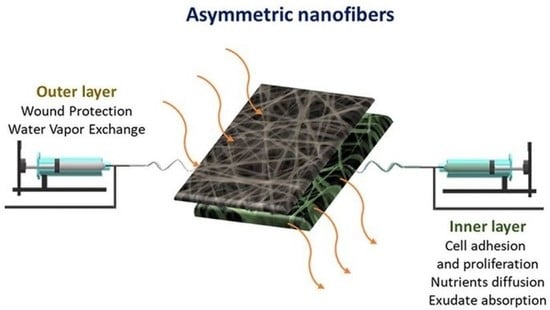
Design of Asymmetric Nanofibers-Membranes Based on Polyvinyl Alcohol and Wool-Keratin for Wound Healing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanofiber Preparation
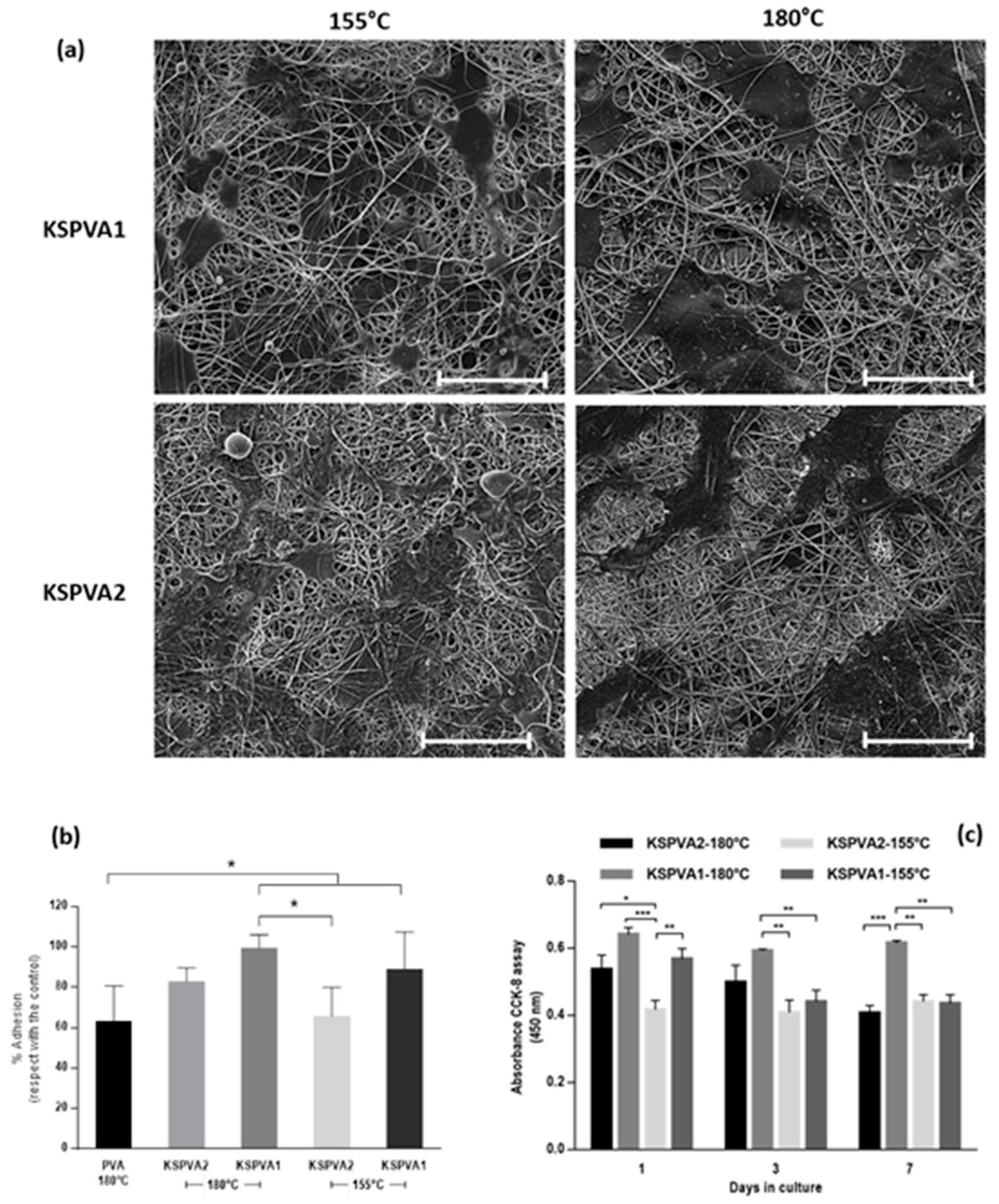
2.2. Morphological Characterization (SEM)
2.3. Water Contact Angle (WCA)
2.4. Water Uptake
2.5. Fourier-Transform Infrared Spectroscopy Analysis (FTIR)
2.6. Cell Culture Tests
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borena, B.M.; Martens, A.; Broeckx, S.Y.; Meyer, E.; Chiers, K.; Duchateau, L.; Spaas, J.H. Regenerative Skin Wound Healing in Mammals: State-of-the-Art on Growth Factor and Stem Cell Based Treatments. Cell. Physiol. Biochem. 2015, 36, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Lalley, A.L. Tissue engineering of skin and regenerative medicine for wound care. Burn. Trauma 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afsharian, Y.P.; Rahimnejad, M. Bioactive electrospun scaffolds for wound healing applications: A comprehensive review. Polym. Test. 2021, 93, 106952. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Hussein, K.H.; Casettari, L.; Khalil, K.A.; Hamdy, A.S. Fabrication of novel high performance ductile poly(lactic acid) nanofiber scaffold coated with poly(vinyl alcohol) for tissue engineering applications. Mater. Sci. Eng. C 2016, 60, 143–150. [Google Scholar] [CrossRef]
- You, Y.; Park, W.H.; Ko, B.M.; Min, B.-M. Effects of PVA sponge containing chitooligosaccharide in the early stage of wound healing. J. Mater. Sci. Mater. Electron. 2004, 15, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-H.; Yang, M.-C. Evaluation of glucan/poly(vinyl alcohol) blend wound dressing using rat models. Int. J. Pharm. 2008, 346, 38–46. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R.; Upadhyay, N.K.; Surekha, P.; Roy, P.K. Synthesis, characterization and efficacy of chemically crosslinked PVA hydrogels for dermal wound healing in experimental animals. J. Appl. Polym. Sci. 2009, 111, 1400–1408. [Google Scholar] [CrossRef]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yong, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S.; et al. Development of polyvinyl alcohol–sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef]
- Niranjan, R.; Kaushik, M.; Prakash, J.; Venkataprasanna, K.S.; Arpana, C.; Balashanmugam, P.; Venkatasubbu, G.D. Enhanced wound healing by PVA/Chitosan/Curcumin patches: In vitro and in vivo study. Colloids Surfaces B Biointerfaces 2019, 182, 110339. [Google Scholar] [CrossRef]
