Metallization by Sputtering to Improve the Bond Strength between Zirconia Ceramics and Resin Cements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
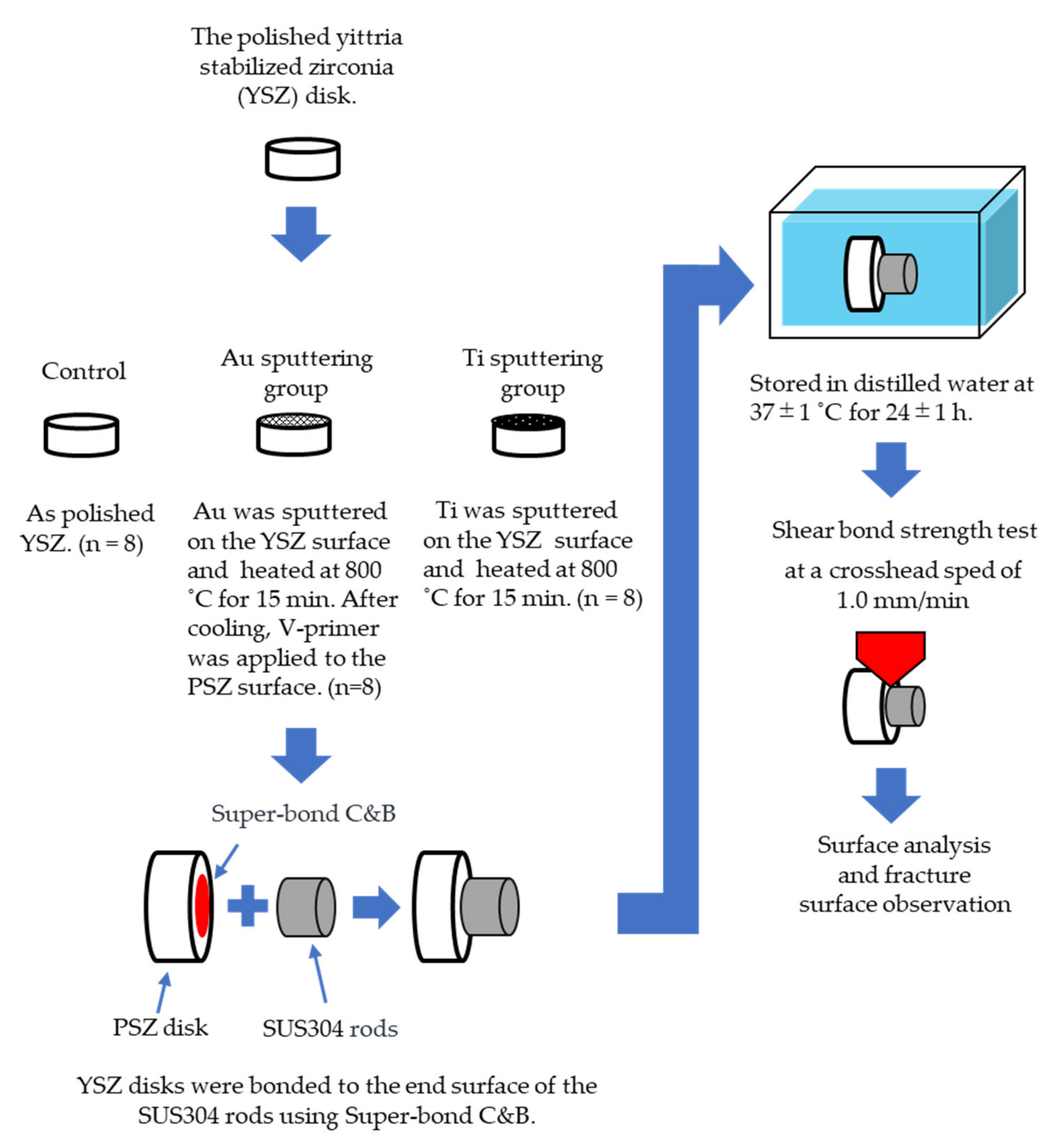
2.2. Surface Treatment of YSZ
- Control group: Polished YSZ was used as the control.
