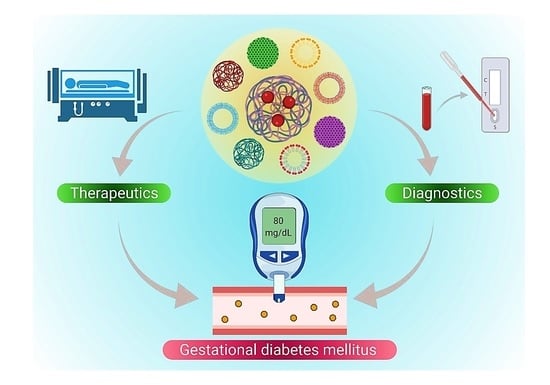
Theranostic Advances of Bionanomaterials against Gestational Diabetes Mellitus: A Preliminary Review
Abstract
:1. Introduction
2. Diagnosis of GDM
2.1. Potential Biomarkers
2.2. Role of Nanotechnology in GDM Diagnosis
3. Nanotechnology for Treatment of GDM
3.1. Use of Metallic NPs for Treatment of GDM
3.2. Use of Polymeric NPs for GDM Treatment
4. Challenges in GDM Diagnosis by Use of Nanosensors
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of diabetes mellitus and cardiovascular disease. Curr. Cardiol. Rep. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Seshiah, V.; Balaji, V.; Balaji, M.S.; Sanjeevi, C.; Green, A. Gestational diabetes mellitus in India. Japi 2004, 52, 707–711. [Google Scholar]
- Mumtaz, M. Gestational diabetes mellitus. Malays. J. Med. Sci. 2000, 7, 4–9. [Google Scholar]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2003, 26, S103–S105. [Google Scholar] [CrossRef] [Green Version]
- Metzger, B.E.; Contreras, M.; Sacks, D.; Watson, W.; Dooley, S.; Foderaro, M.; Niznik, C.; Bjaloncik, J.; Catalano, P.; Dierker, L. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational diabetes mellitus: Mechanisms, treatment, and complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care 2007, 30, S141–S146. [Google Scholar] [CrossRef] [Green Version]
- Mahalakshmi, M.M.; Bhavadharini, B.; Kumar Maheswari, R.M.A.; Jebarani, S.; Ninov, L.; Kayal, A.; Malanda, B.; Belton, A.; Uma, R.; Mohan, V. Current practices in the diagnosis and management of gestational diabetes mellitus in India (WINGS-5). Indian J. Endocrinol. Metab. 2016, 20, 364. [Google Scholar] [PubMed] [Green Version]
- Nelson, R.L. Oral glucose tolerance test: Indications and limitations. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 1988; pp. 263–269. [Google Scholar]
- Agarwal, M.M. Gestational diabetes mellitus: An update on the current international diagnostic criteria. World J. Diabetes 2015, 6, 782. [Google Scholar] [CrossRef]
- Advertising Association. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S137–S143. [Google Scholar] [CrossRef] [Green Version]
- Rowan, J.A.; Hague, W.M.; Gao, W.; Battin, M.R.; Moore, M.P. Metformin versus insulin for the treatment of gestational diabetes. N. Engl. J. Med. 2008, 358, 2003–2015. [Google Scholar] [CrossRef] [Green Version]
- Eades, C.E.; Cameron, D.M.; Evans, J.M. Prevalence of gestational diabetes mellitus in Europe: A meta-analysis. Diabetes Res. Clin. Pract. 2017, 129, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Moumaris, M.; Bretagne, J.-M.; Abuaf, N. Nanomedical Devices and Cancer Theranostics. Open Nanomed. Nanotechnol. J. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Shakeri-Zadeh, A.; Zareyi, H.; Sheervalilou, R.; Laurent, S.; Ghaznavi, H.; Samadian, H. Gold nanoparticle-mediated bubbles in cancer nanotechnology. J. Control. Release 2020, 330, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Cash, K.J.; Clark, H.A. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 2010, 16, 584–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramaniam, B.; Prateek; Ranjan, S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and Antiviral Functional Materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics. ACS Pharmacol. Transl. Sci. 2021, 4, 8–54. [Google Scholar] [CrossRef]
- Miri, A.; Beiki, H.; Najafidoust, A.; Khatami, M.; Sarani, M. Cerium oxide nanoparticles: Green synthesis using Banana peel, cytotoxic effect, UV protection and their photocatalytic activity. Bioprocess Biosyst. Eng. 2021, 44, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.; Sarani, M.; Khatami, M. Nickel-doped cerium oxide nanoparticles: Biosynthesis, cytotoxicity and UV protection studies. RSC Adv. 2020, 10, 3967–3977. [Google Scholar] [CrossRef] [Green Version]
