Morphological and Biochemical Identification
2.2.2. Molecular Identification
2.3. Biosynthesis of AgNPs
2.4. AgNP Characterization
2.5. In Vitro Cytotoxicity of AgNPs against Normal Cell Line
2.6. In Vitro Antifungal Activity of AgNPs
2.7. Ultrastructure Study
2.8. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of the Bacterial Isolate Bacillus sp. MAE 6
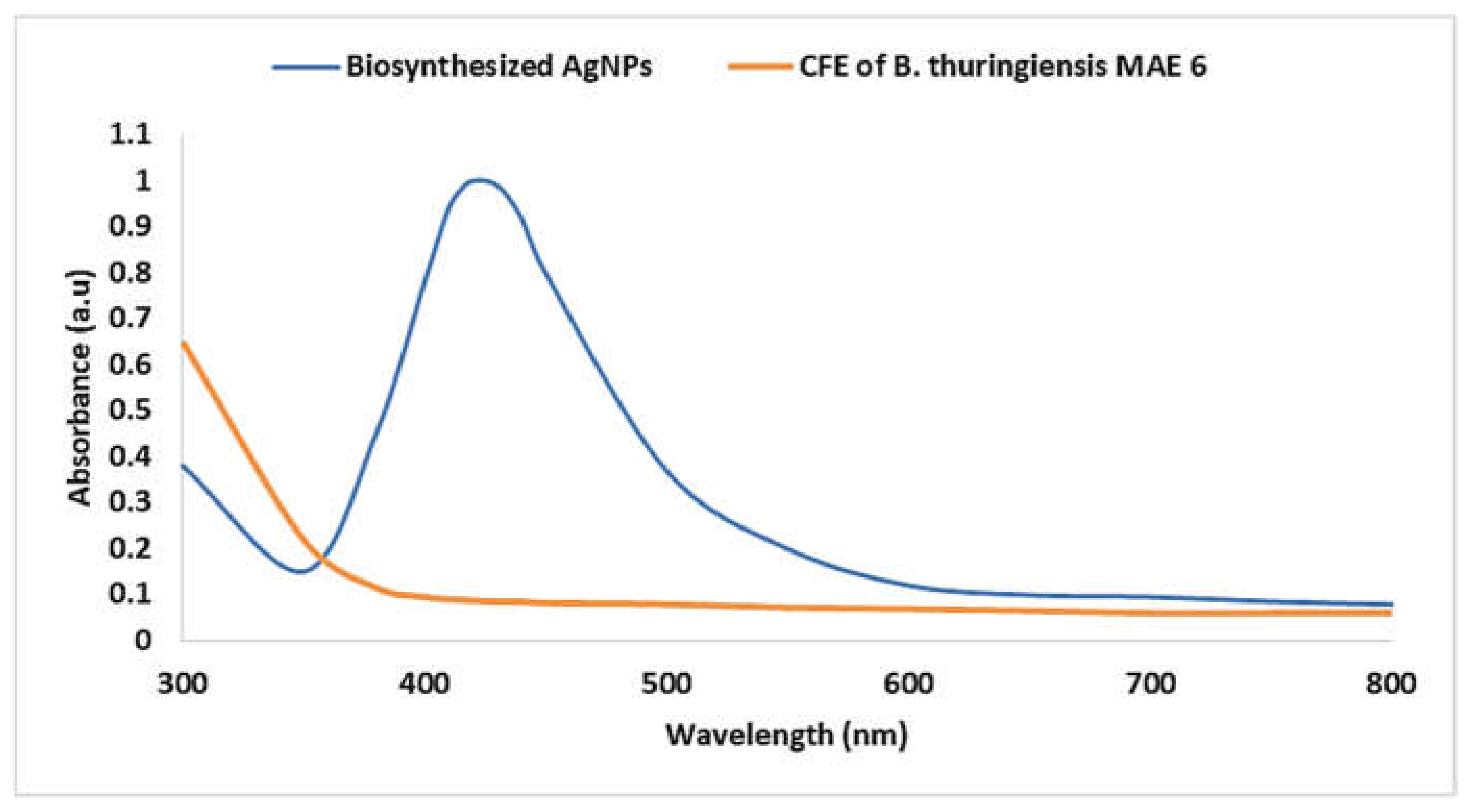
3.2. Biosynthesis of AgNPs Using CFE of B. thuringiensis
3.3. Characterization of Biosynthesized AgNPs
3.3.1. XRD Analysis
3.3.2. FTIR Analysis
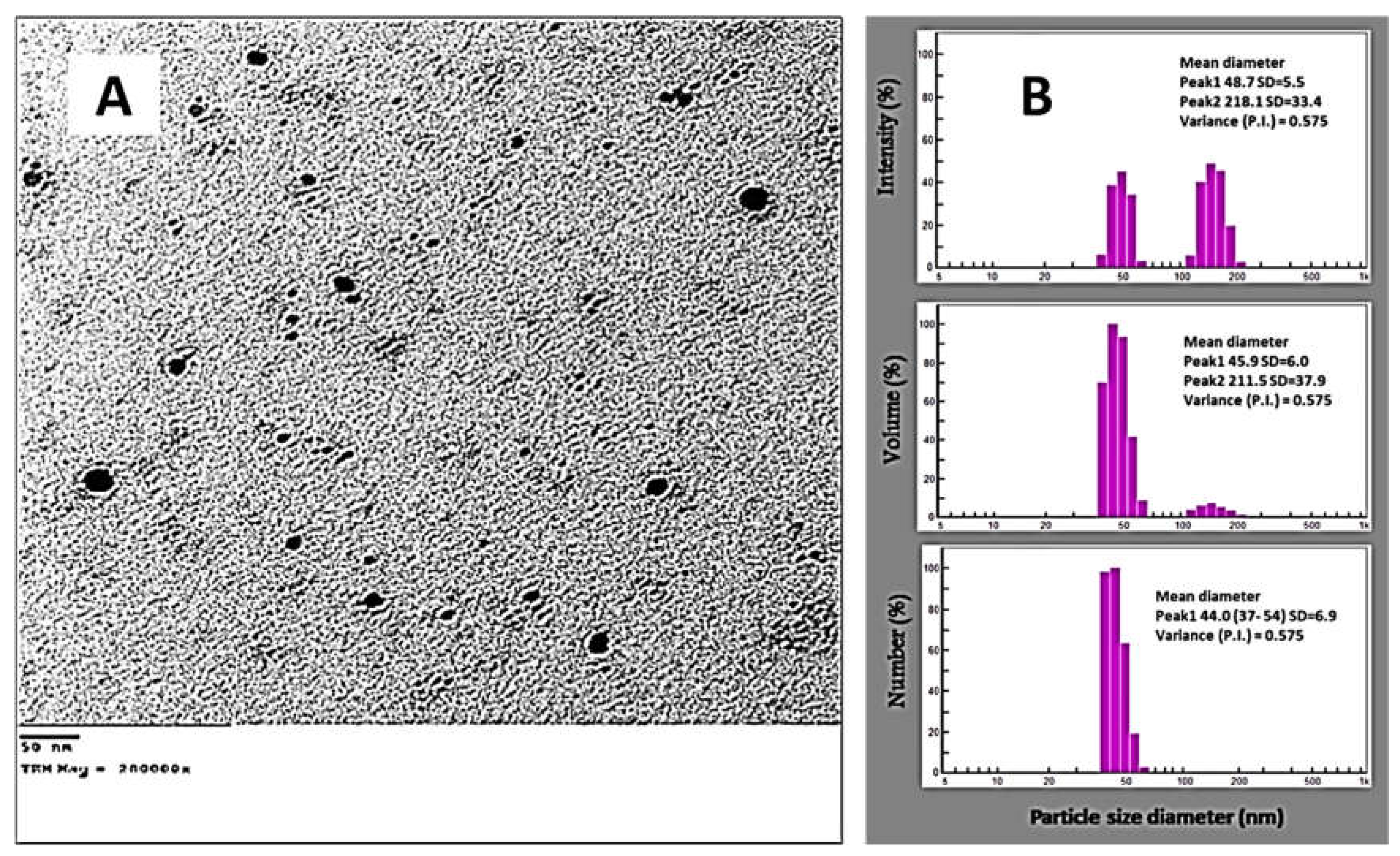
3.3.3. DLS and TEM Analyses
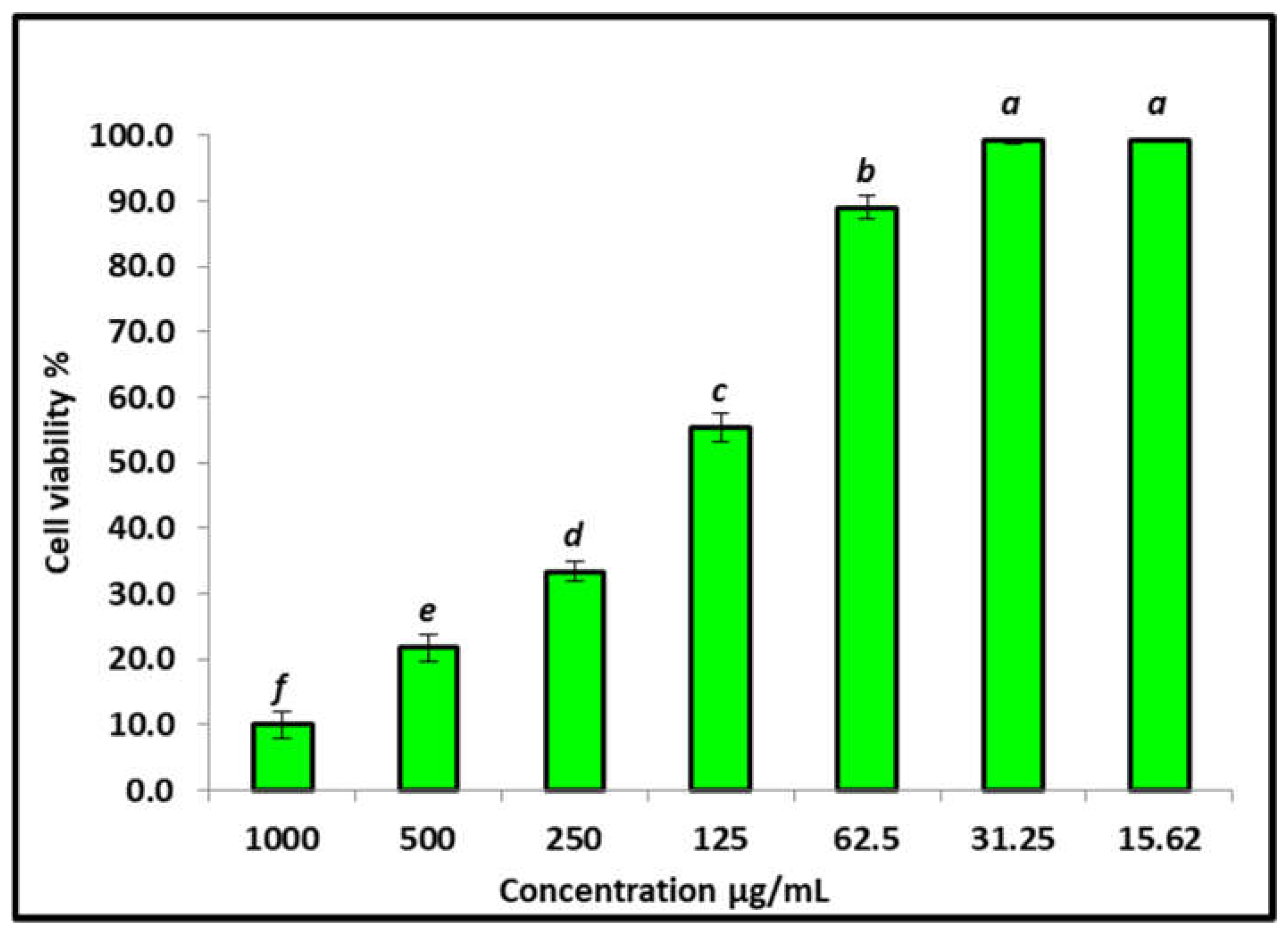
3.4. Effect of the AgNPs on the Vero Cells’ Viability Using MTT Assay
3.5. Antifungal Activity
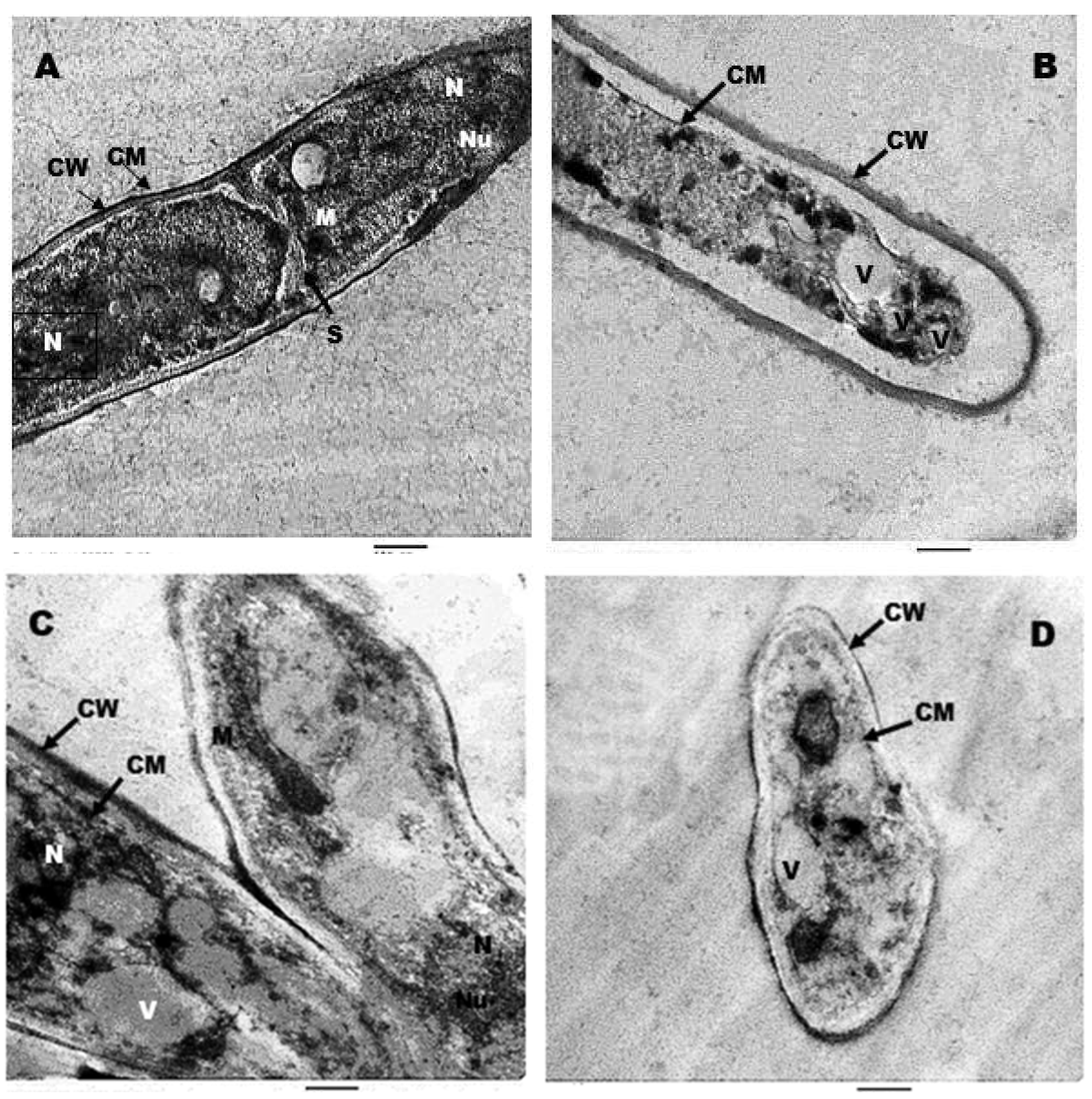
3.6. Ultrastructure Studies by TEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habschied, K.; Krstanović, V.; Zdunić, Z.; Babić, J.; Mastanjević, K.; Šarić, G.K. Mycotoxins biocontrol methods for healthier crops and stored products. J. Fungi 2021, 7, 348. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Camejo, L.A.; Zuluaga-Montero, A.; Morris, V.; Rodríguez, J.A.; Lázaro-Escudero, M.T.; Bayman, P. Fungal diversity in Sahara dust: Aspergillus sydowii and other opportunistic pathogens. Aerobiologia 2022, 38, 367–378. [Google Scholar] [CrossRef]
- Seyedmousavi, S. Aspergillosis in humans and animals. In Recent Trends in Human and Animal Mycology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 81–98. [Google Scholar]
- Salazar, F.; Bignell, E.; Brown, G.D.; Cook, P.C.; Warris, A. Pathogenesis of respiratory viral and fungal coinfections. Clin. Microbiol. Rev. 2022, 35, e00094-21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kausar, M.; Singh, A.; Singh, R. Biological contaminants in the indoor air environment and their impacts on human health. Air Qual. Atmos. Health 2021, 14, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Dacrory, S.; Hashem, A.H.; Hasanin, M. Synthesis of cellulose based amino acid functionalized nano-biocomplex: Characterization, antifungal activity, molecular docking and hemocompatibility. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100453. [Google Scholar] [CrossRef]
- Miller, A.S.; Wilmott, R.W. The Pulmonary Mycoses. In Kendig’s Disorders of the Respiratory Tract in Children; Elsevier: Amsterdam, The Netherlands, 2019; pp. 507–527.e503. [Google Scholar]
- Sugui, J.A.; Kwon-Chung, K.J.; Juvvadi, P.R.; Latge, J.-P.; Steinbach, W.J. Aspergillus fumigatus and related species. Cold Spring Harb. Perspect. Med. 2015, 5, a019786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, A.; Alam, A. Mycotoxins, Mycotoxicosis and Managing Mycotoxin Contamination: A Review. Bio-Manag. Postharvest Dis. Mycotoxigenic Fungi 2020, 161–180. [Google Scholar] [CrossRef]