- Bi, H.; Feng, T.; Li, B.; Han, Y. In Vitro and In Vivo Comparison Study of Electrospun PLA and PLA/PVA/SA Fiber Membranes for Wound Healing. Polymers 2020, 12, 839. [Google Scholar] [CrossRef] [Green Version]
- Jatoi, A.W.; Ogasawara, H.; Kim, I.S.; Ni, Q.-Q. Polyvinyl alcohol nanofiber based three phase wound dressings for sustained wound healing applications. Mater. Lett. 2019, 241, 168–171. [Google Scholar] [CrossRef]
- Khabbaz, B.; Solouk, A.; Mirzadeh, H. Polyvinyl alcohol/soy protein isolate nanofibrous patch for wound-healing applications. Prog. Biomater. 2019, 8, 185–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarino, V.; Cirillo, V.; Ambrosio, L. Bicomponent electrospun scaffolds to design extracellular matrix tissue analogs. Expert Rev. Med. Devices 2015, 13, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Maya, I.; Guarino, V.; Almaguer-Flores, A.; Alvarez-Perez, M.A.; Varesano, A.; Vineis, C. Highly polydisperse keratin rich nanofibers: Scaffold design and in vitro characterization. J. Biomed. Mater. Res. Part A 2019, 107, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Maya, I.; Varesano, A.; Vineis, C.; Guarino, V. Comparative Study on Protein-Rich Electrospun Fibers for in Vitro Applications. Polymers 2020, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Varesano, A.; Vineis, C.; Tonetti, C.; Sanchez Ramirez, D.O.; Mazzuchetti, G. Chemical and physical modifications of electrospun keratin nanofibers induced by heating treatments. J. Appl. Polym. Sci. 2014, 131, 40532. [Google Scholar] [CrossRef]
- Hong, K.H.; Park, J.L.; Sul, I.H.; Youk, J.H.; Kang, T.J. Preparation of antimicrobial poly(vinyl alcohol) nanofibers containing silver nanoparticles. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2468–2474. [Google Scholar] [CrossRef]
- Varesano, A.; Vineis, C.; Tonetti, C.; Sanchez Ramirez, D.O.; Mazzuchetti, G.; Ortelli, S.; Blosi, M.; Costa, A. Multifunctional Hybrid Nanocomposite Nanofibers Produced by Colloid Electrospinning from Water Solutions. Curr. Nanosci. 2014, 11, 41–48. [Google Scholar] [CrossRef]
- van de Weert, M.; Haris, P.I.; Hennink, W.E.; Crommelin, D.J. Fourier Transform Infrared Spectrometric Analysis of Protein Conformation: Effect of Sampling Method and Stress Factors. Anal. Biochem. 2001, 297, 160–169. [Google Scholar] [CrossRef]
- Cobb, J.S.; Zai-Rose, V.; Correia, J.J.; Janorkar, A.V. FT-IR Spectroscopic Analysis of the Secondary Structures Present during the Desiccation Induced Aggregation of Elastin-Like Polypeptide on Silica. ACS Omega 2020, 5, 8403–8413. [Google Scholar] [CrossRef] [PubMed]