- Nazaripour, E.; Mousazadeh, F.; Moghadam, M.D.; Najafi, K.; Borhani, F.; Sarani, M.; Ghasemi, M.; Rahdar, A.; Iravani, S.; Khatami, M. Biosynthesis of lead oxide and cerium oxide nanoparticles and their cytotoxic activities against colon cancer cell line. Inorg. Chem. Commun. 2021, 131, 108800. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, J. Long-term glycemic control and prevention of diabetes complications in vivo using oleic acid-grafted-chitosan-zinc-insulin complexes incorporated in thermosensitive copolymer. J. Control. Release 2020, 323, 161–178. [Google Scholar] [CrossRef]
- Saï, P.; Damgé, C.; Rivereau, A.; Hoeltzel, A.; Gouin, E. Prophylactic Oral Administration of Metabolically Active Insulin Entrapped in Isobutylcyanoacrylate Nanocapsules Reduces the Incidence of Diabetes inNonobese DiabeticMice. J. Autoimmun. 1996, 9, 713–721. [Google Scholar] [CrossRef]
- Sharma, B.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Gupta, V.K.; Thakur, V.K. Titania Modified Gum Tragacanth Based Hydrogel Nanocomposite for Water Remediation. J. Environ. Chem. Eng. 2021, 9, 104608. [Google Scholar] [CrossRef]
- Shalaby, T.I.; El-Refaie, W.M. Bioadhesive chitosan-coated cationic nanoliposomes with improved insulin encapsulation and prolonged oral hypoglycemic effect in diabetic mice. J. Pharm. Sci. 2018, 107, 2136–2143. [Google Scholar] [CrossRef]
- Narjinary, M.; Rana, P.; Sen, A.; Pal, M. Enhanced and selective acetone sensing properties of SnO2-MWCNT nanocomposites: Promising materials for diabetes sensor. Mater. Des. 2017, 115, 158–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Gao, X.; Chang, L.; Chen, Z.; Mei, X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater. Sci. Eng. C 2021, 120, 111671. [Google Scholar] [CrossRef] [PubMed]
- Sona, P. Nanoparticulate drug delivery systems for the treatment of diabetes. Dig. J. Nanomater. Biostruct. (DJNB) 2010, 5, 411–418. [Google Scholar]
- Kerry, R.G.; Mahapatra, G.P.; Maurya, G.K.; Patra, S.; Mahari, S.; Das, G.; Patra, J.K.; Sahoo, S. Molecular prospect of type-2 diabetes: Nanotechnology based diagnostics and therapeutic intervention. Rev. Endocr. Metab. Disord. 2021, 22, 421–451. [Google Scholar] [CrossRef]
- Jeffery, C.J. Engineering periplasmic ligand binding proteins as glucose nanosensors. Nano Rev. 2011, 2, 5743. [Google Scholar] [CrossRef]
- Wang, L.; Yun, X.; Stanacevic, M.; Gouma, P. An Acetone Nanosensor For Non-invasive Diabetes Detection. In Aip Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2009; pp. 206–208. [Google Scholar]
- Le, L.V.; Chendke, G.S.; Gamsey, S.; Wisniewski, N.; Desai, T.A. Near-infrared optical nanosensors for continuous detection of glucose. J. Diabetes Sci. Technol. 2020, 14, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Wang, M.; Li, P.; Ding, C.; Zhang, W.; Wang, H.; Tang, B. Copolymer-Based Fluorescence Nanosensor for In Situ Imaging of Homocysteine in the Liver and Kidney of Diabetic Mice. Anal. Chem. 2020, 92, 16221–16228. [Google Scholar] [CrossRef]
- Huang, C.; Hao, Z.; Qi, T.; Pan, Y.; Zhao, X. An integrated flexible and reusable graphene field effect transistor nanosensor for monitoring glucose. J. Mater. 2020, 6, 308–314. [Google Scholar] [CrossRef]
- Wahab, A.W.; Karim, A.; La Nafie, N.; Sutapa, I.W. Synthesis of silver nanoparticles using muntingia calabura L. Leaf extract as bioreductor and applied as glucose nanosensor. Orient. J. Chem. 2018, 34, 3088. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer nanotechnology: A new revolution for cancer diagnosis and therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Pashazadeh-Panahi, P.; Baradaran, B.; Maleki, A.; Hejazi, M.; Mokhtarzadeh, A.; de la Guardia, M. Recent advances on nanomaterial based electrochemical and optical aptasensors for detection of cancer biomarkers. TrAC Trends Anal. Chem. 2018, 100, 103–115. [Google Scholar] [CrossRef]