- Hendrickson, J.A.; Hu, C.; Aitken, S.L.; Beyda, N. Antifungal resistance: A concerning trend for the present and future. Curr. Infect. Dis. Rep. 2019, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current insights on antifungal therapy: Novel nanotechnology approaches for drug delivery systems and new drugs from natural sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.M.; Dacrory, S.; Hashem, A.H.; Attia, M.S.; Hasanin, M.; Fouda, H.M.; Kamel, S.; ElSaied, H. Protective role of zinc oxide nanoparticles based hydrogel against wilt disease of pepper plant. Biocatal. Agric. Biotechnol. 2021, 35, 102083. [Google Scholar] [CrossRef]
- Silva, L.P.; Silveira, A.P.; Bonatto, C.C.; Reis, I.G.; Milreu, P.V. Silver nanoparticles as antimicrobial agents: Past, present, and future. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 577–596. [Google Scholar]
- Salem, S.S.; Ali, O.M.; Reyad, A.M.; Abd-Elsalam, K.A.; Hashem, A.H. Pseudomonas indica-Mediated Silver Nanoparticles: Antifungal and Antioxidant Biogenic Tool for Suppressing Mucormycosis Fungi. J. Fungi 2022, 8, 126. [Google Scholar] [CrossRef]
- Hasanin, M.; Elbahnasawy, M.A.; Shehabeldine, A.M.; Hashem, A.H. Ecofriendly preparation of silver nanoparticles-based nanocomposite stabilized by polysaccharides with antibacterial, antifungal and antiviral activities. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2021, 34, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional Silver Nanoparticles Based on Chitosan: Antibacterial, Antibiofilm, Antifungal, Antioxidant, and Wound-Healing Activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef]
- Salem, S.S.; Hashem, A.H.; Sallam, A.-A.M.; Doghish, A.S.; Al-Askar, A.A.; Arishi, A.A.; Shehabeldine, A.M. Synthesis of Silver Nanocomposite Based on Carboxymethyl Cellulose: Antibacterial, Antifungal and Anticancer Activities. Polymers 2022, 14, 3352. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.C.; Viana, A.D.; Menezes, F.G.; Neto, A.O.W.; Melo, M.C.N.; Gasparotto, L.H. Biospectroscopy and chemometrics as an analytical tool for comparing the antibacterial mechanism of silver nanoparticles with popular antibiotics against Escherichia coli. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 253, 119558. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Badmus, J.; Oyemomi, S.; Adedosu, O.; Yekeen, T.; Azeez, M.; Adebayo, E.; Lateef, A.; Badeggi, U.; Botha, S.; Hussein, A. Photo-assisted bio-fabrication of silver nanoparticles using Annona muricata leaf extract: Exploring the antioxidant, anti-diabetic, antimicrobial, and cytotoxic activities. Heliyon 2020, 6, e05413. [Google Scholar] [CrossRef] [PubMed]
- Pourzahedi, L.; Vance, M.; Eckelman, M.J. Life cycle assessment and release studies for 15 nanosilver-enabled consumer products: Investigating hotspots and patterns of contribution. Environ. Sci. Technol. 2017, 51, 7148–7158. [Google Scholar] [CrossRef]