- Au sputtering group: Au was sputtered on the YSZ surface using an ion coater (IB-II, Eiko, Japan) for 15 min. After sputtering, the YSZ specimens were heated at 800 °C for 15 min in a porcelain furnace (JELENKO LT ‖, Morita, Osaka, Japan) in air, and then cooled to room temperature. After cooling, V-primer (Sun Medical, Shiga, Japan)—which enhances the bonding strength—was applied to the YSZ surface according to the manufacturer’s instructions.
- Ti sputtering group: Ti was sputtered on the YSZ surface using a high-frequency sputtering unit (SBR1104E, ULVAC, Kanagawa, Japan) at 150 W for 8 h. After sputtering, the YSZ disks and the specimens in the Au sputtering group were heated to 800 °C for 15 min in a porcelain furnace in air and were then cooled to room temperature.
2.3. Bonding Procedures
2.4. Measurement of Shear Bond Strength
2.5. Surface Analysis, Fracture Surface Observation, and Line Analysis
2.6. Statistical Analysis
3. Results
3.1. Shear Bond Strength
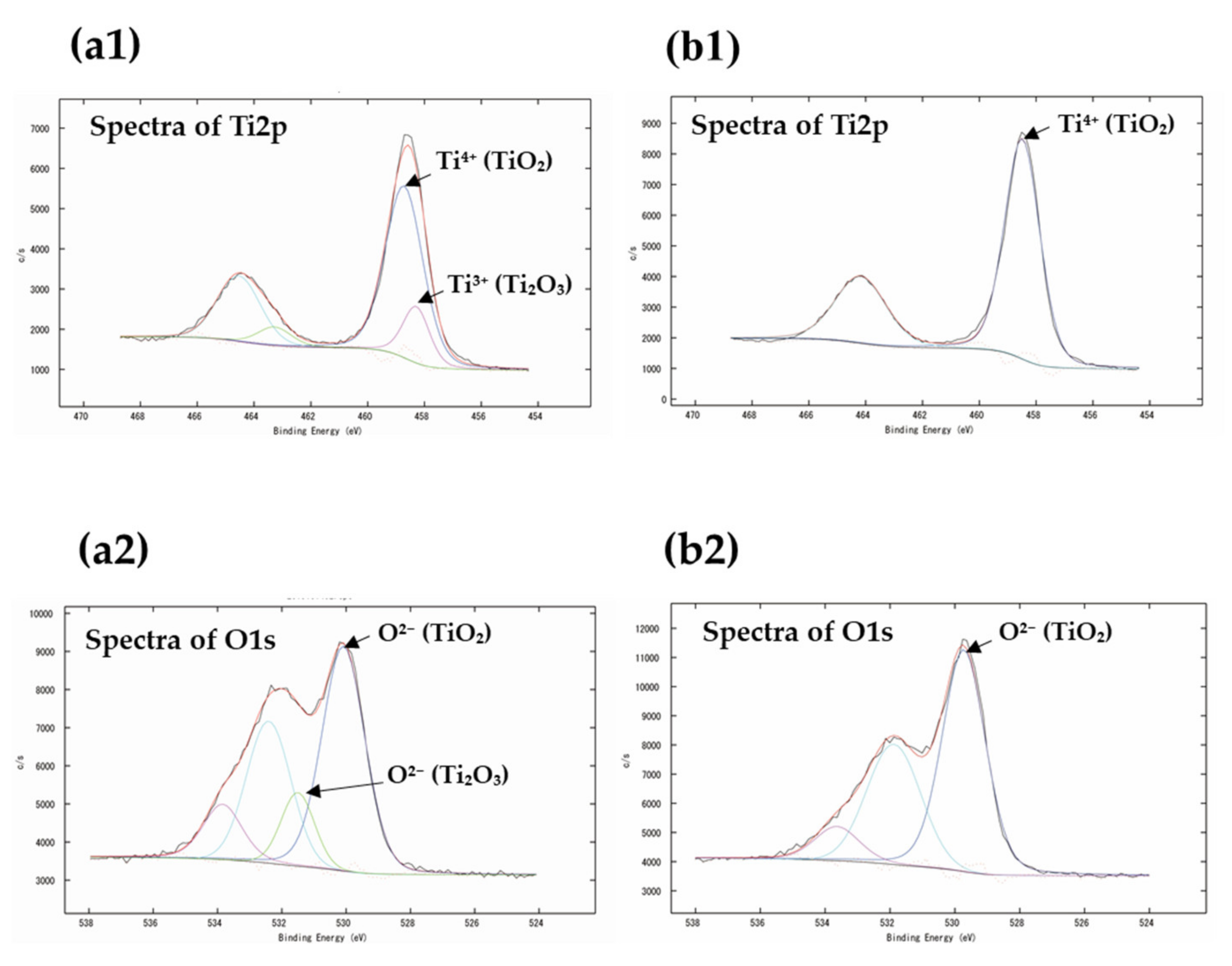
3.2. XPS Analysis
3.3. Fracture Surface Observation
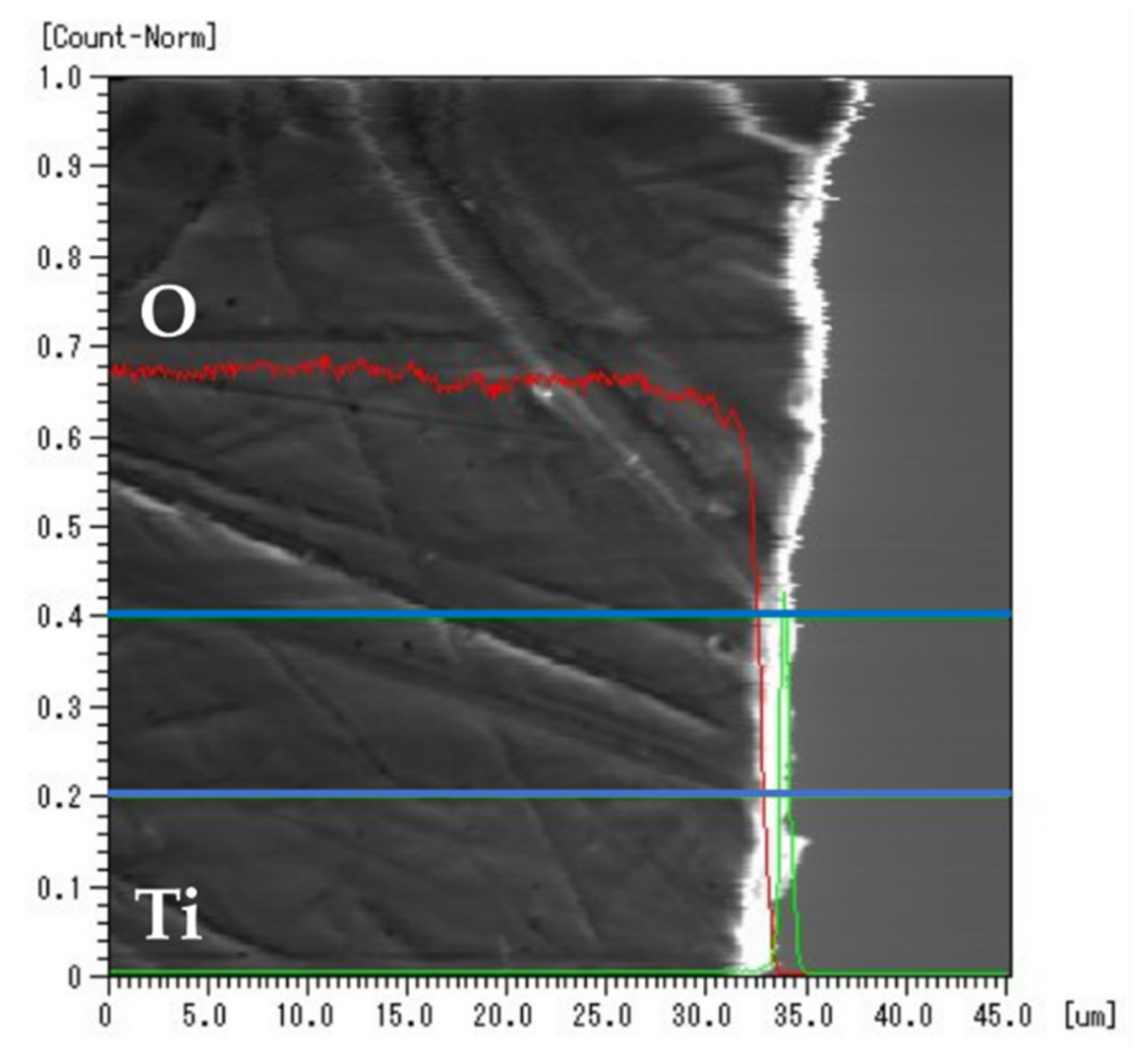
3.4. EPMA Elemental Mapping
3.5. EPMA Line Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrguth, M.; Wichmann, M.; Reich, S. The aesthetics of all-ceramic veneered and monolithic CAD/CAM crowns. J. Oral Rehabil. 2005, 32, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Ayash, G.M.; Ossman, E.; Segaan, L.G.; Rayyan, M.; Joukhadar, C. Influence of core color on final shade reproduction of zirconia crown in single central incisor situation–An in vivo study. J. Clin. Exp. Dent. 2020, 12, 46–51. [Google Scholar] [CrossRef]
- Brunot-Gohin, C.; Duval, J.L.; Verbeke, S.; Belanger, K.; Pezron, I.; Kugel, G.; Laurent-Maquin, D.; Gangloff, S.; Egles, C. Biocompatibility study of lithium disilicate and zirconium oxide ceramics for esthetic dental abutments. J. Periodontal. Implant Sci. 2016, 46, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beuer, F.; Schweiger, J.; Eichberger, M.; Kappert, H.F.; Gernet, W.; Edelhoff, D. High-strength CAD/CAM-fabricated veneering material sintered to zirconia copings—A new fabrication mode for all-ceramic restorations. Dent. Mater. 2009, 25, 121–128. [Google Scholar] [CrossRef]