- Du, S.; Lv, Y.; Li, N.; Huang, X.; Liu, X.; Li, H.; Wang, C.; Jia, Y.-F. Biological investigations on therapeutic effect of chitosan encapsulated nano resveratrol against gestational diabetes mellitus rats induced by streptozotocin. Drug Deliv. 2020, 27, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, H.M.; Lappas, M.; Georgiou, G.M.; Marita, A.; Bryant, V.J.; Hiscock, R.; Permezel, M.; Khalil, Z.; Rice, G.E. Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol. 2008, 45, 157–165. [Google Scholar] [CrossRef]
- Nanda, S.; Savvidou, M.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat. Diagn. 2011, 31, 135–141. [Google Scholar] [CrossRef]
- Rodrigo, N.; Glastras, S.J. The emerging role of biomarkers in the diagnosis of gestational diabetes mellitus. J. Clin. Med. 2018, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Bogdanet, D.; Reddin, C.; Murphy, D.; Doheny, H.C.; Halperin, J.A.; Dunne, F.; O’Shea, P.M. Emerging Protein Biomarkers for the Diagnosis or Prediction of Gestational Diabetes—A Scoping Review. J. Clin. Med. 2021, 10, 1533. [Google Scholar] [CrossRef] [PubMed]
- Sedgh, G.; Singh, S.; Hussain, R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud. Fam. Plan. 2014, 45, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Seshiah, V.; Balaji, V.; Balaji, M.S.; Panneerselvam, A.; Thamizharasi, M.; Arthi, T. Glycemic level at the first visit and prediction of GDM. JAPI 2007, 55, 630–632. [Google Scholar] [PubMed]
- Subramani, K.; Pathak, S.; Hosseinkhani, H. Recent trends in diabetes treatment using nanotechnology. Dig. J. Nanomater. Biostructures (DJNB) 2012, 7, 85–95. [Google Scholar]
- Arya, A.K.; Kumar, L.; Pokharia, D.; Tripathi, K. Applications of nanotechnology in diabetes. Dig. J. Nanomater. Biostructures 2008, 3, 221–225. [Google Scholar]
- Ge, Y.; Lakshmipriya, T.; Gopinath, S.C.; Anbu, P.; Chen, Y.; Hariri, F.; Li, L. Glucose oxidase complexed gold-graphene nanocomposite on a dielectric surface for glucose detection: A strategy for gestational diabetes mellitus. Int. J. Nanomed. 2019, 14, 7851. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Zhang, H.; Lakshmipriya, T.; Gopinath, S.C.; Yang, N. Gold nanorod integrated electrochemical sensing for hyperglycaemia on interdigitated electrode. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xie, W.; Luo, Y.; Ding, X.; Fu, B.; Gopinath, S.C.; Xiong, Y. Sensitive silica-alumina modified capacitive non-faradaic glucose sensor for gestational diabetes. Biotechnol. Appl. Biochem. 2021. [Google Scholar] [CrossRef]
- Pandey, I.; Tiwari, J.D. A novel dual imprinted conducting nanocubes based flexible sensor for simultaneous detection of hemoglobin and glycated haemoglobin in gestational diabetes mellitus patients. Sens. Actuators B Chem. 2019, 285, 470–478. [Google Scholar] [CrossRef]
- Lyons, T.J.; Basu, A. Biomarkers in diabetes: Hemoglobin A1c, vascular and tissue markers. Transl. Res. 2012, 159, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, P.A.C.; Ehlert, L.R.; Camargo, J.L. Glycated albumin: A potential biomarker in diabetes. Arch. Endocrinol. Metab. 2017, 61, 296–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belsare, S.; Coté, G. Development of a colorimetric paper fluidic dipstick assay for measurement of glycated albumin to monitor gestational diabetes at the point-of-care. Talanta 2021, 223, 121728. [Google Scholar] [CrossRef]
- Ki, H.; Jang, H.; Oh, J.; Han, G.-R.; Lee, H.; Kim, S.; Kim, M.-G. Simultaneous detection of serum glucose and glycated albumin on a paper-based sensor for acute hyperglycemia and diabetes mellitus. Anal. Chem. 2020, 92, 11530–11534. [Google Scholar] [CrossRef]
- Singh, A.; Subramani, E.; Ray, C.D.; Rapole, S.; Chaudhury, K. Proteomic-driven biomarker discovery in gestational diabetes mellitus: A review. J. Proteom. 2015, 127, 44–49. [Google Scholar] [CrossRef]