- Yugay, Y.; Rusapetova, T.; Mashtalyar, D.; Grigorchuk, V.; Vasyutkina, E.; Kudinova, O.; Zenkina, K.; Trifuntova, I.; Karabtsov, A.; Ivanov, V. Biomimetic synthesis of functional silver nanoparticles using hairy roots of Panax ginseng for wheat pathogenic fungi treatment. Colloids Surf. B Biointerfaces 2021, 207, 112031. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Saied, E.; Salem, S.S.; Al-Askar, A.A.; Elkady, F.M.; Arishi, A.A.; Hashem, A.H. Mycosynthesis of Hematite (α-Fe2O3) Nanoparticles Using Aspergillus niger and Their Antimicrobial and Photocatalytic Activities. Bioengineering 2022, 9, 397. [Google Scholar] [CrossRef]
- Hasanin, M.; Al Abboud, M.A.; Alawlaqi, M.M.; Abdelghany, T.M.; Hashem, A.H. Ecofriendly Synthesis of Biosynthesized Copper Nanoparticles with Starch-Based Nanocomposite: Antimicrobial, Antioxidant, and Anticancer Activities. Biol. Trace Elem. Res. 2022, 200, 2099–2112. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.M.; Salem, S.S.; Khalil, A.M.A.; El-Wakil, D.A.; Fouda, H.M.; Hashem, A.H. Potential of biosynthesized zinc oxide nanoparticles to control Fusarium wilt disease in eggplant (Solanum melongena) and promote plant growth. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2022, 35, 601–616. [Google Scholar] [CrossRef]
- Hashem, A.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Abdelghany, T.M.; Hasanin, M. Synthesis of nanocapsules based on biosynthesized nickel nanoparticles and potato starch: Antimicrobial, antioxidant, and anticancer activity. Starch-Stärke 2022, 74, 2100165. [Google Scholar] [CrossRef]
- Abu-Elghait, M.; Hasanin, M.; Hashem, A.H.; Salem, S.S. Ecofriendly novel synthesis of tertiary composite based on cellulose and myco-synthesized selenium nanoparticles: Characterization, antibiofilm and biocompatibility. Int. J. Biol. Macromol. 2021, 175, 294–303. [Google Scholar] [CrossRef]
- Hasanin, M.; Hashem, A.H.; Lashin, I.; Hassan, S.A.M. In vitro improvement and rooting of banana plantlets using antifungal nanocomposite based on myco-synthesized copper oxide nanoparticles and starch. Biomass Convers. Biorefinery 2021, 1–11. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.A.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef]
- Saied, E.; Hashem, A.H.; Ali, O.M.; Selim, S.; Almuhayawi, M.S.; Elbahnasawy, M.A. Photocatalytic and Antimicrobial Activities of Biosynthesized Silver Nanoparticles Using Cytobacillus firmus. Life 2022, 12, 1331. [Google Scholar] [CrossRef] [PubMed]
- Elbahnasawy, M.A.; ElSayed, E.E.; Azzam, M.I. Newly isolated coliphages for bio-controlling multidrug-resistant Escherichia coli strains. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100542. [Google Scholar] [CrossRef]
- Van de Loosdrecht, A.; Beelen, R.; Ossenkoppele, G.; Broekhoven, M.; Langenhuijsen, M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef]