- Troullier, A.; Reinstädler, D.; Dupont, Y.; Naumann, D.; Forge, V. Transient non-native secondary structures during the refolding of alpha-lactalbumin detected by infrared spectroscopy. Nat. Genet. 2000, 7, 78–86. [Google Scholar] [CrossRef]
- Baird, G.; Farrell, C.; Cheung, J.; Semple, A.; Blue, J.; Ahl, P.L. FTIR Spectroscopy Detects Intermolecular β-Sheet Formation Above the High Temperature Tm for Two Monoclonal Antibodies. Protein J. 2020, 39, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Middaugh, C.R.; Offerdahl, T.; Munson, E.; Sane, S.; Rytting, J.H. Spectroscopic evaluation of the stabilization of humanized monoclonal antibodies in amino acid formulations. Int. J. Pharm. 2007, 335, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Belton, D.J.; Plowright, R.; Kaplan, D.L.; Perry, C.C. A robust spectroscopic method for the determination of protein conformational composition–Application to the annealing of silk. Acta Biomater. 2018, 73, 355–364. [Google Scholar] [CrossRef] [Green Version]
- DeFlores, L.P.; Ganim, Z.; Nicodemus, R.A.; Tokmakoff, A. Amide I′−II′ 2D IR Spectroscopy Provides Enhanced Protein Secondary Structural Sensitivity. J. Am. Chem. Soc. 2009, 131, 3385–3391. [Google Scholar] [CrossRef] [PubMed]
- Cardamone, J.M. Investigating the microstructure of keratin extracted from wool: Peptide sequence (MALDI-TOF/TOF) and protein conformation (FTIR). J. Mol. Struct. 2010, 969, 97–105. [Google Scholar] [CrossRef]
- Litvinov, R.; Faizullin, D.A.; Zuev, Y.; Weisel, J.W. The α-Helix to β-Sheet Transition in Stretched and Compressed Hydrated Fibrin Clots. Biophys. J. 2012, 103, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Secundo, A.F.; Guerrieri, N. ATR-FT/IR Study on the Interactions between Gliadins and Dextrin and Their Effects on Protein Secondary Structure. J. Agric. Food Chem. 2005, 53, 1757–1764. [Google Scholar] [CrossRef]
- Holland, B.; Hay, J. The thermal degradation of poly(vinyl alcohol). Polymer 2001, 42, 6775–6783. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, S.J.; Kim, I.Y.; Lee, Y.H.; Kim, S.I. Thermal characteristics of poly(vinyl alcohol) and poly(vinylpyrrolidone) IPNs. J. Appl. Polym. Sci. 2002, 86, 1844–1847. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, J.; Zhou, T. Moving-window two-dimensional correlation infrared spectroscopic study on the dissolution process of poly(vinyl alcohol). Anal. Bioanal. Chem. 2015, 407, 8765–8771. [Google Scholar] [CrossRef]
- Cardamone, J.M. Keratin transamidation. Int. J. Biol. Macromol. 2008, 42, 413–419. [Google Scholar] [CrossRef]