- Ayash, G.; Osman, E.; Segaan, L.; Rayyan, M.; Joukhadar, C. Influence of resin cement shade on the color and translucency of zirconia crowns. J. Clin. Exp. Dent. 2020, 12, e257–e263. [Google Scholar] [CrossRef]
- Tran, D.; Nesbit, M.; Petridis, H. Survey of UK dentists regarding the use of CAD/CAM technology. Br. Dent. J. 2016, 221, 639–644. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hotta, Y.; Kunii, J.; Kuriyama, S.; Tamaki, Y. A review of dental CAD/CAM: Current status and future perspectives from 20 years of experience. Dent. Mater. J. 2009, 28, 44–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleimani, F.; Jalali, H.; Mostafavi, A.S.; Zeighami, S.; Memarian, M. Retention and clinical performance of zirconia crowns: A comprehensive review. Int. J. Dent. 2020, 2020, 8846534. [Google Scholar] [CrossRef]
- Beuer, F.; Edelhoff, D.; Gernet, W.; Sorensen, J.A. Three-year clinical prospective evaluation of zirconia-based posterior fixed dental prostheses (FDPs). Clin. Oral Investig. 2009, 13, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Yanagida, H.; Tanoue, N.; Atsuta, M.; Shimoe, S. Shear bond strength of resin composite veneering material to gold alloy with varying metal surface preparations. J. Prosthet. Dent. 2001, 86, 315–319. [Google Scholar] [CrossRef]
- Shimoe, S.; Peng, T.Y.; Wakabayashi, Y.; Takenaka, H.; Iwaguro, S.; Kaku, M. Laser-milled microslits improve the bonding strength of acrylic resin to zirconia ceramics. Polymers 2020, 12, 817. [Google Scholar] [CrossRef] [Green Version]
- Seker, E.; Kilicarslan, M.A.; Deniz, S.T.; Mumcu, E.; Ozkan, P. Effect of atmospheric plasma versus conventional surface treatments on the adhesion capability between self-adhesive resin cement and titanium surface. J. Adv. Prosthodont. 2015, 7, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Çelik, E.; Şahin, S.C.; Dede, D.