- Hashem, A.H.; Khalil, A.M.A.; Reyad, A.M.; Salem, S.S. Biomedical applications of mycosynthesized selenium nanoparticles using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. 2021, 199, 3998–4008. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Aly Khalil, A.; Hosny Hashem, A. Morphological changes of conidiogenesis in two Aspergillus species. J. Pure Appl. Microbiol. 2018, 12, 2041–2048. [Google Scholar] [CrossRef] [Green Version]
- Amin, B.H.; Amer, A.; Azzam, M.; Abd El-Sattar, N.E.; Mahmoud, D.; Al-Ashaal, S.; Al-Khalaf, A.A.; Hozzein, W.N. Antimicrobial and anticancer activities of Periplaneta americana tissue lysate: An in vitro study. J. King Saud Univ. Sci. 2022, 34, 102095. [Google Scholar] [CrossRef]
- Amin, B. Isolation and characterization of antiprotozoal and antimicrobial metabolite from Penicillium roqueforti. Afr. J. Mycol. Biotech. 2016, 21, 13–26. [Google Scholar]
- Amin, B.H.; Abou-Dobara, M.I.; Diab, M.A.; Gomaa, E.A.; El-Mogazy, M.A.; El-Sonbati, A.Z.; EL-Ghareib, M.S.; Hussien, M.A.; Salama, H.M. Synthesis, characterization, and biological investigation of new mixed-ligand complexes. Appl. Organomet. Chem. 2020, 34, e5689. [Google Scholar] [CrossRef]
- Almalki, M.A.; Khalifa, A.Y. Silver nanoparticles synthesis from Bacillus sp KFU36 and its anticancer effect in breast cancer MCF-7 cells via induction of apoptotic mechanism. J. Photochem. Photobiol. B Biol. 2020, 204, 111786. [Google Scholar] [CrossRef]
- Wilson, J.J.; Lakshmi, M.P.; Sivakumar, T.; Ponmanickam, P.; Sevarkodiyone, S. Green synthesis of silver nanoparticles using Bacillus subtilis (P3) and its Larvicidal, histopathological and biotoxicity efficacy. S. Afr. J. Bot. 2022. [Google Scholar] [CrossRef]
- Ajaz, S.; Ahmed, T.; Shahid, M.; Noman, M.; Shah, A.A.; Mehmood, M.A.; Abbas, A.; Cheema, A.I.; Iqbal, M.Z.; Li, B. Bioinspired green synthesis of silver nanoparticles by using a native Bacillus sp. strain AW1-2: Characterization and antifungal activity against Colletotrichum falcatum Went. Enzym. Microb. Technol. 2021, 144, 109745. [Google Scholar]
- Saied, E.; Fouda, A.; Alemam, A.M.; Sultan, M.H.; Barghoth, M.G.; Radwan, A.A.; Desouky, S.G.; Azab, I.H.E.; Nahhas, N.E.; Hassan, S.E.-D. Evaluate the toxicity of pyrethroid insecticide cypermethrin before and after biodegradation by Lysinibacillus cresolivuorans strain HIS7. Plants 2021, 10, 1903. [Google Scholar] [CrossRef]