- Ai, T.; Chen, F.; Zhou, S.; Zhang, J.; Zheng, H.; Zhou, Y.; Hu, W.; Liu, X.; Li, L.; Lin, J. Magnetic bead-based serum peptidome profiling in patients with gestational diabetes mellitus. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Sokup, S.J. ISOLATION of Urinary Epithelial Cell DNA for the Analysis of 4-Aminobiphenyl DNA Adducts by Nano-LC/ESI-MS/MS. Master’s Thesis, Northeastern University, Boston, MA, USA, 2012. [Google Scholar]
- Buchanan, T.A.; Xiang, A.; Kjos, S.L.; Watanabe, R. What is gestational diabetes? Diabetes Care 2007, 30, S105–S111. [Google Scholar] [CrossRef] [Green Version]
- Sheik, R. Assessment of Major Complications in Pregnancy: A Single Center Study. Asian J. Pharm. Res. Dev. 2017, 5, 1–6. [Google Scholar]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. N.P.G. Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 1056–1058. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef] [Green Version]
- Silva, G.A. Seeing the benefits of ceria. Nat. Nanotechnol. 2006, 1, 92–94. [Google Scholar] [CrossRef]
- Vafaeipour, Z.; Shokrzadeh, M.; Jahani, M.; Shaki, F. Protective Effect of nanoceria against streptozotocin induced mitochondrial dysfunction in embryo of diabetic mice. J. Maz. Univ. Med. Sci. 2015, 25, 109–120. [Google Scholar]
- Najafi, R.; Hosseini, A.; Ghaznavi, H.; Mehrzadi, S.; Sharifi, A.M. Neuroprotective effect of cerium oxide nanoparticles in a rat model of experimental diabetic neuropathy. Brain Res. Bull. 2017, 131, 117–122. [Google Scholar] [CrossRef]
- Guan, B.; Yan, R.; Li, R.; Zhang, X. Selenium as a pleiotropic agent for medical discovery and drug delivery. Int. J. Nanomed. 2018, 13, 7473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei-Kelishadi, M.; Ghasemi, A.; Abdolyosefi, N.N.; Zamani-Doabi, S.; Ramezani, M.; Changizi-Ashtiyani, S.; Rahimi, A. Effects of selenium nanoparticles on kidney and liver functional disorders in streptozotocin-induced diabetic rats. Physiol. Pharmacol. 2017, 21, 155–162. [Google Scholar]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Graft Copolymerization and Applications of Chitosan: A Review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Hassan, I.; Ebaid, H.; Al-Tamimi, J.; Habila, M.A.; Alhazza, I.M.; Rady, A.M. Selenium nanoparticles mitigate diabetic nephropathy and pancreatopathy in rat offspring via inhibition of oxidative stress. J. King Saud Univ.-Sci. 2021, 33, 101265. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, S.; Fan, W.; Jiang, Y.; Yang, J.; Tong, Z.; Jiang, G. Dual responsive block copolymer coated hollow mesoporous silica nanoparticles for glucose-mediated transcutaneous drug delivery. Chin. J. Chem. Eng. 2021, in press. [Google Scholar] [CrossRef]
- Dave, P.N.; Gor, A. Natural polysaccharide-based hydrogels and nanomaterials: Recent trends and their applications. Handb. Nanomater. Ind. Appl. 2018, 36–66. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, Y.; Jia, Q.; Guo, N.; Wang, Z.; Wang, Y. Novel greener approached synthesis of polyacrylic nanoparticles for therapy and care of gestational diabetes. Drug Deliv. 2020, 27, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Guo, Y.; Wu, H.; Huang, Y.; Xie, D.; Qin, J. Conceivable Protective Role of Murraya koenigii Leaf Extract Loaded Poly (D, L-lactic-co-glycolic acid)-Gold-Nanoparticles on the Gestational Diabetes Mellitus of Rats Induced by Streptozotocin. Sci. Adv. Mater. 2020, 12, 87–92. [Google Scholar] [CrossRef]
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R. Managing diabetes with nanomedicine: Challenges and opportunities. Nat. Rev. Drug Discov. 2015, 14, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Khalil, O.S. Non-Invasive Monitoring of Diabetes. In Glucose Sensing; Springer: Berlin/Heidelberg, Germany, 2006; pp. 165–199. [Google Scholar]