- Jackson, M.; Mantsch, H.H. The Use and Misuse of FTIR Spectroscopy in the Determination of Protein Structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Erra, P.; Gómez, N.; Dolcet, L.M.; Juliá, M.R.; Lewis, D.M.; Willoughby, J.H. FTIR Analysis to Study Chemical Changes in Wool Following a Sulfitolysis Treatment. Text. Res. J. 1997, 67, 397–401. [Google Scholar] [CrossRef]
- Mansur, H.; Sadahira, C.M.; Souza, A.N.; Mansur, A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Raksa, A.; Utke, R.; Ruksakulpiwat, C.; Numpaisal, P.-O.; Ruksakulpiwat, Y. Morphological and chemical characterization of electrospun silk fibroin/polyvinyl alcohol nanofibers. Second. Mater. Res. Soc. Thail. Int. Conf. 2020, 2279, 080004. [Google Scholar] [CrossRef]
- Park, C.; Carlson, M.J.; Goddard, W.A. Solvent Effects on the Secondary Structures of Proteins. J. Phys. Chem. A 2000, 104, 2498–2503. [Google Scholar] [CrossRef] [Green Version]
- Christensen, L.F.B.; Nowak, J.S.; Sønderby, T.V.; Frank, S.A.; Otzen, D.E. Quantitating denaturation by formic acid: Imperfect repeats are essential to the stability of the functional amyloid protein FapC. J. Biol. Chem. 2020, 295, 13031–13046. [Google Scholar] [CrossRef]
- Colomer, I.; Batchelor-McAuley, C.; Odell, B.; Donohoe, T.J.; Compton, R.G. Hydrogen Bonding to Hexafluoroisopropanol Controls the Oxidative Strength of Hypervalent Iodine Reagents. J. Am. Chem. Soc. 2016, 138, 8855–8861. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Guo, T.; Yang, X.; Deng, J.; Zhu, L.; Wang, B.; Hao, S. Keratin nanoparticles-coating electrospun PVA nanofibers for potential neural tissue applications. J. Mater. Sci. Mater. Med. 2019, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Han, S.; Zhang, R.; Liu, G.; Wu, J. Progress in electrospun composite nanofibers: Composition, performance and applications for tissue engineering. J. Mater. Chem. B 2019, 7, 7075–7089. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.R.; Guarino, V.; Oliveira, M.J.; Ribeiro, C.C.; A Barbosa, M.; Ambrosio, L.; Pêgo, A.P. Ibuprofen-loaded poly(trimethylene carbonate-co-ε-caprolactone) electrospun fibres for nerve regeneration. J. Tissue Eng. Regen. Med. 2016, 10, E154–E166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Babaeijandaghi, F.; Shabani, I.; Seyedjafari, E.; Naraghi, Z.S.; Vasei, M.; Haddadi-Asl, V.; Hesari, K.K.; Soleimani, M. Accelerated Epidermal Regeneration and Improved Dermal Reconstruction Achieved by Polyethersulfone Nanofibers. Tissue Eng. Part A 2010, 16, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation 2018 Impurities: Guideline for residual solvents Q3C (R7). Curr. Step 2005, 4, 509.