- Alsamhary, K.I. Eco-friendly synthesis of silver nanoparticles by Bacillus subtilis and their antibacterial activity. Saudi J. Biol. Sci. 2020, 27, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Tufail, S.; Liaqat, I.; Ali, S.; Ulfat, M.; Shafi, A.; Sadiqa, A.; Iqbal, R.; Ahsan, F. Bacillus licheniformis (MN900686) Mediated Synthesis, Characterization and Antimicrobial Potential of Silver Nanoparticles. J. Oleo Sci. 2022, 71, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Mujaddidi, N.; Nisa, S.; Al Ayoubi, S.; Bibi, Y.; Khan, S.; Sabir, M.; Zia, M.; Ahmad, S.; Qayyum, A. Pharmacological properties of biogenically synthesized silver nanoparticles using endophyte Bacillus cereus extract of Berberis lyceum against oxidative stress and pathogenic multidrug-resistant bacteria. Saudi J. Biol. Sci. 2021, 28, 6432–6440. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, M.; Khorrami, S.; Ravan, H. Identification of Bacillus thuringiensis bacterial strain isolated from the mine soil as a robust agent in the biosynthesis of silver nanoparticles with strong antibacterial and anti-biofilm activities. Biocatal. Agric. Biotechnol. 2019, 18, 101047. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, W.-H.; Song, Y.-P.; Wang, Q.; Kan, Y.-F.; Wang, S.-Y.; Xia, J.-L.; Bilal, M.; Zhu, X.-Y.; Wang, Z.-X. Characterization, antimicrobial, and antioxidant potentialities of first-time isolated silver nanoparticles synthesizing protein secreted by Lysinibacillus sphaericus. Process Biochem. 2022, 122, 230–237. [Google Scholar] [CrossRef]
- Nallal, V.U.M.; Prabha, K.; VethaPotheher, I.; Ravindran, B.; Baazeem, A.; Chang, S.W.; Otunola, G.A.; Razia, M. Sunlight-driven rapid and facile synthesis of Silver nanoparticles using Allium ampeloprasum extract with enhanced antioxidant and antifungal activity. Saudi J. Biol. Sci. 2021, 28, 3660–3668. [Google Scholar] [CrossRef]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, Y.J.; Im, G.B.; Zhu, J.; Wu, Y.; Liu, Y.; Bhang, S.H. Inorganic nanoparticles applied as functional therapeutics. Adv. Funct. Mater. 2021, 31, 2008171. [Google Scholar] [CrossRef]
- Dube, P.; Meyer, S.; Madiehe, A.; Meyer, M. Antibacterial activity of biogenic silver and gold nanoparticles synthesized from Salvia africana-lutea and Sutherlandia frutescens. Nanotechnology 2020, 31, 505607. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, T.M.; Yassin, M.A.; El-Samawaty, A.R.M.; Elgorban, A.M. Silver nanoparticles synthesized by Nigrospora oryzae showed antifungal activity. Saudi J. Biol. Sci. 2021, 28, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; Elgorban, A.M.; El-Samawaty, A.E.-R.M.; Almunqedhi, B.M. Biosynthesis of silver nanoparticles using Penicillium verrucosum and analysis of their antifungal activity. Saudi J. Biol. Sci. 2021, 28, 2123–2127. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Noman, M.; Niazi, M.B.K.; Mahmood, F.; Manzoor, I.; Zhang, Y.; Li, B.; Yang, Y.; Yan, C. Silver nanoparticles synthesized by using Bacillus cereus SZT1 ameliorated the damage of bacterial leaf blight pathogen in rice. Pathogens 2020, 9, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Soliman, A.M.; Abdel-Latif, W.; Shehata, I.H.; Fouda, A.; Abdo, A.M.; Ahmed, Y.M. Green approach to overcome the resistance pattern of Candida spp. using biosynthesized silver nanoparticles fabricated by Penicillium chrysogenum F9. Biol. Trace Elem. Res. 2021, 199, 800–811. [Google Scholar]