Ö. Effect of surface treatments on the bond strength of indirect resin composite to resin matrix ceramics. J. Adv. Prosthodont. 2019, 11, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchimoto, Y.; Yoshida, Y.; Mine, A.; Nakamura, M.; Nishiyama, N.; Van Meerbeek, B.; Suzuki, K.; Kuboki, T. Effect of 4-MET- and 10-MDP-based primers on resin bonding to titanium. Dent. Mater. J. 2006, 25, 120–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, H.; Kamada, K.; Tanoue, N.; Atsuta, M. Effect of thione primers on bonding of noble metal alloys with an adhesive resin. J. Dent. 2000, 28, 287–293. [Google Scholar] [CrossRef]
- Tzanakakis, E.G.C.; Tzoutzas, I.G.; Koidis, P.T. Is there a potential for durable adhesion to zirconia restorations? A systematic review. J. Prosthet. Dent. 2016, 115, 9–19. [Google Scholar] [CrossRef]
- Oba, Y.; Koizumi, H.; Nakayama, D.; Ishii, T.; Akazawa, N.; Matsumura, H. Effect of silane and phosphate primers on the adhesive performance of a tri-n-butylborane initiated luting agent bonded to zirconia. Dent. Mater. J. 2014, 33, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Inokoshi, M.; Vanmeensel, K.; Zhang, F.; De Munck, J.; Eliades, G.; Minakuchi, S.; Naert, I.; Van Meerbeek, B.; Vleugels, J. Aging resistance of surface-treated dental zirconia. Dent. Mater. 2015, 31, 182–194. [Google Scholar] [CrossRef]
- Gomes, A.L.; Castillo-Oyagüe, R.; Lynch, C.D.; Montero, J.; Albaladejo, A. Influence of sandblasting granulometry and resin cement composition on microtensile bond strength to zirconia ceramic for dental prosthetic frameworks. J. Dent. 2013, 41, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Ren, K.; Wang, Y. Reversible joining of zirconia to titanium alloy. Ceram. Int. 2019, 45, 2509–2515. [Google Scholar] [CrossRef]
- Singh, M.; Shpargel, T.P.; Asthana, R. Brazing of stainless steel to yttria-stabilized zirconia using gold-based brazes for solid oxide fuel cell applications. Int. J. Appl. Ceram. Technol. 2007, 4, 119–133. [Google Scholar] [CrossRef]