- Mosher, C.Z.; Brudnicki, P.A.P.; Gong, Z.; Childs, H.R.; Lee, S.W.; Antrobus, R.M.; Fang, E.C.; Schiros, T.N.; Lu, H.H. Green electrospinning for biomaterials and biofabrication. Biofabrication 2021, 13, 035049. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Bao, Z.; Wu, J. Injectable baicalin/F127 hydrogel with antioxidant activity for enhanced wound healing. Chin. Chem. Lett. 2020, 31, 1817–1821. [Google Scholar] [CrossRef]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings. Mini-Rev. Med. Chem. 2018, 18, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Khodir, W.W.A.; Guarino, V.; Alvarez-Perez, M.; Cafiero, C.; Ambrosio, L. Trapping tetracycline-loaded nanoparticles into polycaprolactone fiber networks for periodontal regeneration therapy. J. Bioact. Compat. Polym. 2013, 28, 258–273. [Google Scholar] [CrossRef]
- Khodir, W.K.W.A.; Razak, A.H.A.; Ng, M.H.; Guarino, V.; Susanti, D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Salinas, S.; Evangelopoulos, M.; Gámez-Herrera, E.; Arruebo, M.; Irusta, S.; Taraballi, F.; Mendoza, G.; Tasciotti, E. Electrospun anti-inflammatory patch loaded with essential oils for wound healing. Int. J. Pharm. 2020, 577, 119067. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.A.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef]
- Akhmetova, A.; Heinz, A. Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics 2020, 13, 4. [Google Scholar] [CrossRef]
- Vineis, C.; Maya, I.C.; Mowafi, S.; Varesano, A.; Sanchez Ramirez, D.O.; Taleb, M.A.; Tonetti, C.; Guarino, V.; El-Sayed, H. Synergistic effect of sericin and keratin in gelatin based nanofibers for in vitro applications. Int. J. Biol. Macromol. 2021, 190, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Guarino, V.; Cochis, A.; Varesano, A.; Maya, I.C.; Vineis, C.; Rimondini, L.; Spriano, S. Aligned keratin submicrometric-fibers for fibroblasts guidance onto nanogrooved titanium surfaces for transmucosal implants. Mater. Lett. 2018, 229, 1–4. [Google Scholar] [CrossRef]
- Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.W.A.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J. Funct. Biomater. 2021, 12, 59. [Google Scholar] [CrossRef]
- Chen, B.; Xing, Y.; Yu, W.; Liu, H. Wool keratin and silk sericin composite films reinforced by molecular network reconstruction. J. Mater. Sci. 2017, 53, 5418–5428. [Google Scholar] [CrossRef]
- Yao, C.-H.; Lee, C.-Y.; Huang, C.-H.; Chen, Y.-S.; Chen, K.-Y. Novel bilayer wound dressing based on electrospun gelatin/keratin nanofibrous mats for skin wound repair. Mater. Sci. Eng. C 2017, 79, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Arab-Bafrani, Z.; Karimi, F.; Javid, N. Designing hybrid nanofibers based on keratin-poly (vinyl alcohol) and poly (ε-caprolactone) for application as wound dressing. J. Ind. Text. 2021, 1528083721988978. [Google Scholar] [CrossRef]
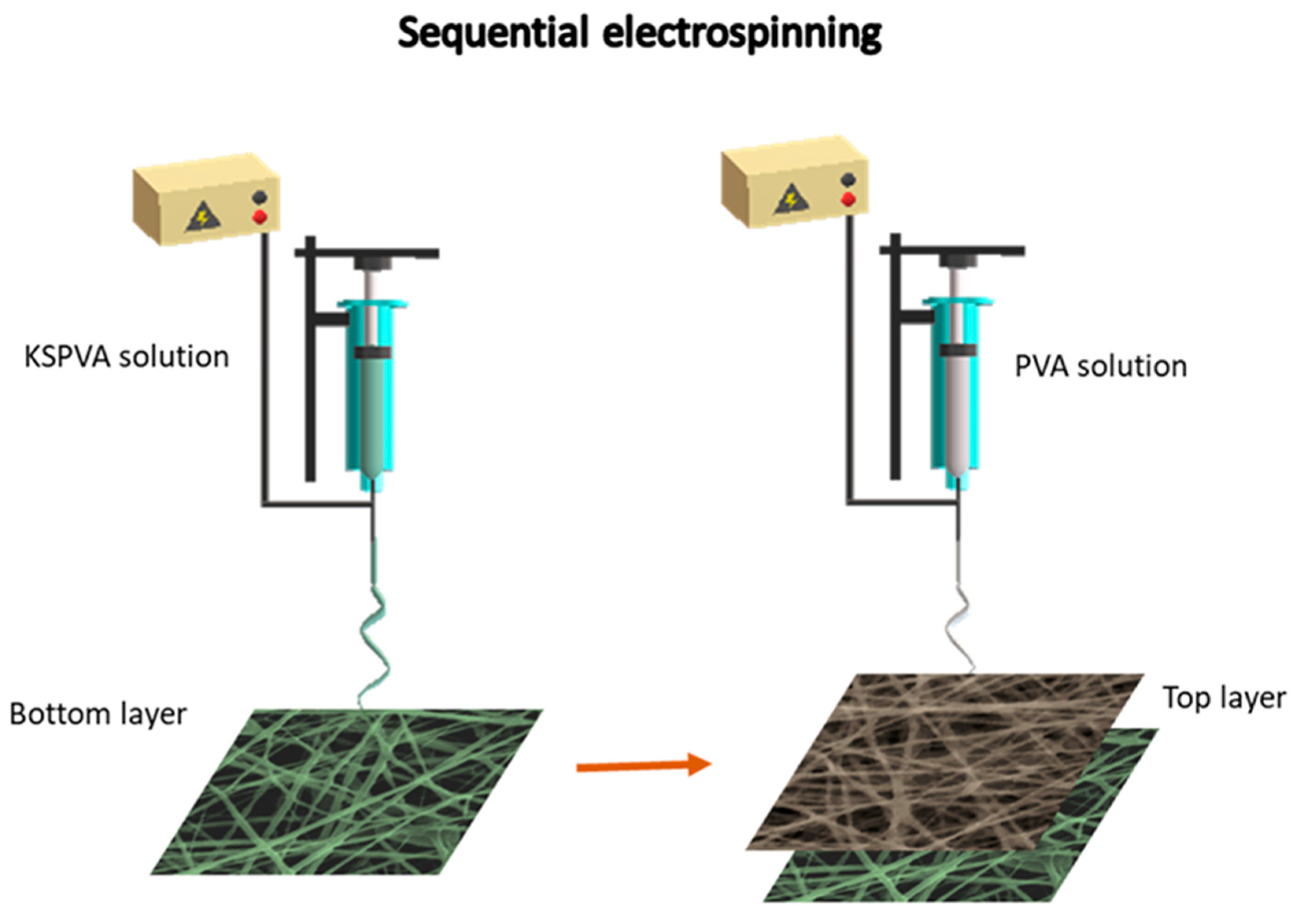


| Samples | Solvent | Total Polymer Concentration (% wt) | Polymer Blend (% wt) | Flow Rate (mL min−1) | Tip Collector Distance (cm) | Voltage (kV) | Needle Inner Diameter (mm) |
|---|---|---|---|---|---|---|---|
| KSPVA1 (Bottom-layer) | Water | 8.75 | 17/83 KS/PVA | 0.015 | 30 | 25 | 0.4 |
| KSPVA2 (Bottom-layer) | Water | 10.00 | 33/67 KS/PVA | 0.015 | 25 | 25 | 0.4 |
| PVA (Up-layer) | Water | 9.00 | 100 PVA | 0.008 | 30 | 23 | 0.4 |
| Sample | Thermal Post-Treatment | WCAti (°), 0 s | WCAtf (°), 3.3 s | Δ (°), WCAti—WCAtf |
|---|---|---|---|---|
| PVA | 155 °C—3 min | 50.2 ± 2.4 | 36.6 ± 1.4 | 14 |
| KSPVA1 | 155 °C—3 min | 78.3 ± 4.0 | 36.7 ± 10.1 | 42 |
| KSPVA2 | 155 °C—3 min | 52.6 ± 3.3 | 18.3 ± 6.1 | 34 |
| PVA | 180 °C—2 h | 67.4 ± 4.1 | 51.6 ± 5.3 | 16 |
| KSPVA1 | 180 °C—2 h | 131.0 ± 4.8 | 123.7 ± 8.7 | 7 |
| KSPVA2 | 180 °C—2 h | 127.8 ± 3.1 | 107.1 ± 6.1 | 21 |
| Sample | Thermal Post-Treatment | |
|---|---|---|
| 155 °C—3 min | 180 °C—2 h | |
| Pure PVA | 3.4% | 3.3% |
| KSPVA1 | 4.9% | 8.5% |
| KSPVA2 | 16.4% | 9.7% |
| Thermal Post-Treatment | Sample | ||