- El-Naggar, N.E.-A.; Hussein, M.H.; El-Sawah, A.A. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci. Rep. 2017, 7, 10844. [Google Scholar] [CrossRef] [Green Version]
- Taran, M.; Rad, M.; Alavi, M. Characterization of Ag nanoparticles biosynthesized by Bacillus sp. HAI4 in different conditions and their antibacterial effects. J. Appl. Pharm. Sci. 2016, 6, 094–099. [Google Scholar]
- Shu, M.; He, F.; Li, Z.; Zhu, X.; Ma, Y.; Zhou, Z.; Yang, Z.; Gao, F.; Zeng, M. Biosynthesis and antibacterial activity of silver nanoparticles using yeast extract as reducing and capping agents. Nanoscale Res. Lett. 2020, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- Lashin, I.; Fouda, A.; Gobouri, A.A.; Azab, E.; Mohammedsaleh, Z.M.; Makharita, R.R. Antimicrobial and in vitro cytotoxic efficacy of biogenic silver nanoparticles (Ag-NPs) fabricated by callus extract of Solanum incanum L. Biomolecules 2021, 11, 341. [Google Scholar] [CrossRef]
- Jalali, E.; Maghsoudi, S.; Noroozian, E. A novel method for biosynthesis of different polymorphs of TiO2 nanoparticles as a protector for Bacillus thuringiensis from Ultra Violet. Sci. Rep. 2020, 10, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavun, V.; van der Veen, M.A.; Repo, E. Selective recovery and separation of rare earth elements by organophosphorus modified MIL-101 (Cr). Microporous Mesoporous Mater. 2021, 312, 110747. [Google Scholar] [CrossRef]
- Stani, C.; Vaccari, L.; Mitri, E.; Birarda, G. FTIR investigation of the secondary structure of type I collagen: New insight into the amide III band. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 118006. [Google Scholar] [CrossRef] [PubMed]
- Csakvari, A.C.; Moisa, C.; Radu, D.G.; Olariu, L.M.; Lupitu, A.I.; Panda, A.O.; Pop, G.; Chambre, D.; Socoliuc, V.; Copolovici, L. Green synthesis, characterization, and antibacterial properties of silver nanoparticles obtained by using diverse varieties of Cannabis sativa leaf extracts. Molecules 2021, 26, 4041. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Alshameri, A.W.; Owais, M. Antibacterial and cytotoxic potency of the plant-mediated synthesis of metallic nanoparticles Ag NPs and ZnO NPs: A Review. OpenNano 2022, 8, 100077. [Google Scholar] [CrossRef]
- Weng, X.; Yang, K.; Owens, G.; Chen, Z. Biosynthesis of silver nanoparticles using three different fruit extracts: Characterization, formation mechanism and estrogen removal. J. Environ. Manag. 2022, 316, 115224. [Google Scholar] [CrossRef]
- Elbahnasawy, M.A.; Shehabeldine, A.M.; Khattab, A.M.; Amin, B.H.; Hashem, A.H. Green biosynthesis of silver nanoparticles using novel endophytic Rothia endophytica: Characterization and anticandidal activity. J. Drug Deliv. Sci. Technol. 2021, 62, 102401. [Google Scholar] [CrossRef]