- Pimenta, J.S.; Buschinelli, A.J.A.; do Nascimento, R.M.; Martinelli, A.E.; Remmel, J. Joining of zirconia mechanically metallized with titanium. Cerâmica 2010, 56, 212–221. [Google Scholar] [CrossRef]
- Lin, K.L.; Singh, M.; Asthana, R.; Lin, C.H. Interfacial and mechanical characterization of yttria-stabilized zirconia (YSZ) to stainless steel joints fabricated using Ag-Cu-Ti interlayers. Ceram. Int. 2014, 40, 2063–2071. [Google Scholar] [CrossRef]
- Kwon, M.S.; Oh, S.Y.; Cho, S.A. Two-body wear comparison of zirconia crown, gold crown, and enamel against zirconia. J. Mech. Behav. Biomed. Mater. 2015, 47, 21–28. [Google Scholar] [CrossRef]
- Fage, S.W.; Muris, J.; Jakobsen, S.S.; Thyssen, J.P. Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermatitis 2016, 74, 323–345. [Google Scholar] [CrossRef] [Green Version]
- Bishara, S.E.; Ajlouni, R.; Laffoon, J.F. Effect of thermocycling on the shear bond strength of a cyanoacrylate orthodontic adhesive. Am. J. Orthod. Dentofac. Orthop. 2003, 123, 21–24. [Google Scholar] [CrossRef]
- Okawa, S.; Taka, N.; Aoyagi, Y. Effect of modification with helium atmospheric-pressure plasma and deep-ultraviolet light on adhesive shear strength of fiber-reinforced poly(ether-ether-ketone) polymer. J. Funct. Biomater. 2020, 11, 27. [Google Scholar] [CrossRef]
- Bona, A.D.; Borba, M.; Benetti, P.; Cecchetti, D. Effect of surface treatments on the bond strength of a zirconia-reinforced ceramic to composite resin. Braz. Oral Res. 2007, 21, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, K.; Uenodan, A.; Kajihara, Y.; Murahara, S.; Minesaki, Y.; Minami, H. A study on the development of a primer effective for bonding various non-metallic restoratives—Effects of acidic functional monomers on silane coupling treatment. J. Adhes. Dent. 2018, 36, 141–145. [Google Scholar]
- Aboushelib, M.N.; Wang, H.; Kleverlaan, C.J.; Feilzer, A.J. Fatigue behavior of zirconia under different loading conditions. Dent. Mater. 2016, 32, 915–920. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Fatigue sensitivity of Y-TZP to microscale sharp-contact flaws. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 388–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, D. X-ray photoelectron spectroscopy (XPS). In Handbook of Adhesion, 3rd ed.; Packham, D.E., Ed.; John Wiley & Sons: Chichester, UK, 2005; pp. 621–622. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ida, K.; Togaya, T.; Tsutsumi, S.; Takeuchi, M. Effect of magnesia investments in the dental casting of pure titanium or titanium alloys. Dent. Mater. J. 1982, 1, 8–21. [Google Scholar] [CrossRef] [PubMed]



| Materials/Product Name | Abbreviation | Manufacturer | Composition |
|---|---|---|---|
| Yttria stabilized zirconia/ZR8Y-12.0 | YSZ | AS ONE Co., Ltd. | 92.0% ZrO2, 8.0% Y2O |
| Stainless steel/SM995 | SUS306 | Stainless Hikari Co., Ltd. | 72.0% Fe, 8.0% Ni, 18.0% Cr, 2.0% others |
| Resin cement/Super Bond C&B | SB | SUN MEDICAL Co., Ltd. (Moriyama City, Japan) | 4-META, TBB, PMMA, MMA |
| Adhesive monomer/V primer | VP | SUN MEDICAL Co., Ltd. | 0.1 mol% VBTDT, acetone |
| XPS Binding Energy (eV) | Atomic Concentration (at%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ti2p | O1s | C1s | O1s | Ti2p | Zr3d | |||
| Before heating | 458.7 | 458.3 | 531.5 | 530.1 | 60.19 | 33.63 | 6.10 | 0.09 |
| After heating | 458.5 | 529.7 | 53.39 | 39.06 | 7.55 | 0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, T.; Aoyagi, Y.; Taka, N.; Kanatani, M.; Uoshima, K. Metallization by Sputtering to Improve the Bond Strength between Zirconia Ceramics and Resin Cements. J. Funct. Biomater. 2021, 12, 62. https://doi.org/10.3390/jfb12040062
Kimura T, Aoyagi Y, Taka N, Kanatani M, Uoshima K. Metallization by Sputtering to Improve the Bond Strength between Zirconia Ceramics and Resin Cements. Journal of Functional Biomaterials. 2021; 12(4):62. https://doi.org/10.3390/jfb12040062
Chicago/Turabian StyleKimura, Tatsuya, Yujin Aoyagi, Norimasa Taka, Mitsugu Kanatani, and Katsumi Uoshima. 2021. "Metallization by Sputtering to Improve the Bond Strength between Zirconia Ceramics and Resin Cements" Journal of Functional Biomaterials 12, no. 4: 62. https://doi.org/10.3390/jfb12040062