|---|---|---|---|
| PVA | KSPVA1 | KSPVA2 | |
| As spun | 0.5813 | 0.7405 | 0.7508 |
| 155 °C—3 min | 0.6779 | 0.7547 | 0.7568 |
| 180 °C—2 h | 0.7611 | 0.7244 | 0.7554 |
| Sample | Intermolecular β-Sheet | Intramolecular β-Sheet | β-Sheet II | β-Turn | Random Coil | α-Helix |
|---|---|---|---|---|---|---|
| KS (lyophilized powder) | 23.9 | 6.5 | 24.2 | 11.3 | 6.7 | 27.4 |
| KSPVA1, as spun | 23.2 | 5.5 | 13.4 | 17.2 | 2.8 | 37.9 |
| KSPVA1, 155 °C—3 min | 24.8 | 5.5 | 10.7 | 16.7 | 1.8 | 40.4 |
| KSPVA1, 180 °C—2 h | 25.8 | 5.6 | 14.1 | 18.5 | 6.0 | 30.0 |
| KSPVA2, as spun | 27.2 | 5.3 | 7.3 | 16.4 | 0.8 | 43.0 |
| KSPVA2, 155 °C—3 min | 27.0 | 5.1 | 13.4 | 13.3 | 1.7 | 39.5 |
| KSPVA2, 180 °C—2 h | 31.9 | 4.7 | 8.9 | 14.7 | 1.7 | 38.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez Ramirez, D.O.; Cruz-Maya, I.; Vineis, C.; Tonetti, C.; Varesano, A.; Guarino, V. Design of Asymmetric Nanofibers-Membranes Based on Polyvinyl Alcohol and Wool-Keratin for Wound Healing Applications. J. Funct. Biomater. 2021, 12, 76. https://doi.org/10.3390/jfb12040076
Sanchez Ramirez DO, Cruz-Maya I, Vineis C, Tonetti C, Varesano A, Guarino V. Design of Asymmetric Nanofibers-Membranes Based on Polyvinyl Alcohol and Wool-Keratin for Wound Healing Applications. Journal of Functional Biomaterials. 2021; 12(4):76. https://doi.org/10.3390/jfb12040076
Chicago/Turabian StyleSanchez Ramirez, Diego O., Iriczalli Cruz-Maya, Claudia Vineis, Cinzia Tonetti, Alessio Varesano, and Vincenzo Guarino. 2021. "Design of Asymmetric Nanofibers-Membranes Based on Polyvinyl Alcohol and Wool-Keratin for Wound Healing Applications" Journal of Functional Biomaterials 12, no. 4: 76. https://doi.org/10.3390/jfb12040076
APA StyleSanchez Ramirez, D. O., Cruz-Maya, I., Vineis, C., Tonetti, C., Varesano, A., & Guarino, V. (2021). Design of Asymmetric Nanofibers-Membranes Based on Polyvinyl Alcohol and Wool-Keratin for Wound Healing Applications. Journal of Functional Biomaterials, 12(4), 76. https://doi.org/10.3390/jfb12040076