- Saied, E.; Eid, A.M.; Hassan, S.E.-D.; Salem, S.S.; Radwan, A.A.; Halawa, M.; Saleh, F.M.; Saad, H.A.; Saied, E.M.; Fouda, A. The catalytic activity of biosynthesized magnesium oxide nanoparticles (Mgo-nps) for inhibiting the growth of pathogenic microbes, tanning effluent treatment, and chromium ion removal. Catalysts 2021, 11, 821. [Google Scholar] [CrossRef]
- Najitha Banu, A.; Balasubramanian, C.; Moorthi, P.V. Biosynthesis of silver nanoparticles using Bacillus thuringiensis against dengue vector, Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2014, 113, 311–316. [Google Scholar] [CrossRef]
- Dharmaraj, D.; Krishnamoorthy, M.; Rajendran, K.; Karuppiah, K.; Annamalai, J.; Durairaj, K.R.; Santhiyagu, P.; Ethiraj, K. Antibacterial and cytotoxicity activities of biosynthesized silver oxide (Ag2O) nanoparticles using Bacillus paramycoides. J. Drug Deliv. Sci. Technol. 2021, 61, 102111. [Google Scholar] [CrossRef]
- Chi, N.T.L.; Veeraragavan, G.R.; Brindhadevi, K.; Chinnathambi, A.; Salmen, S.H.; Alharbi, S.A.; Krishnan, R.; Pugazhendhi, A. Fungi fabrication, characterization, and anticancer activity of silver nanoparticles using metals resistant Aspergillus niger. Environ. Res. 2022, 208, 112721. [Google Scholar]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.; Chowdhury, M.A.; Hossain, N. Green synthesis of hybrid nanoparticles for biomedical applications: A review. Appl. Surf. Sci. Adv. 2022, 11, 100296. [Google Scholar]
- Pavan, S.; Venkatesan, J.; Prabhu, A. Anticancer activity of silver nanoparticles from the aqueous extract of Dictyota ciliolata on non-small cell lung cancer cells. J. Drug Deliv. Sci. Technol. 2022, 74, 103525. [Google Scholar]
- Kiani, Z.; Aramjoo, H.; Chamani, E.; Siami-Aliabad, M.; Mortazavi-Derazkola, S. In vitro cytotoxicity against K562 tumor cell line, antibacterial, antioxidant, antifungal and catalytic activities of biosynthesized silver nanoparticles using Sophora pachycarpa extract. Arab. J. Chem. 2022, 15, 103677. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Selvamani, V. Stability studies on nanomaterials used in drugs. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–444. [Google Scholar]
- Mohmed, A.A.; Saad, E.; Fouda, A.; Elgamal, M.S.; Salem, S.S. Extracellular biosynthesis of silver nanoparticles using Aspergillus sp. and evaluation of their antibacterial and cytotoxicity. J. Appl. Life Sci. Int. 2017, 11, 169–180. [Google Scholar]
- Ioset, J.-R.; Brun, R.; Wenzler, T.; Kaiser, M.; Yardley, V. Drug Screening for Kinetoplastids Diseases. A Training Manual for Screening in Neglected Diseases. 2009. Available online: https://dndi.org/wp-content/uploads/2009/04/kinetoplastid_drug_screening_manual_final.pdf (accessed on 11 November 2022).