- Scognamiglio, V. Nanotechnology in glucose monitoring: Advances and challenges in the last 10 years. Biosens. Bioelectron. 2013, 47, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, W.; Meng, X.; Zhao, W.; Pan, J.; Tang, J.; Huang, Y.; Tao, M.; Liu, F. Heterogeneity of insulin resistance and beta cell dysfunction in gestational diabetes mellitus: A prospective cohort study of perinatal outcomes. J. Transl. Med. 2018, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; Cui, W.; Chaikof, E.L. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008, 8, 1940–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krol, S.; Del Guerra, S.; Grupillo, M.; Diaspro, A.; Gliozzi, A.; Marchetti, P. Multilayer nanoencapsulation. New approach for immune protection of human pancreatic islets. Nano Lett. 2006, 6, 1933–1939. [Google Scholar] [CrossRef]
- Contreras, J.L.; Xie, D.; Mays, J.; Smyth, C.A.; Eckstein, C.; Rahemtulla, F.G.; Young, C.J.; Thompson, J.A.; Bilbao, G.; Curiel, D.T. A novel approach to xenotransplantation combining surface engineering and genetic modification of isolated adult porcine islets. Surgery 2004, 136, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.P.; Hayes, J.L.; Chick, W.L. Encapsulated cell technology. Nat. Biotechnol. 1996, 14, 1107–1111. [Google Scholar] [CrossRef]
- Dolgin, E. Encapsulate this; these precious cells. A team of bioengineers thinks it has discovered one: Insulin-producing islet cells could hold the secret to curing type 1 diabetes—If only scientists could figure out a way to encapsulate and transplant them into the body. But first, the right biocompatible material must be found to hold. Nat. Med. 2014, 20, 9–12. [Google Scholar] [PubMed]
- Sharma, D.; Shandilya, P.; Saini, N.K.; Singh, P.; Thakur, V.K.; Saini, R.V.; Mittal, D.; Chandan, G.; Saini, V.; Saini, A.K. Insights into the Synthesis and Mechanism of Green Synthesized Antimicrobial Nanoparticles, Answer to the Multidrug Resistance. Mater. Today Chem. 2021, 19, 100391. [Google Scholar] [CrossRef]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent Progress in Sodium Alginate Based Sustainable Hydrogels for Environmental Applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, S.D.; Mohapatra, P.C.; Aria, A.I.; Christie, G.; Mishra, Y.K.; Hofmann, S.; Thakur, V.K. Piezoelectric Materials for Energy Harvesting and Sensing Applications: Roadmap for Future Smart Materials. Adv. Sci. 2021, 8, 2100864. [Google Scholar] [CrossRef]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose Nanocrystals: Pretreatments, Preparation Strategies, and Surface Functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef]
- Walsh, T.R.; Knecht, M.R. Biointerface Structural Effects on the Properties and Applications of Bioinspired Peptide-Based Nanomaterials. Chem. Rev. 2017, 117, 12641–12704. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barani, M.; Sargazi, S.; Mohammadzadeh, V.; Rahdar, A.; Pandey, S.; Jha, N.K.; Gupta, P.K.; Thakur, V.K. Theranostic Advances of Bionanomaterials against Gestational Diabetes Mellitus: A Preliminary Review. J. Funct. Biomater. 2021, 12, 54. https://doi.org/10.3390/jfb12040054
Barani M, Sargazi S, Mohammadzadeh V, Rahdar A, Pandey S, Jha NK, Gupta PK, Thakur VK. Theranostic Advances of Bionanomaterials against Gestational Diabetes Mellitus: A Preliminary Review. Journal of Functional Biomaterials. 2021; 12(4):54. https://doi.org/10.3390/jfb12040054
Chicago/Turabian StyleBarani, Mahmood, Saman Sargazi, Vahideh Mohammadzadeh, Abbas Rahdar, Sadanand Pandey, Niraj Kumar Jha, Piyush Kumar Gupta, and Vijay Kumar Thakur. 2021. "Theranostic Advances of Bionanomaterials against Gestational Diabetes Mellitus: A Preliminary Review" Journal of Functional Biomaterials 12, no. 4: 54. https://doi.org/10.3390/jfb12040054
APA StyleBarani, M., Sargazi, S., Mohammadzadeh, V., Rahdar, A., Pandey, S., Jha, N. K., Gupta, P. K., & Thakur, V. K. (2021). Theranostic Advances of Bionanomaterials against Gestational Diabetes Mellitus: A Preliminary Review. Journal of Functional Biomaterials, 12(4), 54. https://doi.org/10.3390/jfb12040054