- Khan, T.; Yasmin, A.; Townley, H.E. An evaluation of the activity of biologically synthesized silver nanoparticles against bacteria, fungi and mammalian cell lines. Colloids Surf. B Biointerfaces 2020, 194, 111156. [Google Scholar] [CrossRef]
- Khalil, N.M.; Abd El-Ghany, M.N.; Rodríguez-Couto, S. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere 2019, 218, 477–486. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zahir, E.; Asghar, M.A.; Iqbal, J.; Rehman, A.A. Facile, one-pot biosynthesis and characterization of iron, copper and silver nanoparticles using Syzygium cumini leaf extract: As an effective antimicrobial and aflatoxin B1 adsorption agents. PLoS ONE 2020, 15, e0234964. [Google Scholar] [CrossRef] [PubMed]
- Dashora, A.; Rathore, K.; Raj, S.; Sharma, K. Synthesis of silver nanoparticles employing Polyalthia longifolia leaf extract and their in vitro antifungal activity against phytopathogen. Biochem. Biophys. Rep. 2022, 31, 101320. [Google Scholar] [CrossRef] [PubMed]
- Al-Otibi, F.; Alfuzan, S.A.; Alharbi, R.I.; Al-Askar, A.A.; Al-Otaibi, R.M.; Al Subaie, H.F.; Moubayed, N.M. Comparative study of antifungal activity of two preparations of green silver nanoparticles from Portulaca oleracea extract. Saudi J. Biol. Sci. 2022, 29, 2772–2781. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, E.A.; Hashem, A.E.G.; Elhifnawi, H.N.; Nada, H.G.; Khattab, R.A. One-pot biosynthesis of silver nanoparticles with potential antimicrobial and antibiofilm efficiency against otitis media–causing pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Kalam, A.; Al-Sehemi, A.G.; Alomary, M.N.; AlYahya, S.; Aziz, M.K.; Srivastava, S.; Alghamdi, S.; Akhtar, S.; Almalki, H.D. Counteraction of biofilm formation and antimicrobial potential of Terminalia catappa functionalized silver nanoparticles against Candida albicans and multidrug-resistant Gram-negative and Gram-positive bacteria. Antibiotics 2021, 10, 725. [Google Scholar] [CrossRef] [PubMed]





| AgNPs | AgNO3 | NS | ||
|---|---|---|---|---|
| A. niger | IZ/mm | 16 | ND ** | ND |
| MIC * | 125 | ND | ND | |
| A. terreus | IZ/mm | 20 | ND | ND |
| MIC | 62.5 | ND | ND | |
| A. flavus | IZ/mm | 26 | ND | ND |
| MIC | 15.62 | ND | ND | |
| A. fumigatus | IZ/mm | 19 | ND | ND |
| MIC | 62.5 | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, A.H.; Saied, E.; Amin, B.H.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Elbahnasawy, M.A. Antifungal Activity of Biosynthesized Silver Nanoparticles (AgNPs) against Aspergilli Causing Aspergillosis: Ultrastructure Study. J. Funct. Biomater. 2022, 13, 242. https://doi.org/10.3390/jfb13040242
Hashem AH, Saied E, Amin BH, Alotibi FO, Al-Askar AA, Arishi AA, Elkady FM, Elbahnasawy MA. Antifungal Activity of Biosynthesized Silver Nanoparticles (AgNPs) against Aspergilli Causing Aspergillosis: Ultrastructure Study. Journal of Functional Biomaterials. 2022; 13(4):242. https://doi.org/10.3390/jfb13040242
Chicago/Turabian StyleHashem, Amr H., Ebrahim Saied, Basma H. Amin, Fatimah O. Alotibi, Abdulaziz A. Al-Askar, Amr A. Arishi, Fathy M. Elkady, and Mostafa A. Elbahnasawy. 2022. "Antifungal Activity of Biosynthesized Silver Nanoparticles (AgNPs) against Aspergilli Causing Aspergillosis: Ultrastructure Study" Journal of Functional Biomaterials 13, no. 4: 242. https://doi.org/10.3390/jfb13040242






